Introduction
The growth and development of the prostate is
regulated by hormones. Abnormal hormonal secretions lead to
prostatic lesions, including benign prostatic hyperplasia (BPH). A
previous study by Alonso-Magdalena et al (1) reported that BPH is not a disease of
prostatic stromal proliferation, but of the accumulation of
mesenchymal-like cells derived from the prostatic epithelium and
endothelium. Therefore, the proliferation of epithelial cells in
the hyperplastic acini is indispensable for benign growth of the
prostate gland.
Bisphenol A (BPA), a xenoestrogen, is a key monomer
and industrial plasticizer, fungicide, flame retardant and
component of epoxy resins used in food packaging, including cans
and metal jar lids (2,3). In humans, data from previous studies
suggests that BPA may induce adverse outcomes and medical
conditions, particularly on the reproductive and metabolic systems.
However, the majority of these studies examine non-occupationally
exposed people, who may be considered ‘low dose’, and thus these
studies support the hypothesis that average levels of BPA exposure
is sufficient to cause toxicity and affect human health (4). ‘Low dose’ has become a widely used term
referring to toxicity studies for BPA that has been considered more
informative about the potential health risk in humans compared with
higher exposure studies (5). There
are numerous animal studies demonstrating causal associations
between BPA exposure and harm.
As an endocrine disruptor, BPA stimulates cellular
responses at low concentrations, below the levels where BPA is
expected to bind to classical nuclear estrogen receptors (ERs). For
example, BPA altered the differentiation pattern of periductal
stromal cells of the ventral prostate by diminishing the expression
of androgen receptors (AR) andprostatic acid phosphatase (PAP)
(6). Additionally, studies have
demonstrated that BPA induces PCa cell migration via the modulation
of the ion channel protein expression involved in calcium entry and
in cancer cell migration (7). In
addition to the adverse effect in vitro, BPA is known to
affect prostate weight in rats (8,9) and elicit
cytokeratin 10 expression in the prostatic epithelium of mice
(10). The administration of 10 µg/kg
BPA to Sprague-Dawley (SD) rats on days 1, 3 and 5 markedly
increased the incidence of adult estrogen-induced prostate
intraepithelial neoplasia compared with control rats (11). We have previously demonstrated that
environmental exposure to a low daily dose, 10 µg/kg intragastric,
of BPA may induce proliferation of the ventral prostate in adult
rats by increasing the estrogen to androgen ratio and upregulating
the expression of prostaglandin D2 synthase to promote the
production of dihydrotestosterone (12). However, a direct association between
low dose BPA and alterations to the prostatic epithelium has not
been investigated.
Based on the aforementioned evidence, it is
hypothesized that environmental exposure to BPA may promote the
proliferation of prostate cells in the ventral prostate. In the
present study, the possible mechanisms of action and the direct
proliferative influence of BPA on primary cultured prostatic
epithelium in rats were examined.
Materials and methods
Ethical standards
All animals in the present study were treated
humanely according to the Guide for the Care and Use of Laboratory
Animals of the Shanghai Institute of Planned Parenthood Research
Animal Care and Use Committee (Shanghai, China).
Animals and housing
Male SD rats aged 10 weeks and weighing 240 g were
purchased from Sino-British SIPPR/BK Laboratory Animal Co., Ltd.,
Shanghai, China. The animals were housed on sawdust bedding in
standard polypropylene cages. Drinking water and a pellet diet
(Shanghai Shilin Science & Tech Co., Ltd, China) were available
ad libitum in glass bottles. The rooms were maintained at
temperatures between 20 and 26°C and 40–70% humidity under a 12:12
h light: dark cycle. Animals were anesthetized with pentobarbital
sodium following 5 days of adaptive breeding. All animal procedures
were approved by the Animal Care and Use Committee of Shanghai
Institute of Planned Parenthood Research (Shanghai, China), and
conformed to the Guide for the Care and Use of Laboratory Animals
(13).
Reagents
BPA (lot no., 162k0715; purity, 95%) and collagenase
II were purchased from Sigma-Aldrich; Epidermal growth factor and
cholera toxin were purchased from Merck KGaA (Darmstadt, Germany).
Insulin and transferrin were purchased from R&D Systems China
Co., Ltd. (Shanghai, China). The RPMI-1640 medium (without phenol
red, with glutamine) was from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). RPMI-1640 medium (with glutamine) was supplied
by Hyclone; GE Healthcare Sciences (Logan, UT, USA). Fetal bovine
serum (FBS) was sourced from Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd. (Hangzhou, China). Mouse anti-pan
cytokeratin (cat. no. BM0034), rabbit anti-androgen receptor (cat.
no. BA0004), rabbit anti-ERα (cat. no. BA0345), rabbit anti-ERβ
(cat. no. BA2210), StreptAvidin Biotin peroxidase Complex (SABC;
SA1022) kit, and 3′3 diaminobenzidine (DAB; cat. no. AR1022) kit
were purchased from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China). Dimethyl sulphoxide (DMSO) was sourced from
Shanghai Shisheng Cell Biology Technology Co., Ltd. (Shanghai,
China). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Molecular Technologies (Kumamoto, Japan). Annexin V-fluorescein
isothiocyante (FITC), propidium iodide (PI) and 5X binding buffer
were purchased from Merck & Co., Inc. (Whitehouse Station, NJ,
USA).
Cell culture
As described previously (14), the ventral prostates were removed,
weighed and cut into 1.0-mm3 blocks. Subsequent to the
addition of 675 U/ml collagenase II, the tissue suspension was
blown 3 times with a straw and incubated at 37°C for 1 h. The
suspension was then cooled to 4°C and looped through 200-, 300- and
400-mesh sieves to obtain individual cells. The cells were
collected subsequent to centrifugation at 4°C at 1,500 x g for 5
min, and dispersed in RPMI-1640 at a concentration of 5×105
cells/ml. The cells were seeded in RPMI-1640 culture medium
supplemented with 10% FBS, 100 µg/ml penicillin, 100 U/ml
streptomycin, 10 µg/l epidermal growth factor, 10 µg/l cholera
toxin, 5 mg/l transferrin and 5 mg/l insulin, and cultured at 37°C
with 5% CO2, changing the culture medium every 3–5
days.
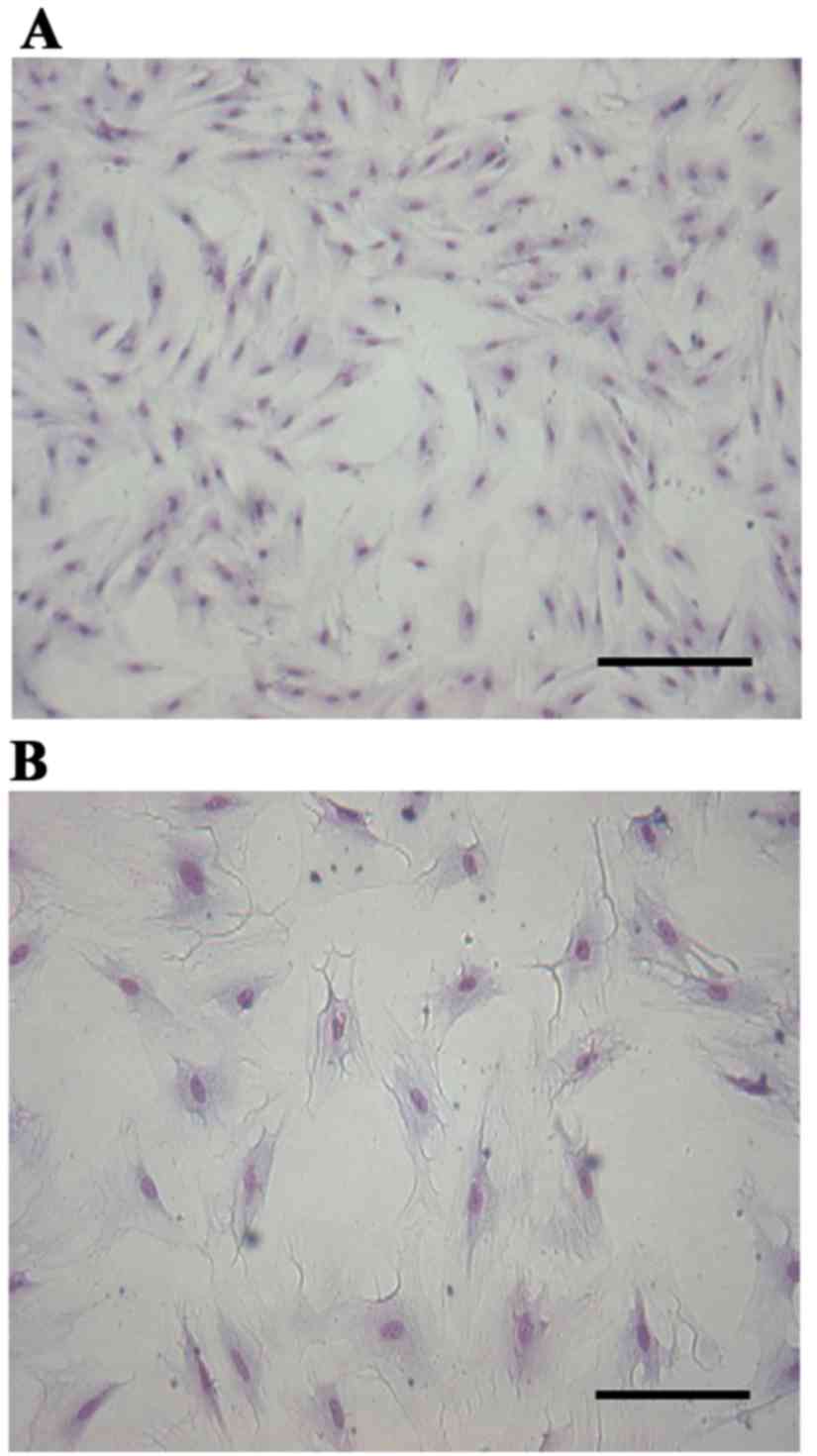
Cell identification
The cells were dispersed at a density of 1×105/ml
and seeded in 12-well plates for culturing. The medium was removed
subsequent to 12 days of adherent growth. The slides were fixed
with 10% formalin for 60 min and endogenous peroxidase was quenched
with hydrogen peroxide in methanol solution (fresh, 30%
H2O2; methyl alcohol; dilution, 1:50) for 30
min. The cells were washed with distilled water and immersed in 5%
bovine serum albumin for 20 min at room temperature to block the
non-specific binding sites prior to incubation with mouse anti-pan
cytokeratin (dilution, 1:100) in a wet box at 4°C overnight.
Biotin-labeled goat anti-rabbit IgG (contained within the SABC kit;
ready-to-use) and SABC kit were added successively at 37°C for 20
min, followed by staining with a DAB kit. The sections were
counterstained with hematoxylin and dehydrated, washed, mounted and
observed under a Motic inverted microscope.
Treatment
Theprostatic epithelium suspension was seeded in
96-well plates and 24-well plates, and each hole was filled with
200 µl suspension. The culture medium was replaced every 3 days.
Subsequent to 12 days cultivation, the culture medium was
substituted with phenol red-free RPMI-1640 without serum, and BPA
was added to make a final concentration of 0.01, 0.1, 1, 10, 100
and 1000 nM. This solution was maintained for 72 h, subsequently
the cells were processed for subsequent experiments. DMSO was used
as the control, and the final concentration of the solution was
below 0.5%.
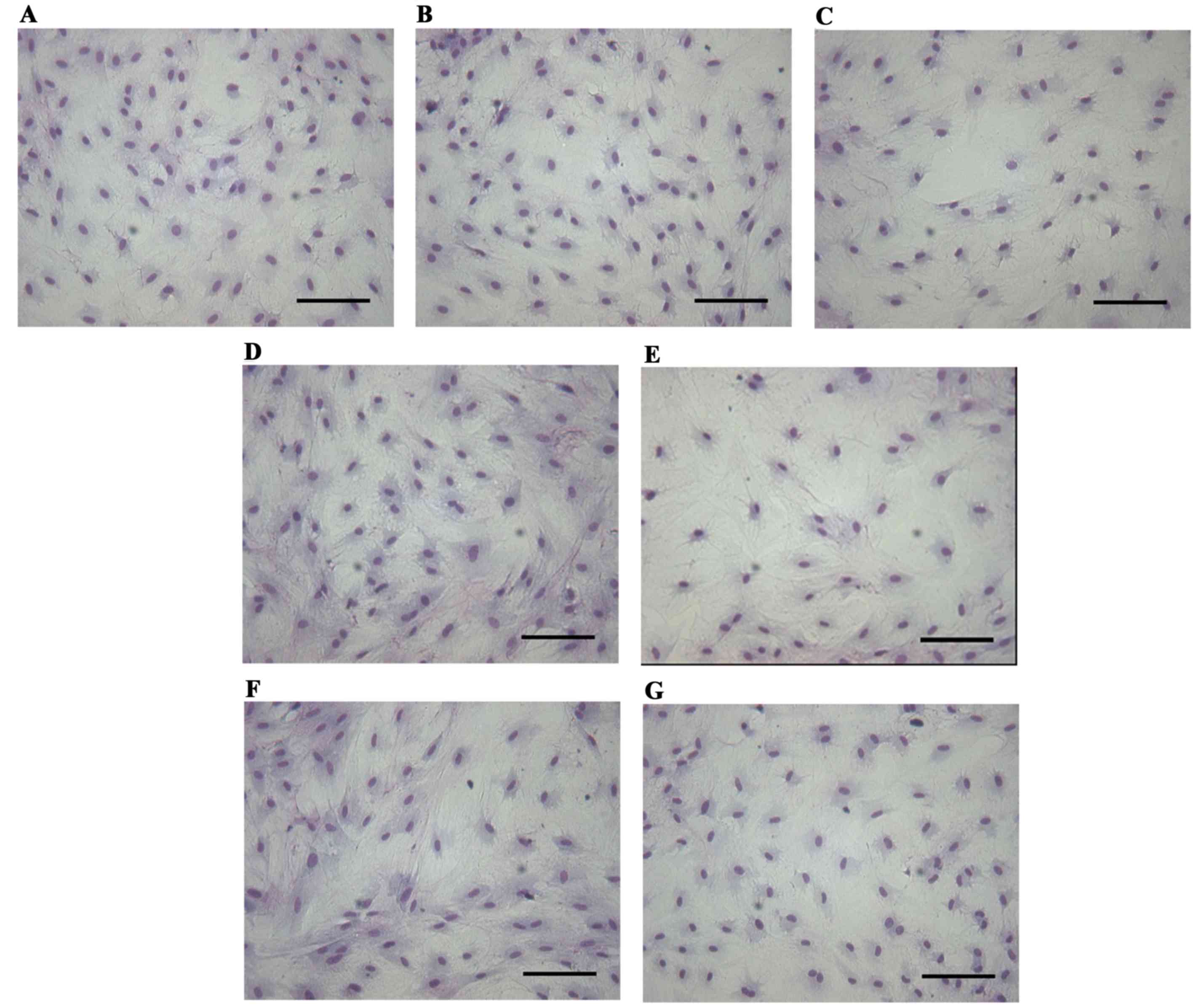
Cell morphological analysis by
Giemsa
The medium of prostatic epithelium inoculated in
24-well plates was discarded subsequent to treatment. The plates
were then washed twice with physiological saline prior to fixation
with 95% ethanol for 30 min and staining with Giemsa for 15 min.
The reactions were halted with water and the images were captured
with a Motic inverted microscope.
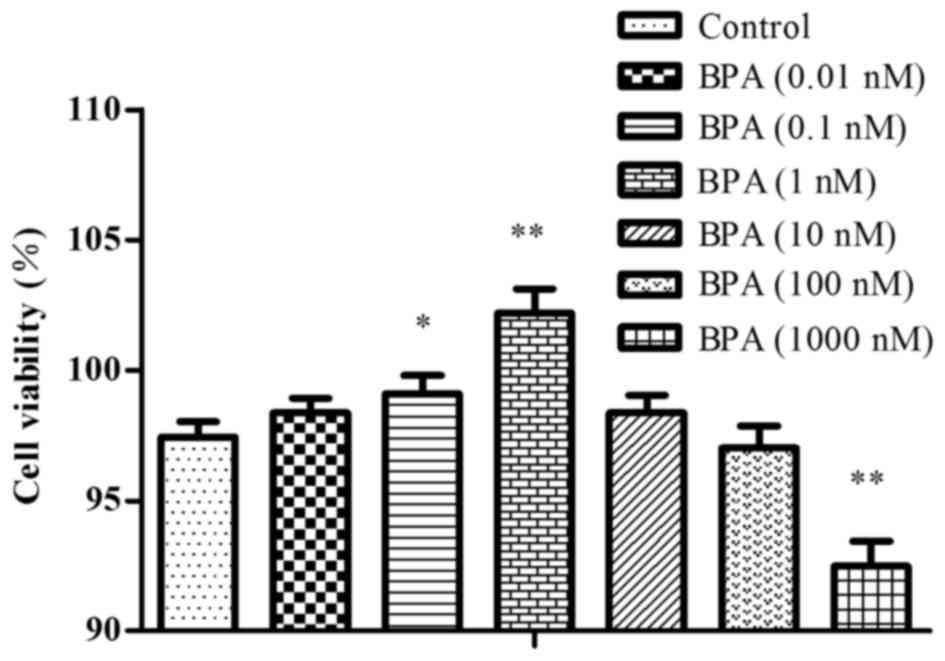
Cell viability assay by CCK-8
The supernatant of prostatic epithelium inoculated
in the 96-well plates was discarded, and the cell viability was
detected by the WST-8 assay with CCK-8, according to the protocol
of the manufacturer. Absorbance was measured at 450 nm using a
microplate reader. The cell viability was calculated using the
formula: Cell viability (%) = [(the experimental value-the blank
value) / (the control value-the blank value)] x100. A cell
viability chart was then drawn.
Detection of apoptosis by flow
cytometry
Subsequent to producing the cell viability chart,
the proliferation mechanism of BPA on prostate epithelial cells was
investigated. The cells were treated with 0.01, 0.1 and 1 nM BPA
for 72 h, respectively, and the prostatic epithelia were then
digested to obtain single cells at a density of 1×106/ml and
centrifuged at 4°C and 1,500 x g for 5 min. The supernatant was
discarded and the cells were washed with the binding buffer,
centrifuged (4°C, 1,500 x g) and incubated with 100 µl Annexin
V-FITC for 10 min at room temperature. Washing and centrifugation
(4°C, 1,500 x g) were repeated, followed by the addition of PI. The
mixture was incubated for 20 min at 4°C, and cell apoptosis was
detected by flow cytometry (FACSCalibur™, BD Biosciences, Franklin
Lakes, NJ, USA).
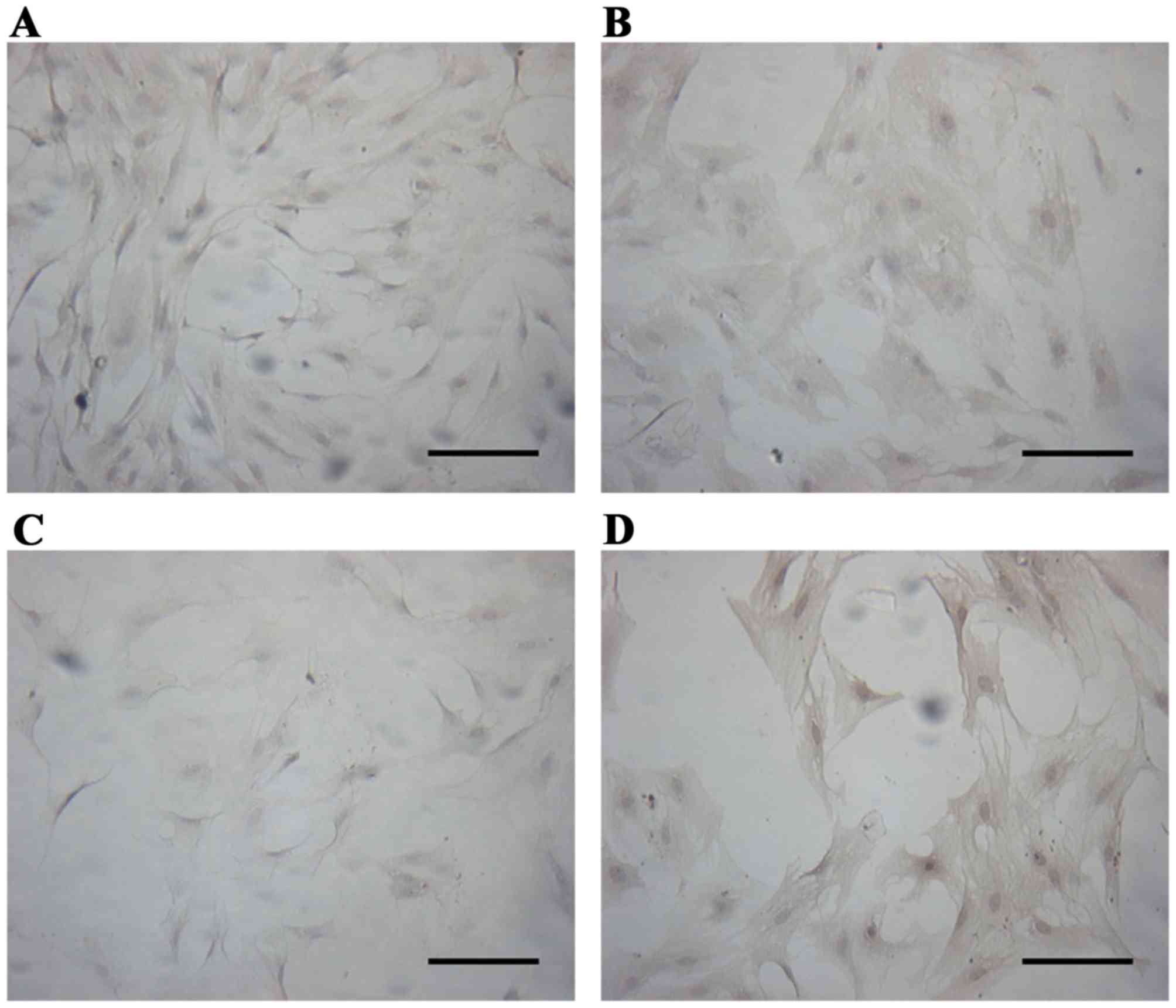
Evaluation of AR and ER expression by
immunocytochemistry analysis
The cells were dispersed at a density of 1×105/ml
and seeded in 12-well plates for culturing. The medium was removed
subsequent to 12 days of adherent growth. The slides were fixed
with 10% formalin for 60 min and endogenous peroxidase was quenched
with hydrogen peroxide in methanol solution (fresh, 30%
H2O2; methyl alcohol, dilution, 1:50) for 30
min. The cells were washed with distilled water and immersed in 5%
bovine serum albumin for 20 min at room temperature to block the
non-specific binding sites prior to incubation with the
anti-androgen receptor, anti-ERα and anti-ERβ primary antibodies
(dilution, 1:100) in a wet box at 4°C overnight. Biotin-labeled
Goat Anti-Rabbit IgG and SABC were added successively at 37°C for
20 min, followed by staining with a DAB kit. The sections were
counterstained with hematoxylin and dehydrated, washed, mounted,
and observed under a Motic inverted microscope. Finally, 120 cells
in each group were randomly selected to obtain the mean values with
the Motic Images Advanced 3.2 software (Motic, Kowloon, Hong
Kong).
Statistical analysis
Data were analyzed using SPSS version 11.0 (SPSS,
Inc., Chicago, IL, USA) and expressed as the mean ± standard
deviation of three experimental repeats. Statistical comparisons
were performed by one-factor analysis of variance. If statistically
significant, the differences between control and treatment groups
were tested by the least-squares means test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell morphological analysis
Giemsa staining revealed that the cultured cells
were flattened and polygonal, the nucleus was approximately oval
and located in the center of the cytoplasm and the cells were
closely linked and appeared to grow in clusters. All these features
conformed to the features of epithelial cells (Fig. 1). Subsequent to treatment of the
prostatic epithelium with 0.01–1,000 nM BPA for 72 h, no marked
changes in cell morphology were observed compared with the control
group (Fig. 2).
Cell viability
The cells were exposed to 0.01–1,000 nM BPA, and
cell viability was tested by the CCK-8 method. At doses of 0.01–1
nM BPA, the cells exhibited growth-promoting activity and the cell
survival rate increased as BPA dose increased. In the cells exposed
to a dose of BPA >1 nM, there was less growth promotion, and
there was growth-nhibition with the dose of 1,000 nM, as presented
in Fig. 3.
Cell apoptosis
In Annexin V-FITC/PI double staining, the apoptotic
cells resisted staining by PI, whilst the necrotic cells did not.
All DNA with damaged membranes were dyed fluorescent red by PI,
whilst the cells with intact membranes were not dyed. Therefore,
these cells did not emit a red fluorescence signal in the early
stages of apoptosis, which was exhibited by the normal living
cells. These results demonstrated that the apoptosis rate in 0.01–1
nM BPA-treated groups was lower, and the quantity of living cells
was higher compared with in the control group, which was consistent
with the trends in cell viability, as summarized in Table I.
 | Table I.Effects of BPA on the apoptosis of
prostate epithelial cells. |
Table I.
Effects of BPA on the apoptosis of
prostate epithelial cells.
| Dose (nM) | Necrotic (%) | Living cells (%) | Apoptotic cells
(%) |
|---|
| Control | 3.15 | 89.64 | 4.87 |
| BPA (0.01) | 0.70 | 96.14a |
2.72a |
| BPA (0.1) | 1.86 | 94.09a |
2.53a |
| BPA (1) | 1.14 | 93.92a |
2.77a |
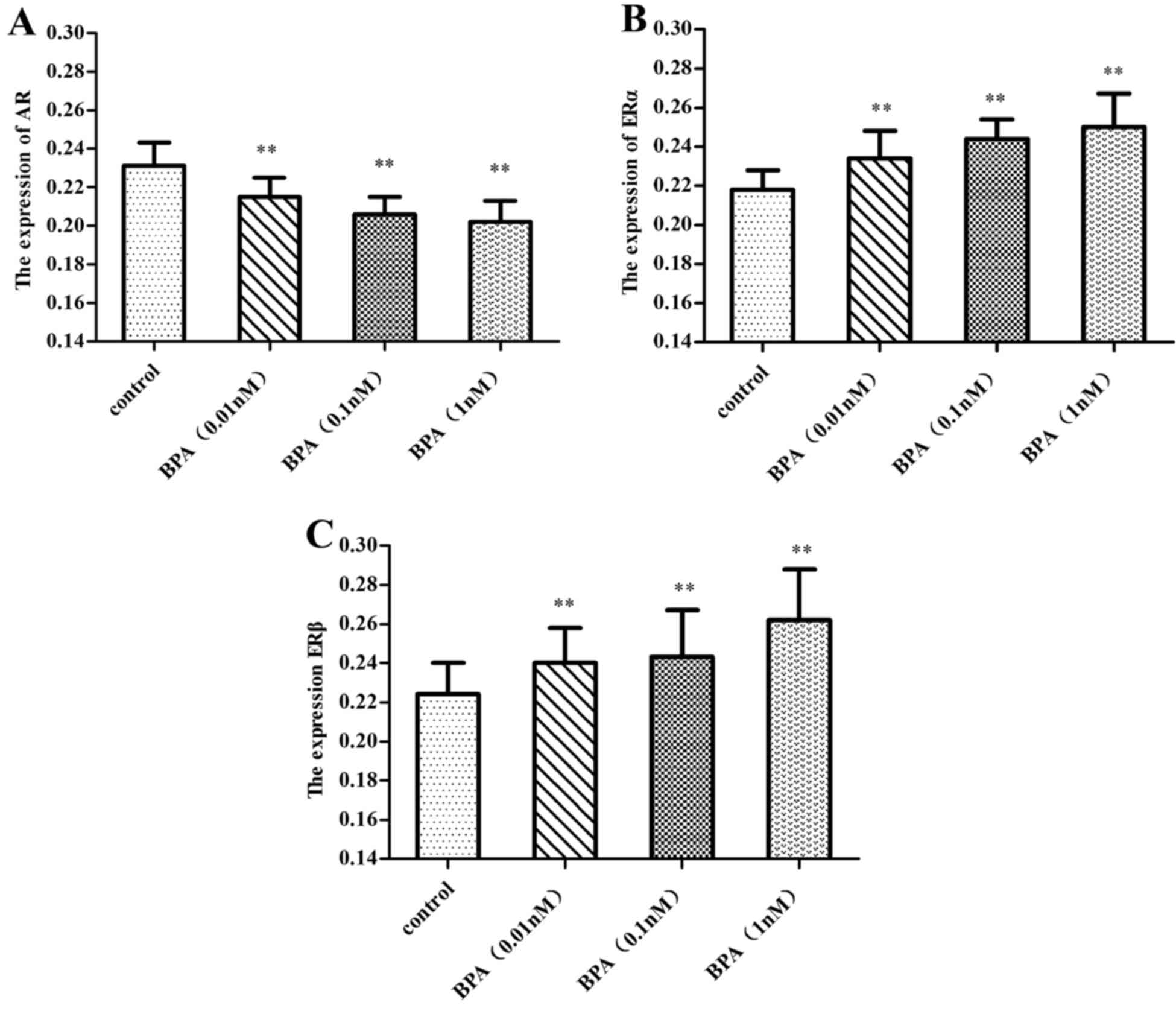
Expressions of AR and ER in prostate
epithelial cells
Immunocytochemistry analysis revealed that a 0.01–1
nM dose of BPA downregulated the expression of AR (P<0.01), and
this inhibition increased with increasing doses of BPA.
Simultaneously, BPA upregulated the expression of ERα and ERβ
(P<0.01), and the expression of ERβ increased with increasing
dose of BPA, while the expression of ERα decreased slightly, as
illustrated in Figs. 4–7.
Discussion
The exposure to low doses of BPA, an endocrine
disruptor, has been demonstrated to induce proliferation of the
prostate and aggravation of testosterone-induced BPH in rats, by
affecting the prostatic epithelium of the ventral prostate
(15). In the present study, prostate
epithelial cells were cultured successfully. Prostatic epithelial
cells of rats in a primary cultured system were established
smoothly under the experimental conditions, which permitted
additional investigation into the pathogenesis of BPH and the
mechanism of action of BPA.
Similar to hormones, EDCs are generally reported to
demonstrate a bi-phasic dose response as they are stimulating at
low doses and inhibiting at high doses. U-shaped or inverted
U-shaped non-monotonic dose-response curves are used to demonstrate
this effect. For example, Gualtieri et al (16) used Sertoli cells exposed to series of
doses, 0.5 nM-100 µM, of BPA, to demonstrate that only intermediate
doses, 10–50 µM, enhanced cell viability through increasing the
levels of cell-protecting glutathione. In the present study, cell
viability testing by the CCK-8 method revealed that BPA elicited
bi-phasic dose responses, as 0.01–1 nM BPA promoted cell growth,
but 10–1,000 nM elicited growth inhibition. The inverse U-shape
dose-response curve maybe more marked if the dose interval was
diminished. However, the present study focused on the effect of BPA
treatment on cell proliferation activity, because it was more
applicable. A dose of 1 nM BPA was equivalent to the BPA detected
in serum in environmental exposures (17), which suggests that BPA may promote
prostate cell growth directly at an environmental level. This
result was consistent with another study that revealed that 1 nM
BPA promoted the hyperplasia of testosterone-dependent prostate
cancer cells (18).
Although BPA has been reported to be involved in
apoptosis of numerous types of cells, the exposure dose and cell
lines may be important factors in how the chemical affects
apoptosis. For instance, 100 µg/ml BPA caused comparable apoptosis
by increasing cytosolic Ca2+ level, reducing the
transmembrane mitochondrial potential, increasing caspase −8, −9,
−3 activities and poly (ADP-ribose) Polymerase-1 cleavage in
peripheral blood mononuclear cells (19). Zhou at al (20) revealed that in vitro 0.1, 1, 5
and 10 µg/ml BPA exposure significantly inhibited germ cell nest
breakdown by altering the expression of key ovarian apoptotic
genes. BPA appears to inhibit apoptosis at low doses but promotes
apoptosis at high doses, which conforms to the effect on cell
viability. The present study demonstrated that inhibition of
apoptosis was observed in the prostate epithelial cells exposed to
0.01–1 nM BPA, whilst BPA doses of >1 nM may only exhibit slight
cytotoxic effects (21). Therefore,
apoptosis may participate in the effects of BPA on the prostate
within an appropriate range of exposures.
BPA is considered to exhibit weak estrogenic
activity based on the relative binding affinity of the compound for
nuclear receptors ERα and ERβ, which is ~1,000-10,000 times less
than the affinity of the compound for estradiol (22). ERα exists in the nucleus and is
distributed mainly in the prostatic stroma; it may be detected in
the epithelium subsequent to exposure to estrogen. ERβ is a member
of the estrogen receptor family and the nuclear receptor
superfamily (23). ERα and ERβ may be
involved in the regulation of transcription (24). Generally, ERα promotes the hyperplasia
of prostatic epithelial cells, whilst ERβ possesses
anti-proliferative effects (25),
which contributes to the maintenance of a dynamic balance between
promoting and inhibiting cell growth. BPA combines with ER
(26,27), and the responses of ERα and ERβ to BPA
differ as BPA binds to ERα with a lesser affinity compared with
ERβ. In the present study, a combination of BPA and ER led to an
increase in the level of ER expression, which correspondingly
promoted cell proliferation and inhibition. However, the greatest
effect was exhibited in the rates of cell proliferation in the
cells treated with lower doses, 0.01–1 nM, of BPA. Conversely, with
the dose increasing, the inhibition may enhance. In addition to
binding to ERs, BPA exposure has been demonstrated to interfere
with the thyroid hormone pathway by binding to thyroid hormone
receptor (28).
The prostate is an androgen-dependent organ, and AR
serves a pivotal role in regulating the function, growth and
differentiation of the prostate gland. In the present study, it was
revealed that 0.01–1 nM BPA reduced the expression of AR. BPA
exhibits strong antiandrogenic activity, both in vitro and
in vivo, with a lower affinity for AR compared with ER
(29,30). The AR is a ligand-activated
transcription factor and binds to specific elements of the androgen
response on target genes to stimulate transcription (31), but this activity is effectively
ablated by BPA, leading to the disruption of transcription and
androgen-independent prostate cancer cell proliferation (32). Environmental exposures to low doses of
BPA activated a mutated AR and promoted testosterone-dependent cell
proliferation, and that BPA acts as a ligand for the AR mutant and
stimulates cell proliferation at doses of ≤1 nM (18). However, there is a debate concerning
whether upregulated or downregulated expression of AR is important.
The weak estrogenic and antiandrogenic activity of BPA possibly
results in an imbalance of estrogen and androgen, leading to an
overall increase in the relative levels of estradiol, which is
considered a factor leading to the development of BPH (33).
In conclusion, the present study suggests that
environmental exposure to BPA directly promoted the proliferation
of prostate cells, and that this effect may have been achieved by
downregulating the expression of AR and upregulating the expression
of ER in cells, inhibiting cell apoptosis. However, the complicated
crosstalk amongst different signaling pathways of BPA on the
prostate and the possible mechanisms by which BPA activates ER and
antagonizes AR functions, alongside the pathways that are involved
in apoptosis require future investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no., 21007041), the
Shanghai Public Service Platform of Research & Development
(grant no., 13DZ2291300) and the Talents Development Foundation of
Shanghai Municipality (grant no., 201372).
References
|
1
|
Alonso-Magdalena P, Brössner C, Reiner A,
Cheng G, Sugiyama N, Warner M and Gustafsson JA: A role for
epithelial-mesenchymal transition in the etiology of benign
prostatic hyperplasia. Proc Natl Acad Sci USA. 106:pp. 2859–2863.
2009; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tyl RW, Myers CB, Marr MC, Thomas BF,
Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC,
et al: Three-generation reproductive toxicity study of dietary
bisphenol A in CD Sprague-Dawley rats. Toxicol Sci. 68:121–146.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Biedermann S, Tschudin P and Grob K:
Transfer of bisphenol A from thermal printer paper to the skin.
Anal Bioanal Chem. 398:571–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vandenberg LN, Chahoud I, Heindel JJ,
Padmanabhan V, Paumgartten FJ and Schoenfelder G: Urinary,
circulating, and tissue biomonitoring studies indicate widespread
exposure to bisphenol A. Environ Health Perspect. 118:1055–1070.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vandenberg LN, Colborn T, Hayes TB,
Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS,
Welshons WV, et al: Hormones and endocrine-disrupting chemicals:
Low-dose effects and nonmonotonic dose responses. Endocr Rev.
33:378–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramos JG, Varayoud J, Sonnenschein C, Soto
AM, Muñoz-de-Toro M and Luque EH: Prenatal exposure to low doses of
bisphenol A alters the periductal stroma and glandular cell
function in the rat ventral prostate. Biol Reprod. 65:1271–1277.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Derouiche S, Warnier M, Mariot P, Gosset
P, Mauroy B, Bonnal JL, Slomianny C, Delcourt P, Prevarskaya N and
Roudbaraki M: Bisphenol A stimulates human prostate cancer cell
migration via remodelling of calcium signalling. Springerplus.
2:542013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi O and Oishi S: Testicular
toxicity of dietary 2,2-bis(4-hydroxyphenyl)propane (bisphenol A)
in F344 rats. Arch Toxicol. 75:42–51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herath CB, Jin W, Watanabe G, Arai K,
Suzuki AK and Taya K: Adverse effects of environmental toxicants,
octylphenol and bisphenol A, on male reproductive functions in
pubertal rats. Endocrine. 25:163–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogura Y, Ishii K, Kanda H, Kanai M, Arima
K, Wang YZ and Sugimura Y: Bisphenol A induces permanent squamous
change in mouse prostatic epithelium. Differentiation. 75:745–756.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ho SM, Tang WY, de Frausto J Belmonte and
Prins GS: Developmental exposure to estradiol and bisphenol A
increases susceptibility to prostate carcinogenesis and
epigenetically regulates phosphodiesterase type 4 variant 4. Cancer
Res. 66:5624–5632. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu J, Huang D, Su X, Yan H and Sun ZY:
Oral administration of low-dose bisphenol A promotes proliferation
of ventral prostate and upregulates prostaglandin D2 synthase
expression in adult rats. Toxicol Ind Health. pii:Jun 18–2015.(Epub
ahead of print).
|
|
13
|
Wang JF, Zhou YLIUJH, et al: Guide for the
Care and Use of Laboratory Animals. Shanghai Scientific &
Technical Publishers (SSTP); Shanghai, China: 2012, View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mckeehan WL, Adams PS and Rosser MP:
Direct mitogenic effects of insulin, epidermal growth factor,
glucocorticoid, cholera toxin, unknown pituitary factors and
possibly prolactin, but not androgen, on normal rat prostate
epithelial cells in serum-free, primary cell culture. Cancer Res.
44:1998–2010. 1984.PubMed/NCBI
|
|
15
|
Wu JH, Jiang XR, Liu GM, Liu XY, He GL and
Sun ZY: Oral exposure to low-dose bisphenol A aggravates
testosterone-induced benign hyperplasia prostate in rats. Toxicol
Ind Health. 27:810–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gualtieri AF, Iwachow MA, Venara M, Rey RA
and Schteingart HF: Bisphenol A effect onglutathione synthesis and
recycling in testicular Sertoli cells. J Endocrinol. 5:e102–e109.
2011.
|
|
17
|
Wetherill YB, Hess-Wilson JK, Comstock CE,
Shah SA, Buncher CR, Sallans L, Limbach PA, Schwemberger S, Babcock
GF and Knudsen KE: Bisphenol A facilitates bypass of androgen
ablation therapy in prostate cancer. Mol Cancer Ther. 5:3181–3190.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hess-Wilson JK, Webb SL, Daly HK, Leung
YK, Boldison J, Comstock CE, Sartor MA, Ho SM and Knudsen KE:
Unique bisphenol A tanscriptom in prostate cancer: Novel effects on
ERbeta expression that correspond to androgen receptor mutation
status. Environ Health Perspect. 115:1646–1653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mokra K, Kocia M and Michałowicz J:
Bisphenol A and its analogs exhibit different apoptotic potential
in peripheral blood mononuclear cells (in vitro study). Food Chem
Toxicol. 84:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou C, Wang W, Peretz J and Flaws JA:
Bisphenol A exposure inhibits germ cell nest breakdown by reducing
apoptosis in cultured neonatal mouse ovaries. Reprod Toxicol.
57:87–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iida H, Maehara K, Doiguchi M, Mōri T and
Yamada F: Bisphenol A-induced apoptosis of cultured rat Sertoli
cells. Reprod Toxicol. 17:457–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuiper GG, Lemmen JG, Carlsson B, Corton
JC, Safe SH, van der Saag PT, van der Burg B and Gustafsson JK:
Interaction of estrogenic chemicals and phytoestrogens with
estrogen receptor β. Endocrinology. 139:4252–4263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Omoto Y, Imamov O, Warner M and Gustafsson
JA: Estrogen receptor alpha and imprinting of the neonatal mouse
ventral prostate by estrogen. Proc Natl Acad Sci USA. 102:pp.
1484–1489. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams JY, Leav I, Lau KM, Ho SM and
Pfluege SM: Expression of estrogen receptor beta in the fetal,
neonatal, and prepubertal human prostate. Prostate. 52:69–81. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taylor JA, Richter CA, Ruhlen RL and vom
Saal FS: Estrogenic environmental chemicals and drugs: Mechanisms
for effects on the developing male urogenital system. J Steroid
Biochem Mol Biol. 127:83–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vandenberg LN, Maffini MV, Sonnenschein C,
Rubin BS and Soto AM: Bisphenol A and the great divide: A review of
controversies in the field of endocrine disruption. Endocr Rev.
30:75–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weng YI, Hsu PY, Liyanarachchi S, Liu J,
Deatherage DE, Huang YW, Zuo T, Rodriguez B, Lin CH, Cheng AL and
Huang TH: Epigenetic influences of low-dose bisphenol A in primary
human breast epithelial cells. Toxicol Appl Pharmacol. 248:111–121.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rubin BS: Bisphenol A: An endocrine
disruptor with widespread exposure and multiple effects. J Steroid
Biochem Mol Biol. 127:27–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luccio-Camelo DC and Prins GS: Disruption
of androgen receptor signaling in males by environmental chemicals.
J Steroid Biochem Mol Biol. 127:74–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bonefeld-Jørgensen EC, Long M, Hofmeister
MV and Vinggaard AM: Endocrine-disrupting potential of bisphenol A,
bisphenol A dimethacrylate, 4-nnonylphenol, and 4-n-octylphenol in
vitro: New data and a brief review. Environ Health Perspect. 115
Suppl 1:S69–S76. 2007. View
Article : Google Scholar
|
|
31
|
Wetherill YB, Fisher NL, Staubach A,
Danielsen M, de Vere White RW and Knudsen KE: Xenoestrogen action
in prostate cancer: Pleiotropic effects dependent on androgen
receptor status. Cancer Res. 65:54–65. 2005.PubMed/NCBI
|
|
32
|
Wetherill YB, Petre CE, Monk KR, Puga A
and Knudsen KE: The xenoestrogen bisphenol A induces inappropriate
androgen receptor activation and mitogenesis in prostatic
adenocarcinoma cells. Mol Cancer Ther. 1:515–524. 2002.PubMed/NCBI
|
|
33
|
Lee CH, Akin-Olugbade O and Kirschenbaum
A: Overview of prostate anatomy, histology, and pathology.
Endocrinol Metab Clin North Am. 40565–575. (viii-xi)2011.
View Article : Google Scholar : PubMed/NCBI
|