Introduction
Gastric cancer has one of the highest incidence
rates of all types of cancer globally, and the second highest
mortality rate of all types of malignant tumor (1). As gastric cancer is frequently
asymptomatic, the majority of tumor invasion and metastasis events
occur prior to diagnosis of the patient at hospital. The high
incidence of tumor metastasis and low sensitivity to chemotherapy
in gastric cancer are important factors that restrict the
improvement of gastric cancer survival rates (2). Epithelial-mesenchymal transition (EMT)
is an important biological process through which malignant tumor
cells derived from the epithelium obtain migration and invasion
abilities. Following EMT, tumor cells lose epithelial cell polarity
and their connection to the basement membrane, while their
migration and invasion abilities, resistance to apoptosis and
ability to degrade the extracellular matrix (ECM) are enhanced
(3). Cofilin 1 is an important
regulatory factor of EMT in tumor cells, demonstrating an
association with the occurrence and development of tumors (4–6). Cofilin 1
may become a novel target for the treatment of malignant tumor
growth and metastasis (7–10). Celastrus orbiculatus Thunb, a
member of the Celastraceae family, is an important medicinal plant
in China. Preliminary experimental studies have identified that the
ethyl acetate extract of C. orbiculatus Thunb (COE) may
significantly inhibit the proliferation, EMT, invasion and
metastasis abilities of tumor cells (11,12).
However, the molecular mechanisms underlying the inhibition of EMT
in tumor cells by COE remains unclear, and studies investigating
the regulation of EMT by COE through Cofilin 1 pathways in tumors
have not been performed at present. Identifying the mechanisms
underlying COE-induced inhibition of tumor EMT processes and
metastasis has significance for the identification and development
of novel antitumor agents in traditional Chinese medicine (TCM). On
the basis of previous studies by our group (11,12), the
present study examined EMT processes from the aspect of the
cytoskeleton. The present study also revealed the mechanism
underlying COE-induced inhibition of gastric cancer metastasis and
invasion, which provides a basis for the development of novel
antitumor TCM.
Materials and methods
Drugs
C. orbiculatus Thunb was purchased from
Guangzhou Zhixin Pharmaceutical Co. Ltd. (Guangzhou, China) in July
2014 and stored at 4°C. It was identified as Celastraceae by
Professor Qin Minjian of China Pharmaceutical University (Nanjing,
China). The extraction, purification and identification of the COE
compounds was performed as described previously (13). COE was prepared at the Department of
Chinese Materia Medica Analysis, China Pharmaceutical University
(Nanjing, China). A detailed description of the preparation
procedure has been described previously (14,15).
Briefly, dried stems of C. orbiculatus were pulverized and
extracted using 95% ethanol 3 times; the final extract was obtained
by filtering, removing ethanol, and vacuum cold-drying the final
extracts at 4°C for 6 h. Finally, the extract was condensed,
purified and lyophilized into powder at 4°C, and stored at 4°C
thereafter. The COE micro-powder was dissolved in dimethyl
sulfoxide (DMSO) to 1% and was further diluted with RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to
different concentrations (10, 20, 40, 80, 160 and 320 mg/l) prior
to use. The final concentration of DMSO in the medium did not
exceed 0.1%.
Reagents and antibodies
RPMI-1640 medium and fetal bovine serum (FBS) were
acquired from Gibco; Thermo Fisher Scientific, Inc. MTT and
tetramethylrhodamine (TRITC)-conjugated Phalloidin, an actin
staining agent, were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Recombinant transforming growth factor
(TGF)-β1 was obtained from R&D Systems, Inc. (Minneapolis, MN,
USA). Matrigel was purchased from BD Biosciences (San Jose, CA,
USA). Antibodies against epithelial (E)-cadherin (cat no. 3195),
neural (N)-cadherin (cat no. 4061), Cofilin 1 (cat no. 5175),
vimentin (cat no. 5741), MMP-2 (cat no. 4022), MMP-9 (cat no.
13667) and β-actin (cat no. 3700) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Other chemicals used
of analytical grade were from commercial sources.
Cell culture
Human gastric cancer AGS cells were acquired from
the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences, Shanghai Institute of Cell Biology (Shanghai, China). AGS
cells were cultured in RPMI-1640 medium containing 10% FBS and
maintained at 37°C in a humidified incubator in an atmosphere of 5%
CO2. Cell morphology was visualized at x100
magnification with an optical microscope (IX72; Olympus
Corporation, Tokyo, Japan).
EMT model
105 AGS cells were seeded in 6-well
plates for 12 h, then RPMI-1640 containing a concentration of 10
µg/l TGF-β1 was added into each well, and cultured at 37°C for an
additional 24 h. To confirm the establishment of the model, the
culture medium was removed, cells were washed twice with PBS and
cell lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton
X-100] was added. After 15 min, all the liquid was collected into
1.5 ml EP tubes, and centrifuged at 8,000 x g at 4°C for 15 min.
The BCA kit (Beyotime Institute of Biotechnology, Haimen, China)
was used to quantify the protein, and total protein (50 µg) was
separated using electrophoresis with a constant voltage of 110 V
for 90 min in 10% SDS-PAGE gel. Following separation, proteins were
transferred to a polyvinylidene (PVDF) membrane by electricity. The
PVDF membrane was then washed 3 times, for 15 min each time, with
1X TBST (containing 0.1% Tween-20) wash buffer, then 5% skimmed
milk powder solution was added for 2 h at room temperature. The
membranes were subsequently washed again 3 times, for 15 min each
time, with 1X TBST wash buffer. Primary antibodies were diluted to
1:1,000 with 5% skimmed milk powder solution and incubated with the
membranes overnight at 4°C, which were then washed 3 times, for 15
min each time, with 1X TBST wash buffer. Secondary antibodies were
diluted to 1:1,000 in 5% skimmed milk powder solution and incubated
for 2 h at room temperature, then the membranes were washed 3
times, for 15 min each time, with 1X TBST wash buffer. A Bio-Rad
protein gel imaging analysis system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to detect protein bands of the EMT
biomarkers.
MTT measurement of cell viability and
proliferation
AGS cells were plated at a density of 1,000 to
10,000 cells/well in a 96-well plate, and incubated at 37°C for 12
h. AGS cells were grouped into a negative control group
(untreated), a model group (10 µg/l TGF-β1) and COE groups of
different concentrations (10, 20, 40, 80 and 160 mg/l). The cells
were cultured at 37°C for an additional 24, 48 or 72 h following
the treatments. At 24, 48 or 72 h, cells were incubated with
RPMI-1640 medium containing 0.5 mg/ml MTT at 37°C for 4 h. Formazan
crystals were dissolved with 150 µl DMSO. The absorbance of each
well, including blanks, was measured at 490 nm in an automatic
microplate reader subsequent to 10 min oscillation. The formula of
the cell growth inhibition rate was as follows: Inhibition rate =
(1 - absorbance of COE group/absorbance of blank control group)
x100%.
Cell invasion and migration
assays
Cell invasion and migration assays were performed
using a Transwell membrane (Corning Incorporated, Corning, NY, USA)
according to the manufacturer's protocol. For the invasion assay,
Matrigel was applied to the upper chamber. AGS cells
(104) were seeded into the upper chamber and treated
with RPMI-1640 medium containing different concentrations (10, 20,
40, 80 and 160 mg/l) of COE. Media containing 10% FBS and TGF-β1
were added to the lower chamber for 24 h as a chemoattractant. At
the end of the treatment, the cells on the upper surface of the
membrane were removed by cotton swabs and cells invading across the
Matrigel to the lower surface of the membrane were fixed with 95%
methanol and stained with 0.1% crystal violet. Images were captured
under a microscope at x200 magnification (Nikon Corporation, Tokyo,
Japan) and invading cells were quantified by manual counting 5
fields of view. Migration assays were performed using the same
procedure, except that the polycarbonate membrane was not coated
with Matrigel. Each experiment was repeated 3 times.
Association between Cofilin 1
expression and EMT
AGS cells were passaged and cultured in RPMI-1640
medium until they reached 50–60% confluence. Cultured cells were
fixed with 4% paraformaldehyde in 1X PBS for 15–20 min at room
temperature. Following 2 washes, cultured cells was permeabilized
with 0.1% Triton X-100 for 1–5 min at room temperature. Following 2
washes, blocking solution [5% bovine serum albumin (BSA) in 1X PBS]
was applied to the cultured cells for 30 min at room temperature.
Primary antibodies (Anti-Cofilin 1) were diluted to 1:500 with 5%
BSA and incubated at 4°C for 12–18 h. Following an additional 2
washes with 1X wash buffer, fluorescein isothiocyanate-labeled
(1:500; AP124F) and TRITC-conjugated anti-Phalloidin secondary
antibodies (1:100; SC-209) were diluted with 1X PBS immediately
prior to use and incubated for 30–60 min at room temperature.
Detection of Cofilin 1, matrix
metalloproteinase (MMP)-2 and MMP-9
105 AGS cells were seeded into 6-well
plates. A negative control group, model group and groups of
different concentrations (20, 40 or 80 mg/l) of COE were prepared
and incubated at 37°C for 1 h. Then 10 µg/l TGF-β1 was added into
each well and incubated at 37°C for an additional 24 h. Proteins
were extracted from each group as previously described. A Bio-Rad
protein gel imaging analysis system (Bio-Rad Laboratories, Inc.)
was used to detect protein bands.
Cell microfilament cytoskeleton
staining
AGS cells (105) were seeded into 6-well
plates. A negative control group, model group and groups of
different concentrations (mass concentration of 20, 40, 80 mg/l) of
COE were prepared. Cells were passaged and cultured in RPMI-1640
medium until they reached 50–60% confluence. Cultured cells were
fixed with 4% paraformaldehyde in 1X PBS for 15–20 min at room
temperature. Suitable media was washed twice with 1X wash buffer,
and permeabilized with 0.1% Triton X-100 in 1X PBS for 1–5 min at
room temperature. Following 2 washes with 1X wash buffer, cells in
suitable media were covered with dilute TRITC-conjugated Phalloidin
in 1X PBS immediately prior to use, and incubated for 30–60 min at
room temperature to stain the actin. Following this washing step,
nuclei counterstaining was performed by incubating cells with 0.1
µg/ml DAPI for 1–5 min at room temperature, followed by 3 washes
with 1X wash buffer, for 5–10 min each time. Fluorescence images
were captured with a laser scanning confocal microscope (FV3000;
Olympus Corporation).
Statistical analysis
Data processing was performed with SPSS software
(version 16; SPSS Inc., Chicago, IL, USA), using one-way analysis
of variance, followed by a Bonferroni post-hoc test. Data are
expressed as the mean ± standard deviation. P<0.05 and P<0.01
were considered to indicate a statistically significant
difference.
Results
EMT model group
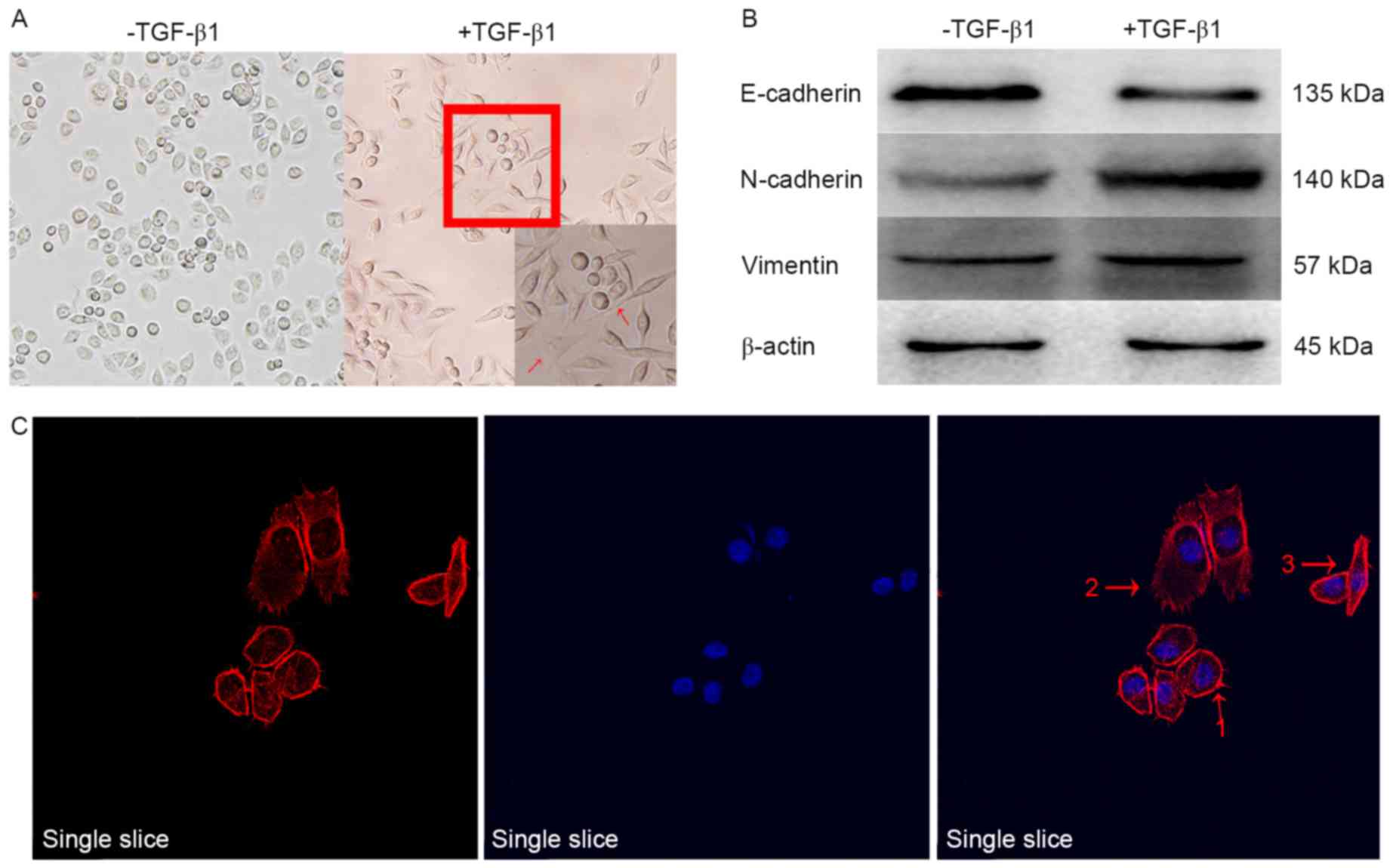
The cell morphology of the EMT model group was
observed (Fig. 1). Marked changes
occurred in the morphology of cells, as visualized under a
microscope. Cells with an originally irregular polygon morphology
form gradually became spindle-shaped or fusiform, with high levels
of pseudopodia identified around the cells, indicating that the
cells had transitioned from epithelial cells into mesenchymal
cells. The cells grew pseudopodia, and moved from their original
congregated formations into dispersed populations, as demonstrated
in Fig. 1A and C. Through EMT, the
epithelial cells lost their epithelial phenotype, including cell
polarity and the connection with the basement membrane. Following
this transformation, cells achieved a mesenchymal phenotype,
including an increased capacity for migration and invasion, an
increased resistance to apoptosis and the ability to degrade the
ECM. Additional examination of the levels of classical
EMT-associated biomarker levels revealed that the expression of the
epithelial biomarker E-cadherin, which mediates cell-to-cell
homogenous adhesion, was markedly decreased while the expression
levels of the interstitial markers N-cadherin and vimentin, which
mediate cell-cell matrix heterogeneous adhesion, were markedly
increased, as demonstrated in Fig.
1B. The results indicated that the classical biological markers
of EMT, induced by TGF-β1, were markedly altered in human AGS
gastric cancer cells compared with untreated controls, which
suggested that the transformation of epithelial cells to
mesenchymal cells occurred. EMT is an important biological process
during which malignant tumor cells derived from epithelial cells
obtain migratory and invasive abilities. TGF-β1 may successfully
induce EMT in the AGS cell line.
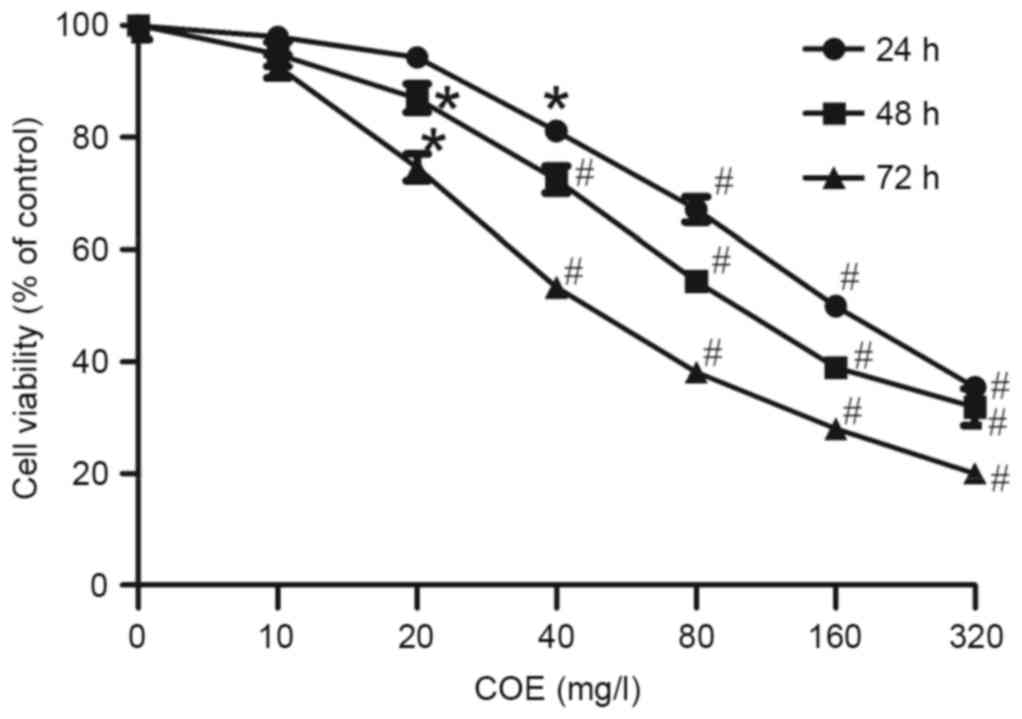
Cell viability
The AGS cell control group grew well in
vitro. Compared with the control group, AGS cells treated with
10–320 mg/l COE exhibited a concentration- and time-dependent
inhibition of viability, as indicated by Fig. 2. Compared with the control group,
cells treated with 40 mg/l COE for 12 and 24 h, and cells treated
with 20 mg/l COE for 48 h exhibited marked growth inhibition
(P<0.05). In order to exclude the cytotoxic effect of COE on EMT
in cells, low concentrations (10, 20, 40 mg/l) of COE were used to
treat AGS cells for 24 h in subsequent experiments to investigate
the inhibitory effect of COE on AGS cell viability. Concomitantly,
the 50% inhibitory concentration of COE was 83.5 mg/l at 24 h.
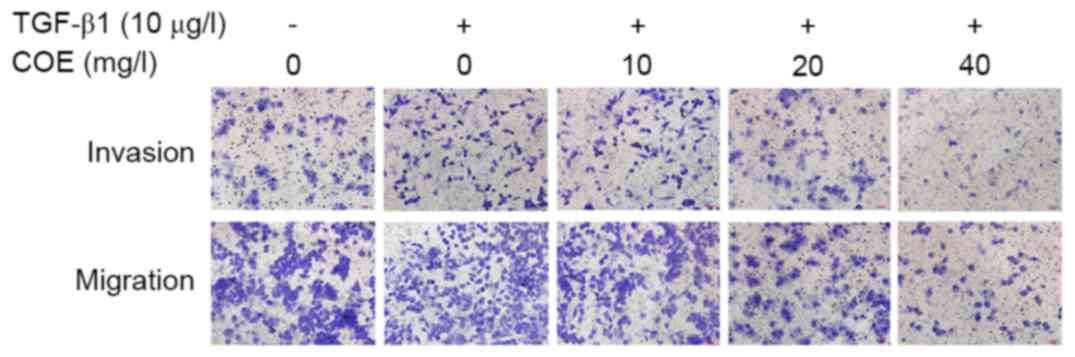
Effect of COE on invasion and
migration of AGS cells
A negative control group, model group and groups
treated with 10, 20 or 40 mg/l COE were prepared. Compared with the
model group, the number of cells invading through the membrane was
significantly decreased following treatment with 10, 20 or 40 mg/l
COE for 24 h (P<0.05), as demonstrated as Fig. 3 and Table
I. COE may significantly inhibit AGS cell invasion and
metastasis.
 | Table I.Inhibition of AGS cell invasion and
metastasis by COE. |
Table I.
Inhibition of AGS cell invasion and
metastasis by COE.
| Transforming growth
factor-β1 (10 µg/l) | COE (mg/l) | No. of invading
cells | No. of migrating
cells |
|---|
| − | 0 | 110.4±7.64 | 221.8±29.56 |
| + | 0 |
137.8±6.38a |
384.2±22.75a |
| + | 10 |
105.4±9.24a,b |
288.4±20.65a,b |
| + | 20 |
77.8±7.92a,b |
191.0±20.11a,b |
| + | 40 |
38.4±13.35a,b |
85.2±18.77a,b |
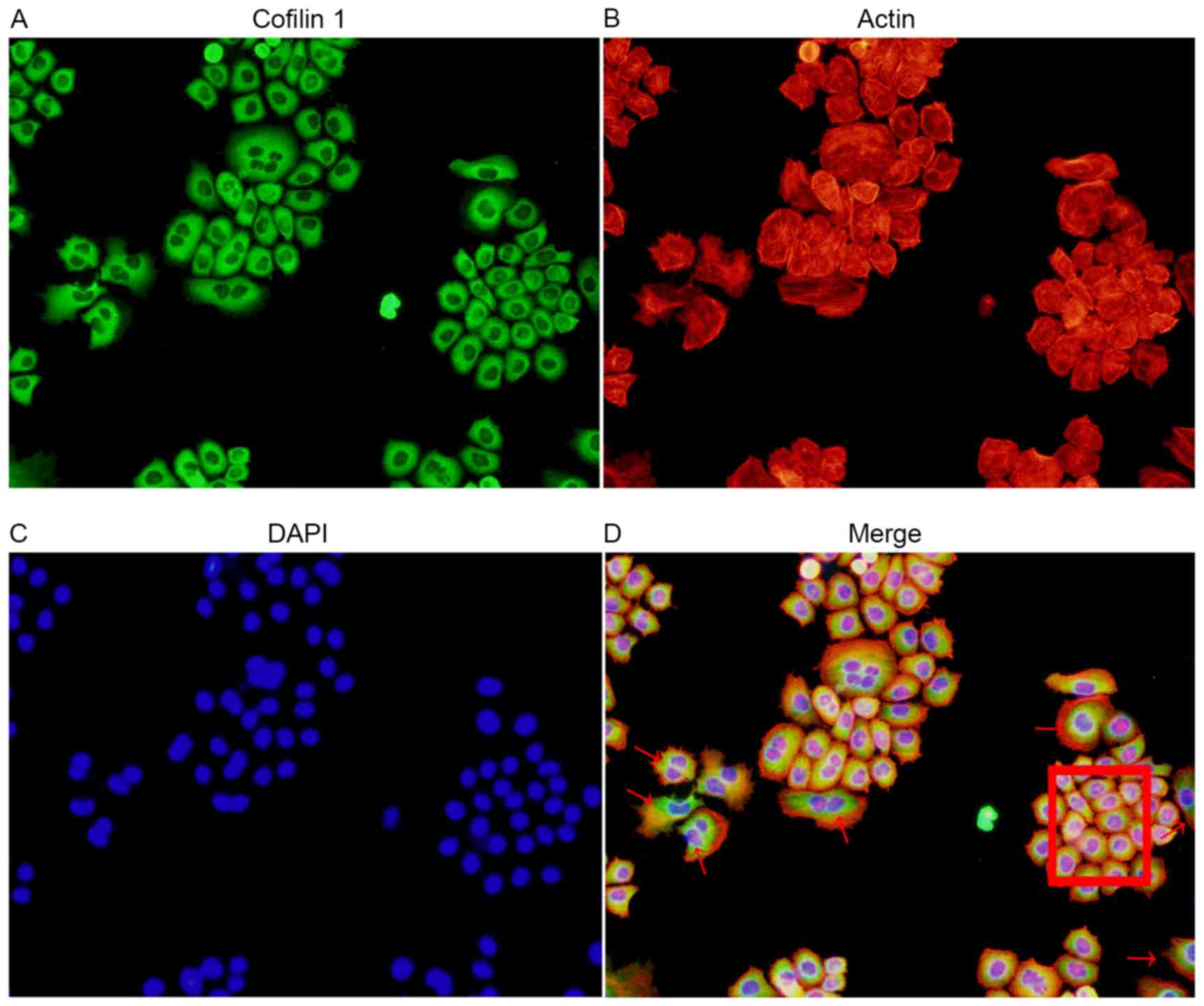
Association between cofilin 1
expression and EMT
Through a combined immunofluorescence and
cytoskeleton staining method, it was clear that an increased level
of Cofilin 1 expression accompanied the stretching out of
lamellipodia and filopodia in the cells undergoing EMT. The high
expression of Cofilin 1 in the EMT cells was demonstrated with dark
green staining, as compared with the normal control cells. A number
of cells demonstrated Cofilin 1 expression in normal control cells
(red box; Fig. 4). This suggested
that Cofilin 1 was directly involved in the process of EMT in AGS
cells, and served an important function.
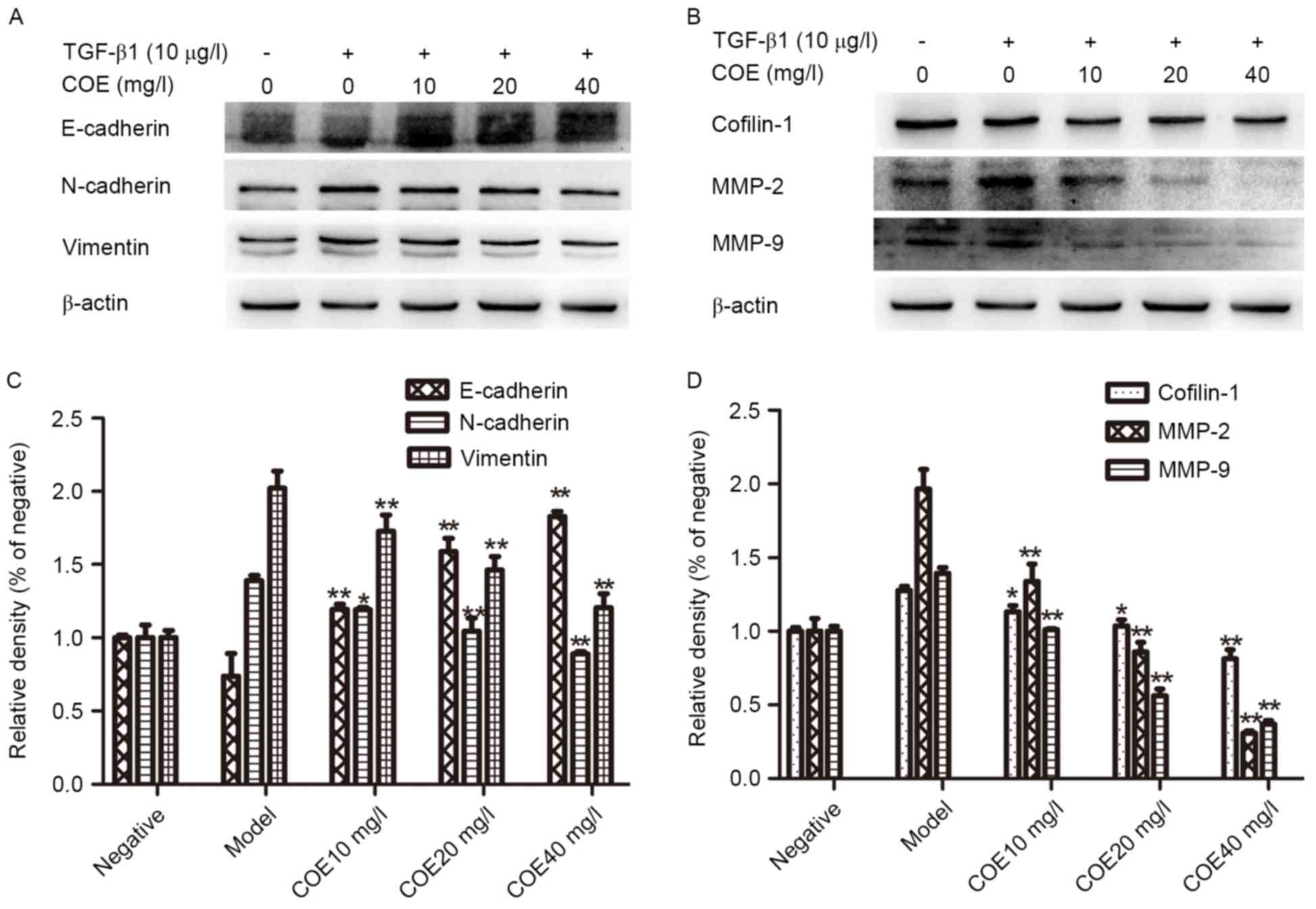
Expressions of cofilin 1, E-cadherin,
N-cadherin, vimentin, MMP-9 and MMP-2
AGS cells were treated with different concentrations
of COE for 24 h. The western blotting results suggested that
Cofilin 1, N-cadherin, vimentin, MMP-2 and MMP-9 protein expression
was markedly decreased with increasing concentrations of COE
compared with the model group, while the expression level of
E-cadherin protein increased, as demonstrated in Fig. 5.
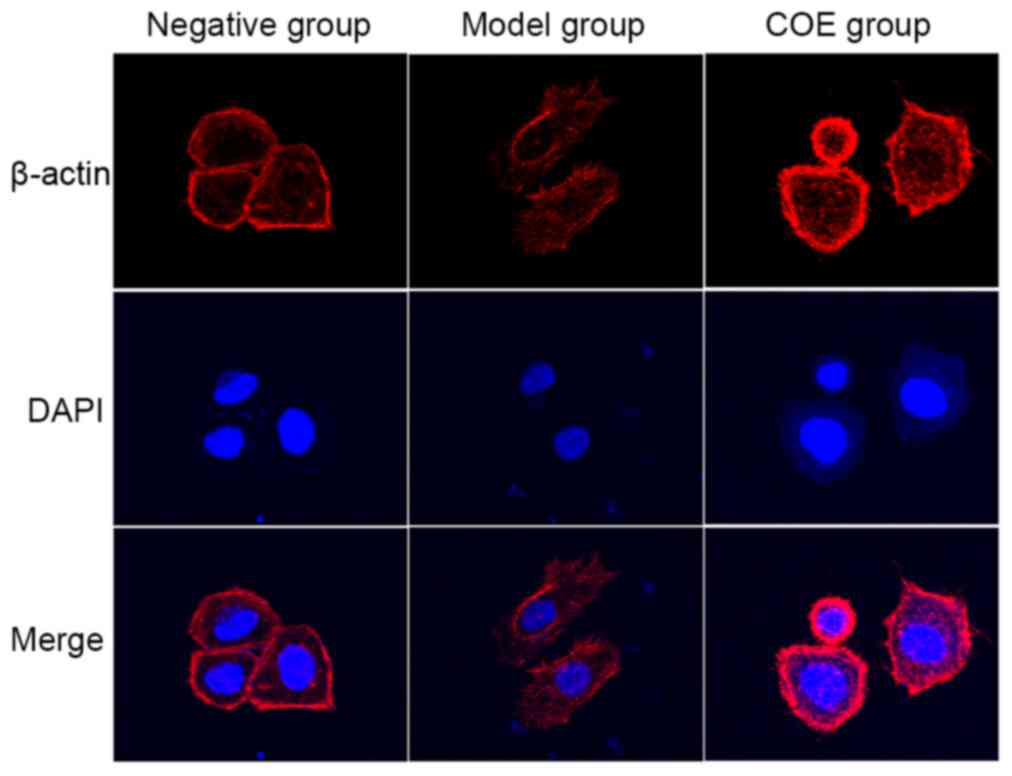
Cytoskeleton staining detected by
laser confocal microscopy
The negative control AGS cell group exhibited a
distribution of microfilaments primarily on the cell membrane and
cytoplasm, connected into a net. Cytoplasmic microtubules were
stained uniformly with red fluorescence, and the cell borders
around were clear with a small amount of microfilament formation
(Fig. 6). Cytoskeleton staining in
the model group demonstrated that the microfilament and skeleton
structures were significantly altered compared with the negative
control group. For example, cytoskeleton staining was darker, the
microfilament grid structure was not clear and there was evidence
of microfilament enrichment on the cell membrane. Cell morphology
changed from an irregular quadrilateral shape into an oval
appearance. The cells possessed increased invasion and metastasis
abilities with long and rich microfilament tubules, a typical EMT
cell form (Fig. 6). Treatment with 40
mg/l COE for 24 h resulted in a restoration of the clear
microfilament grid structure, and the number of microfilament
bundle fibers on the cell membrane was reduced. The number of
microfilament tubules around the cells was reduced, or disappeared
in certain instances, and the cytoskeleton of the cells became
partially dissolved or fractured. This indicated that COE may
inhibit the changes in cytoskeleton and microfilament structure and
distribution in AGS cells, as induced by TGF-β1 (Fig. 6).
Discussion
Cell migration is a key step in malignant tumor
invasion and metastasis events (16).
Tumors spread through tumor cell invasion and migration into
peripheral tissues. It is the primary reason why malignant tumor
recurrence rates are high. EMT is an important biological process
through which malignant tumor cells, derived from epithelium,
achieve migration and invasion abilities (17). During this process, cell morphology
changes; they become flatter and wider, and visible filopodia and
lamellipodia form at the front of polarized cells. Under the action
of the contractile forces of the cells, pseudopodia are stretched
forward and pull the rear cell body forward (18). As an important regulatory factor of
cell migration, Cofilin 1 adjusts the structure of filopodia and
lamellipodia to promote cell migration (19). Clinical studies have revealed that
Cofilin 1 is closely associated with pathological differentiation,
tumor size, lymph node metastasis and clinical stages in gastric
cancer, and serves an important function in the process of invasion
and metastasis in gastric cancer (8,20). As an
important factor regulating tumor cell invasion and metastasis,
Cofilin 1 regulation of EMT is primarily evident in the regulation
of changes to the cell cytoskeleton, which reduces the dependence
on the ECM for adhesive growth (21,22).
Cofilin 1 continuously regulates filamentous actin (F-actin)
depolymerization and aggregation in order to alter the cell
cytoskeleton through nucleating the polymerization of F-actin
fibres (23). Through reconstructing
the front lamellipodium and lamellar structure of cells, this
regulates cell ‘steps’ forward (24).
The highly-localized activities of Cofilin 1 produce pseudopodia
and determine the exact direction of cell movement, serving a
function as the ‘steering wheel’ of cells (25).
Previous studies have identified that COE may
inhibit tumor cell proliferation, invasion and metastasis (26–28). Its
action may occur through inhibiting tumor cell transformation of
epithelial-mesenchymal process to implement, but the specific
mechanism is unclear. An experimental study revealed that COE may
downregulate the expression level of Cofilin 1, thereby reducing
the interaction of Cofilin 1 and F-actin (29). Cofilin 1 inhibits the depolymerization
of F-actin by reducing the dissociation rate of actin monomers from
the end of the fiber. As the depolymerization rate of F-actin is
inhibited the changes to the cytoskeleton are impeded, which
directly inhibits the EMT process of induced changes in morphology
and cell invasion and metastasis abilities.
In the process of tumor invasion and metastasis, MMP
family proteins are the most important proteases that degrade the
ECM. In particular, MMP-2 and MMP-9 are closely associated with
invasion and metastasis in gastric cancer (30). Following EMT in cells, the expression
levels of MMPs rises (31). The
present study revealed that in the control and intervention groups,
the expression levels of Cofilin 1 and MMPs were consistent.
Previous studies have demonstrated that the expression of Cofilin 1
reduces adhesion between the cells and ECM, and that Cofilin 1
activation may extend the actin chain, produce barbs at the ends,
and determine the precise direction of the tumor cell movement.
Silencing Cofilin 1 expression may increase adhesion between the
cells and ECM (32,33). This is in concordance with the results
of the present study. Therefore, COE may reduce Cofilin 1-dependent
changes to the cytoskeleton through directly inhibiting the
expression of Cofilin 1, resulting in the inhibition of the EMT
process of the cells. The suppression of the EMT process in tumor
cells directly leads to decreased MMP-2 and MMP-9 protein
expression levels (30). In the
present study, detecting the expression levels of classic EMT
biomarkers and MMPs indicated a direct inhibition of the EMT
process.
In conclusion, COE may significantly downregulate
Cofilin 1 protein expression levels in human gastric cancer AGS
cells, thus effectively inhibiting alterations of the AGS cell
cytoskeleton and suppressing EMT progress. Additional studies are
required to investigate how to reduce the expression levels of
Cofilin 1 protein.
Acknowledgements
The present study was financially supported by
grants from the National Natural Science Foundation of China (grant
nos. 81573656, 81450051 and 81403232) and the Natural Science
Foundation of Jiangsu Province of China (grant no. BK20141280).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. Ca Cancer J Clin. 66:115–132. 2015. View Article : Google Scholar
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flemban A and Qualtrough D: The potential
role of hedgehog signaling in the luminal/basal phenotype of breast
epithelia and in breast cancer invasion and metastasis. Cancers
(Basel). 7:1863–1884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W, Mouneimne G, Sidani M, Wyckoff J,
Chen X, Makris A, Goswami S, Bresnick AR and Condeelis JS: The
activity status of cofilin is directly related to invasion,
intravasation and metastasis of mammary tumors. J Cell Biol.
173:395–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tahtamouni LH, Shaw AE, Hasan MH, Yasin SR
and Bamburg JR: Non-overlapping activities of ADF and cofilin-1
during the migration of metastatic breast tumor cells. BMC Cell
Biol. 14:452013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han L, Stope MB, de Jesús ML, Oude
Weernink PA, Urban M, Wieland T, Rosskopf D, Mizuno K, Jakobs KH
and Schmidt M: Direct stimulation of receptor-controlled
phospholipase D1 by phospho-cofilin. EMBO J. 26:4189–4202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang WS, Zhong HJ, Xiao DW, Huang X, Liao
LD, Xie ZF, Xu XE, Shen ZY, Xu LY and Li EM: The expression of CFL1
and N-WASP in esophageal squamous cell carcinoma and its
correlation with clinicopathological features. Dis Esophagus.
23:512–521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y, Tang Y and Zhang Y:
Clinicopathological significance of cofilin-1 in gastric cancer
tissues. Cancer Res Prev Treat. 39:295–298. 2012.
|
|
9
|
Cho HJ, Baek KE, Kim IK, Park SM, Choi YL,
Nam IK, Park SH, Im MJ, Yoo JM, Ryu KJ, et al: Proteomics-based
strategy to delineate the molecular mechanisms of RhoGDI2-induced
metastasis and drug resistance in gastric cancer. J Proteome Res.
11:2355–2364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen
J, Guo S and Hisamitsu T: Research on the efficacy of Celastrus
orbiculatus in suppressing TGF-β1-induced epithelial-mesenchymal
transition by inhibiting HSP27 and TNF-α-induced NF-κB/Snail
signaling pathway in human gastric adenocarcinoma. BMC Complement
Altern Med. 14:4332014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian YY, Zhang H, Hou Y, Yuan L, Li GQ,
Guo SY, Hisamits T and Liu YQ: Celastrus orbiculatus extract
inhibits tumor angiogenesis by targeting vascular endothelial
growth factor signaling pathway and shows potent antitumor activity
in hepatocarcinomas in vitro and in vivo. Chin J Integr Med.
18:752–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Qian Y, Liu Y, Li G, Cui P, Zhu
Y, Ma H, Ji X, Guo S and Tadashi H: Celastrus orbiculatus extract
induces mitochondrial-mediated apoptosis in human hepatocellular
carcinoma cells. J Tradit Chin Med. 32:621–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao Ke, Chen Xiaoqing, Wang Qiang, et al:
Research of chemical composition of celastrus orbiculatus Thunb.
Chinese Herbal Med. 38:14552007.
|
|
14
|
Li JJ, Yang J, Lu F, Qi YT, Liu YQ, Sun Y
and Wang Q: Chemical constituents from the stems of Celastrus
orbiculatus. Chin J Nat Med. 10:279–283. 2012. View Article : Google Scholar
|
|
15
|
Zan K, Chen X-Q, Wang Q and Cao L:
Chemical constituents in stem of Celastrus orbiculatus. Chin Trad
Herbal Drugs. 38:14552007.
|
|
16
|
Croisé P, Estay-Ahumada C, Gasman S and
Ory S: Rho GTPases, phosphoinositides, and actin: A tripartite
framework for efficient vesicular trafficking. Small GTPases.
5:e294692014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Felipe Lima J, Nofech-Mozes S, Bayani J
and Bartlett JM: EMT in breast carcinoma - A review. J Clin Med.
5(pii): E652016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Wu L, Liu Q, Chen K and Zhang X:
Impact on growth and invasion of gastric cancer cell lines by
silencing NEDD9. Onco Targets Ther. 8:223–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delorme V, Machacek M, DerMardirossian C,
Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G and
Bokoch GM: Cofilin activity downstream of Pak1 regulates cell
protrusion efficiency by organizing lamellipodium and lamella actin
networks. Dev Cell. 13:646–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Mouneimne G, Sidani M, Wyckoff J,
Chen X, Makris A, Goswami S, Bresnick AR and Condeelis JS: The
activity statusofcofilin is directly related to invasion,
intravasation, and metastasis of mammary tumors. J Cell Biol.
173:395–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabrielsen M, Schuldt M, Munro J, Borucka
D, Cameron J, Baugh M, Mleczak A, Lilla S, Morrice N and Olson MF:
Cucurbitacin covalent bonding to cysteine thiols: The
filamentous-actin severing protein Cofilin1 as an exemplary target.
Cell Commun Signal. 11:582013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oleinik NV, Helke KL, Kistner-Griffin E,
Krupenko NI and Krupenko SA: Rho GTPases RhoA and Rac1 mediate
effects of dietary folate on metastatic potential of A549 cancer
cells through the control of cofilin phosphorylation. J Biol Chem.
289:26383–26394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galkin VE, Orlova A, VanLoock MS, Shvetsov
A, Reisler E and Egelman EH: ADF/cofilin use an intrinsicmode of
F-actin instability to disrupt actin filaments. J Cell Biol.
163:1057–1066. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sidani M, Wessels D, Mouneimne G, Ghosh M,
Goswami S, Sarmiento C, Wang W, Kuhl S, El-Sibai M, Backer JM, et
al: Cofilin determines the migration behavior and turning frequency
of metastatic cancer cells. J Cell Biol. 179:777–791. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delorme V, Machacek M, DerMardirossian C,
Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G and
Bokoch GM: Cofilin activity downstream of Pak1 regulates cell
protrusion efficiency by organizing lamellipodium and lamella actin
networks. Dev Cell. 13:646–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang FR, Hayashi K, Chen IH, Liaw CC,
Bastow KF, Nakanishi Y, Nozaki H, Cragg GM, Wu YC and Lee KH:
Antitumor agents. 228. Five new agarofurans, reissantins A-E, and
cytotoxic principles from Reissantia buchananii. J Nat Prod.
66:1416–1420. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan L, Zhang H, Qian YY, Hou Y, Zhu YD,
Ma H, Guo SY, Tadashi H and Liu YQ: Effects of serum containing
Celastrus orbiculatus extracts on proliferation and VEGF-c
expression in hepatoma cells of mice. Chin J Experimental
Traditional Med Formulae. 17:1572011.
|
|
28
|
Yang Qingwei, Liu Li, Liu Weiwei, et al:
Effects of Celastrus orbiculatus extract on suppressing invasion
and metastasis of human hepatoma 7721 cells. Chin Herbal Med.
40:4342009.
|
|
29
|
Wang Haibo, Qian Yayun, Zhu Yaodong, et
al: Effects of Celastrus orbiculatus extract on suppressing the
epithelial-mesenchymal transition of human gastric adenocarcinoma
cells by inhibiting cofilin 1 signaling pathway. Li Shi Zhen Medi
Materia Medica Res. 27:303–307. 2016.
|
|
30
|
Hua H, Li M, Luo T, Yin Y and Jiang Y:
Matrix metalloproteinases in tumorigenesis: An evolving paradigm.
Cell Mol Life Sci. 68:3853–3868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haibo W, Lide T, Feng J, Hao G, Xiaojun D,
Tengyang N, Jun F, Yanbing D, Weiming X, Yayun Q and Yanqing L:
Cofilin 1 induces the epithelial-mesenchymal transition of gastric
cancer cells by promoting cytoskeletal rearrangement. Oncotarget.
2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hitchcock-Degregori SE: Chemotaxis:
Cofilin in the driver's seat. Curr Biol. 16:R1030–R1032. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Estornes Y, Gay F, Gevrey JC, Navoizat S,
Nejjari M, Scoazec JY, Chayvialle JA, Saurin JC and Abello J:
Differential involvement of destrin and cofilin-1 in the control of
invasive properties of Isreco1 human colon cancer cells. Int J
Cancer. 121:2162–2171. 2007. View Article : Google Scholar : PubMed/NCBI
|