Introduction
Osteosarcoma, which arises from mesenchymal
osteoblasts, is the most common malignant bone cancer in children
and adolescents (1). Despite the
effective use of chemotherapy (2) and
surgical techniques (3) in
controlling osteosarcoma, there remains a mortality of rate of
~30%. In addition, patients with unresectable primary tumors or
clinically evident metastases have a poor prognosis, even in
chemotherapy-responsive cases of osteosarcoma (4,5).
Therefore, novel effective therapeutic approaches for the treatment
of osteosarcoma are required. Low-intensity pulsed ultrasound
(LIPUS) is a non-invasive ultrasound medical technology that uses
low-frequency, low-intensity pulses. LIPUS was previously
demonstrated to promote bone formation and accelerate bone
maturation in cases of bone fracture (6,7),
distraction osteogenesis (8) and
delayed fracture union (9). LIPUS
appears to act by affecting the biological mechanisms of cell
proliferation, gene regulation and cell differentiation (10). Osteosarcoma is characterized by the
production of malignant osteoid matrix and differentiation of
mesenchymal osteoblasts (11). LIPUS
affects osteoblastic differentiation without increasing the number
of osteoblasts (12,13). Therefore, the authors hypothesize that
LIPUS inhibits osteoblastic differentiation by inducing apoptosis
and inhibiting cell growth in osteosarcoma cells. To examine the
proposed antitumor effects of LIPUS, cells from the LM8
osteosarcoma cell line were treated with LIPUS, and the effects on
cell growth, apoptosis, mitochondrial membrane potential and
intracellular signaling molecules were investigated.
Materials and methods
Cells and culture
LM8 mouse osteosarcoma and MC3T3-E1 mouse
osteoblastic cells were obtained from the Japanese Collection of
Research Bioresources Cell Bank (Ibaraki, Japan) and were
maintained in Eagle's minimal essential medium (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) containing 10% heat-inactivated
fetal calf serum, 100 U/ml penicillin and 100 mg/ml streptomycin
(all from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
at 37°C in a humidified incubator containing 5% CO2.
Ultrasound apparatus and
treatment
The Sonic Accelerated Fracture Healing System
ultrasound apparatus (Teijin Ltd., Osaka, Japan) was used with 1.5
MHz frequency pulses, with a pulse width of 200 µs, repeated at 1
kHz, at a spatial average and temporal average intensity of 30
mW/cm, in all sonication experiments. The LIPUS transducer was
placed horizontally on each plate of cells for different times (1,
12, 18 or 24 h). In control experiments, the cells were treated in
the same manner without LIPUS exposure.
Cell proliferation assay
LM8 cells were seeded into 96-well black/clear
plates (Falcon; Corning Incorporated, Corning, NY, USA) at a
density of 1×104 cells/well. MC3T3-E1 cells were seeded
into black/clear 96-well plates at a density of 7.5×103
cells/well. Following incubation overnight at 37°C, the cells were
treated with or without LIPUS for 1, 12, 18 or 24 h. The WST-8
reagent (Cell Counting Kit-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) The WST-8 reagent (Cell Counting Kit; Dojindo Lab,
Tokyo, Japan) was added to each well, and the cells were cultured
for 2 h according to the manufacturer's protocol. Absorbance in
conditioned medium (Eagle's minimal essential medium:
Sigma-Aldrich; Merck KGaA) was monitored at 490 nm using a
microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA).
IC50 values were calculated using the Softmax Pro
software 6 (Molecular Devices LLC).
Measurement of mitochondrial membrane
potential
LM8 cells were seeded into 96-well black/clear
plates at a density of 4×104 cells/well. MC3T3-E1 cells
were seeded into black/clear 96-well plates at a density of
2×104 cells/well. Following incubation overnight at
37°C, the cells were treated with or without LIPUS for 48 h. The
mitochondrial membrane potential of the cells were measured using a
membrane potential cytotoxicity kit (Mito-ID; Enzo Life Sciences,
Inc., Farmingdale, NY, USA) and fluorescence microscopy (IX73;
Olympus Corporation, Tokyo, Japan).
Apoptosis assay and flow
cytometry
LM8 cells (1×106 cells/well) were
cultured in a 35-mm dish (Lumox dish 35; Sarstedt K.K., Tokyo,
Japan) and stimulated with LIPUS at 37°C for 48 h. The LM8 cells
were then treated with trypsin-EDTA, washed with PBS and
resuspended in binding buffer (Medical & Biological
Laboratories Co., Ltd., Nagoya, Japan) according to the
manufacturer's protocol. Cell suspension (85 µl) was then incubated
with 10 µl Annexin V/fluorescein isothiocyanate and 5 µl protein
iodide. After a 15-min incubation at room temperature in the dark,
400 µl binding buffer was added. The cells were analyzed by flow
cytometry (FACSCanto II; BD Biosciences, San Jose, CA, USA) and the
results were analyzed using software (FlowJo version 10.2; Tomy
Digital Biology, Tokyo, Japan).
Terminal deoxynucleotidyl transferase
dUTP nick end labelling (TUNEL) assay
An Apoptosis In Situ Detection kit (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) was used to measure
apoptosis using the TUNEL staining method, according to the
manufacturer's protocol, following treatment of LM8
(1×106) cells with LIPUS at 37°C for 48 h.
TUNEL-positive and TUNEL-negative cells were counted in four random
high-power fields (magnification, ×400) of each section. The rate
of apoptosis was calculated using the following equation:
TUNEL-positive cell number/total cell number ×100 (%).
Screening intracellular apoptosis
signaling
LM8 cells were seeded into a 35-mm dish (Lumox dish
35) at a density of 1×106 cells. Following incubation
overnight at 37°C, the cells were exposed to LIPUS for 24 or 48 h.
The cells were then treated with trypsin-EDTA, rinsed with cold PBS
and solubilized in cell lysate buffer (Cell Signaling Technology,
Inc., Danvers, MA, USA) containing a complete inhibitor cocktail
(Roche Diagnostics GmbH, Mannheim, Germany) and 1 mM PMSF
(phenylmethyl sulfonyl fluoride; Sigma-Aldrich; Merck KGaA) buffer.
Lysates were subsequently rocked gently at 4°C for 30 min.
Following centrifugation at 14,000 × g for 5 min at 4°C, the
supernatants were transferred to test tubes. Sample protein levels
were quantified using Bradford method (Protein Assay; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and then diluted to a
concentration of 1.0 mg/ml and used with the PathScan Stress and
Apoptosis Signaling Antibody Array kit (Cell Signaling Technology,
Inc.) according to the manufacturer's protocol. The detected dots
were visualized using the supplied LumiGLO reagent and enumerated
with the ImageQuant LAS-4000 instrument (GE Healthcare, Chicago,
IL, USA). The relative dot densities were determined with ImageJ
version 1.48 software (National Institutes of Health, Bethesda, MD,
USA), normalized to the relative density of α-tubulin.
Statistical analysis
The significance of differences between groups was
evaluated by a paired t-test. Data are presented as the mean ±
standard deviation of 6–10 replications performed. In all analyses,
P<0.05 was considered to represent a statistically significant
difference. All analyses were performed using the Statview
statistical software package (version 5.0; Abacus Concepts,
Berkley, CA, USA).
Results
Inhibition of cell viability
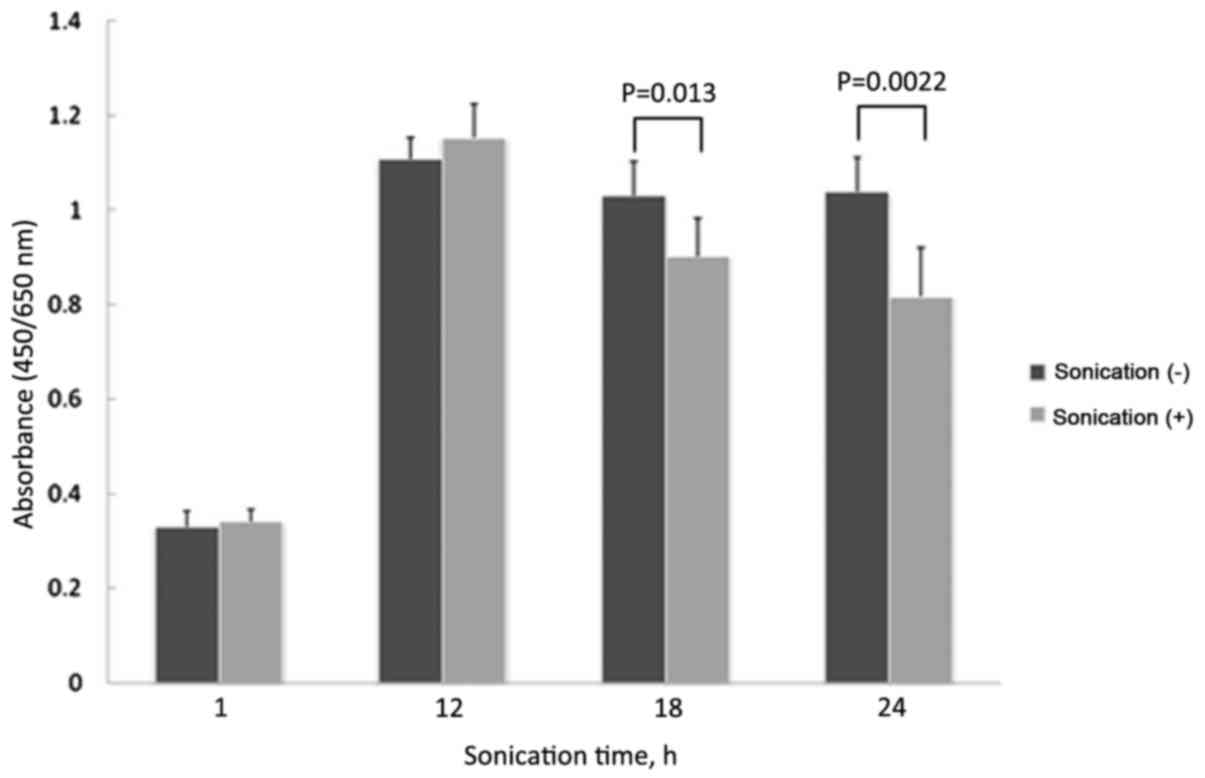
Treatment with LIPUS for 18 or 24 h significantly
inhibited the growth of LM8 cells, compared with no treatment (18
h, P=0.0133; 24 h, P=0.0022). There was no significant difference
in cell growth when treated for 1 or 12 h compared with no
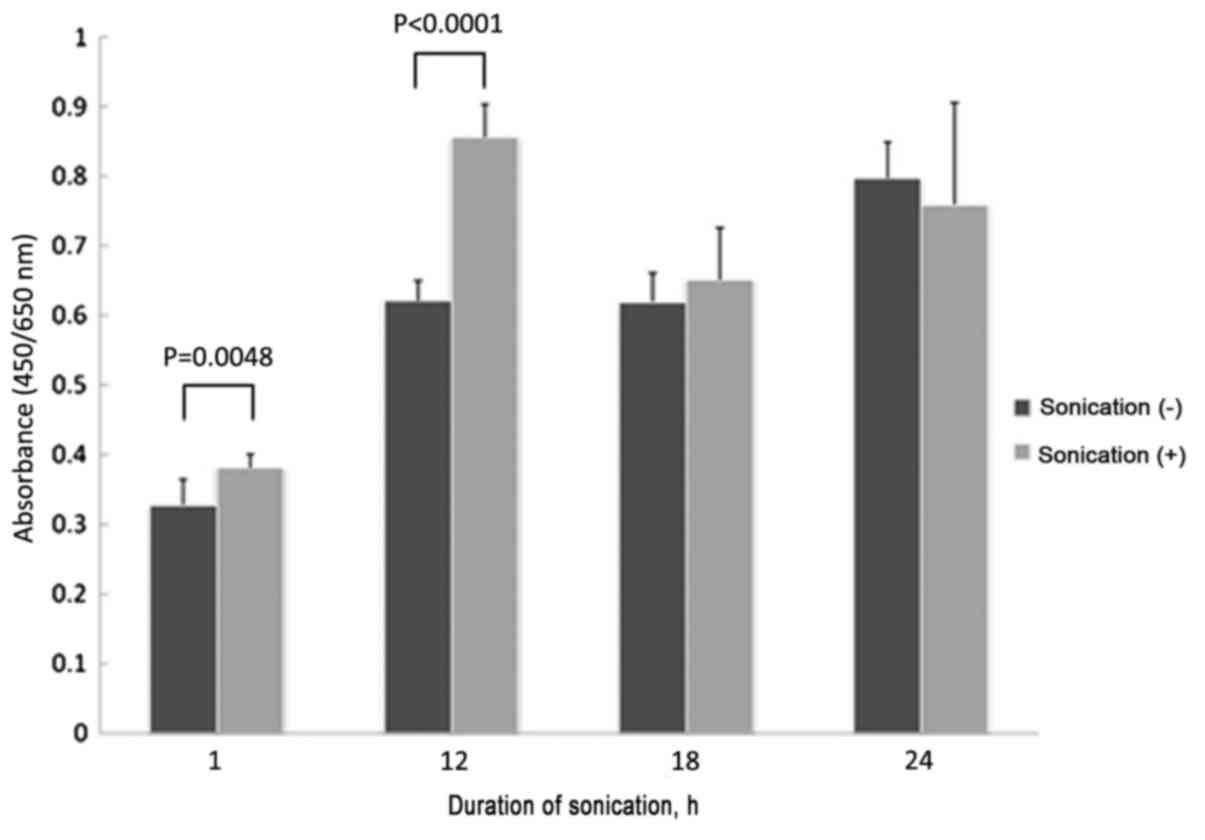
treatment (1 h, P=0.3762; 12 h, P=0.1858; Fig. 1). Treatment with LIPUS for 1 or 12 h
significantly inhibited the growth of MC3T3-E1 cells, compared with
no treatment (1 h, P=0.0048; 12 h, P<0.0001). There was no
significant difference in cell growth when treated for 18 or 24 h
compared with no treatment (18 h, P=0.6574; 24 h, P=0.3606;
Fig. 2).
Effects on mitochondrial membrane
potential
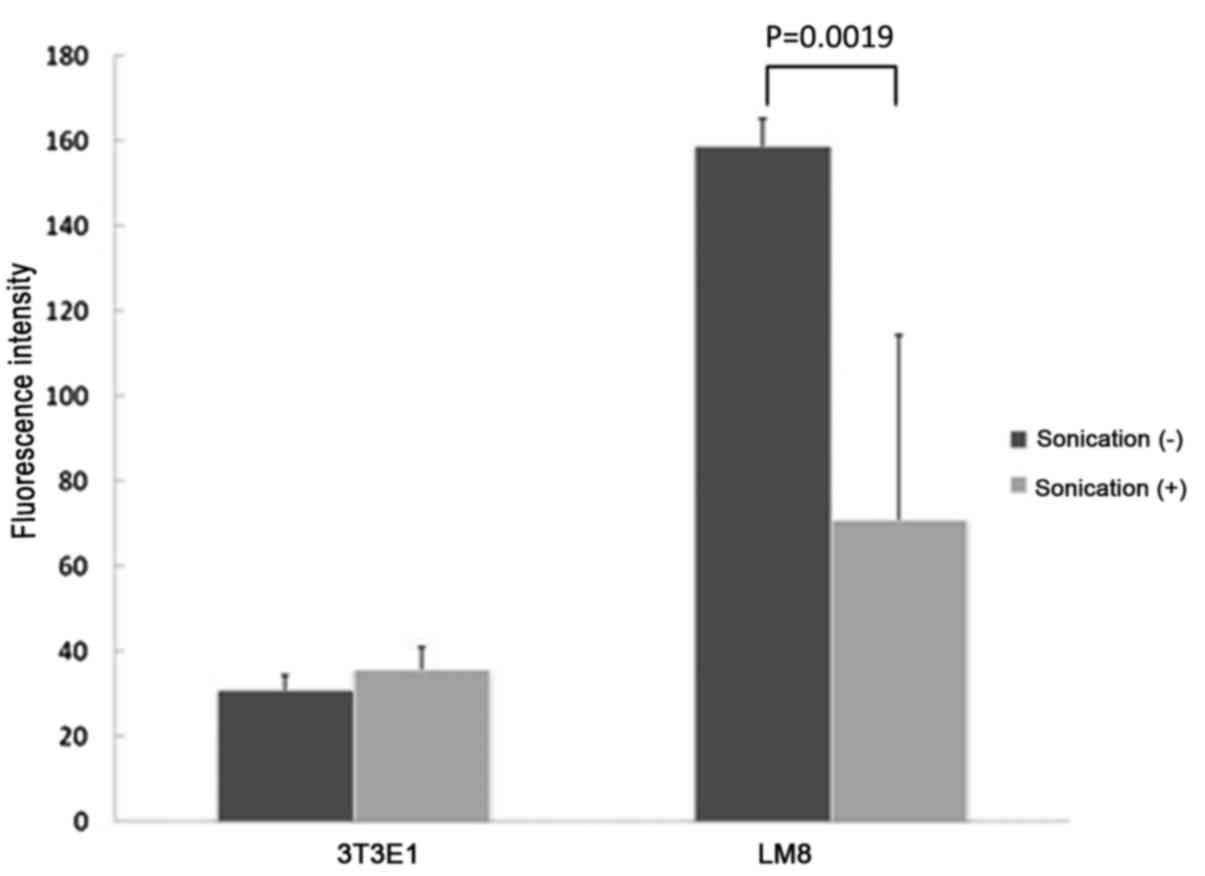
LM8 cells treated with LIPUS for 48 h had a
significantly lower mitochondrial membrane potential compared with
cells without treatment (P=0.0019), but there were no significant
differences in mitochondrial membrane potential between MC3T3-E1
cells with or without LIPUS treatment (P=0.2437; Fig. 3).
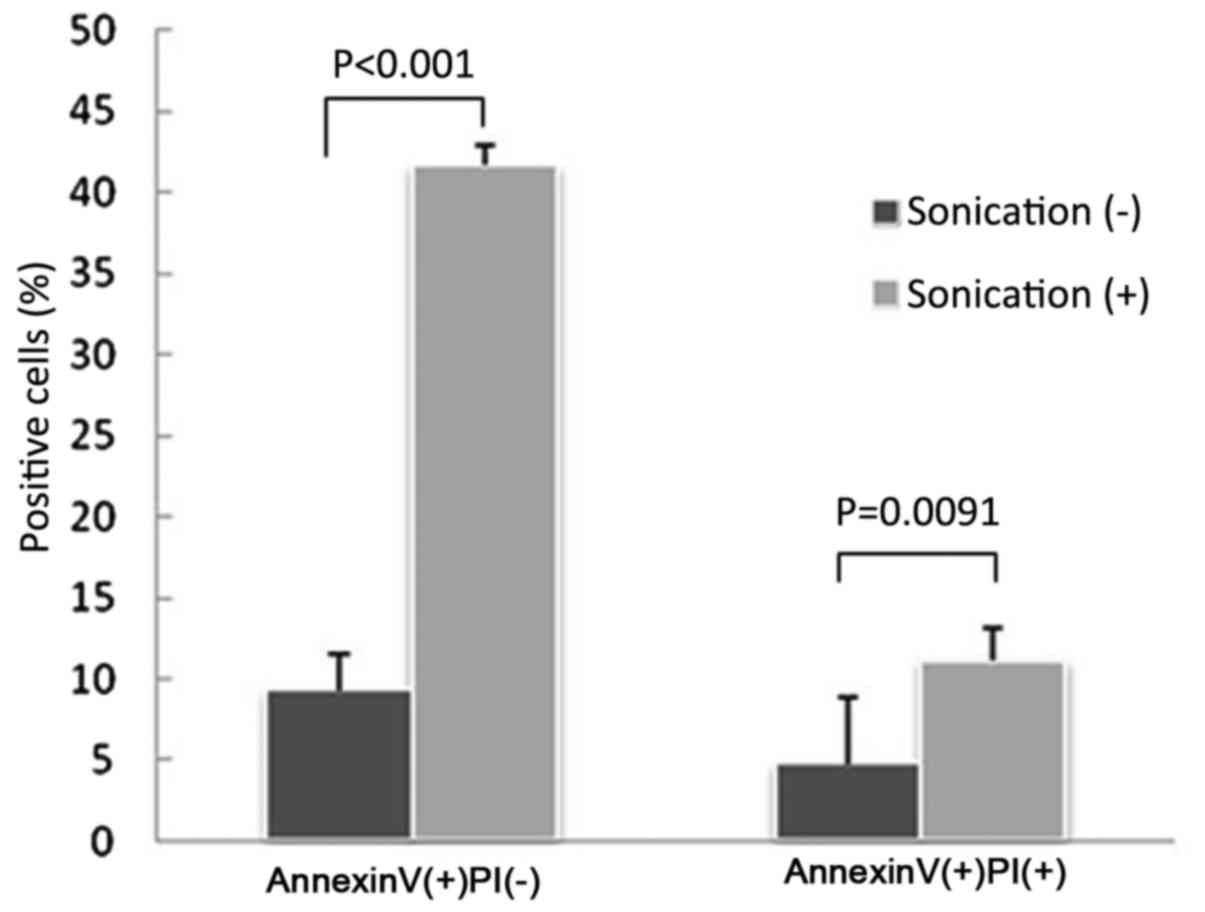
Induction of apoptosis
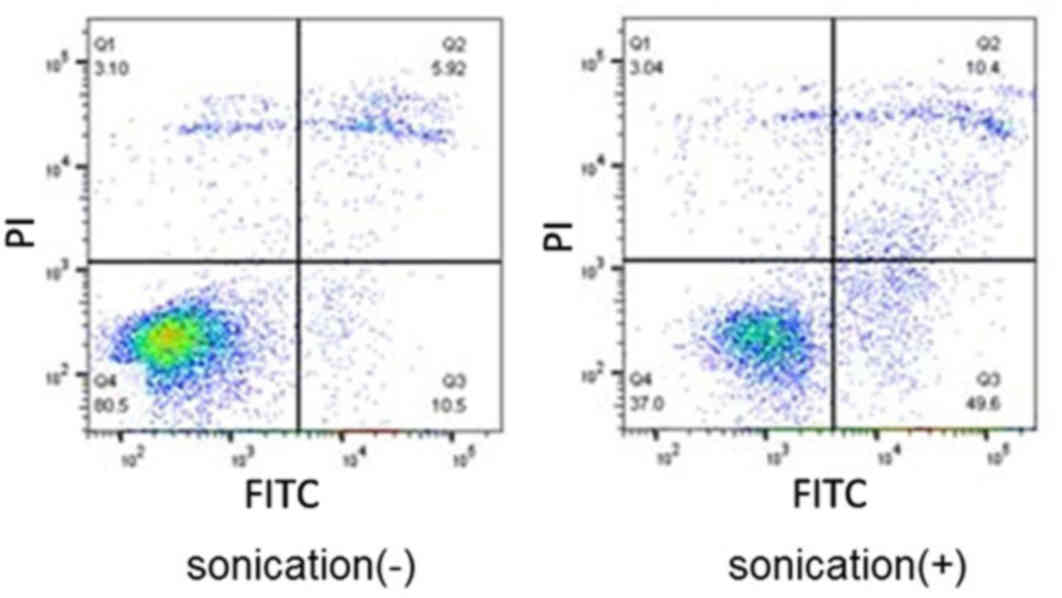
Flow cytometry analysis revealed that the LM8 cells
treated with LIPUS had significantly higher numbers of apoptotic
and necrotic cells compared with cells without treatment (apoptotic
cells, P<0.0001; necrotic cells, P=0.0091; Figs. 4 and 5).
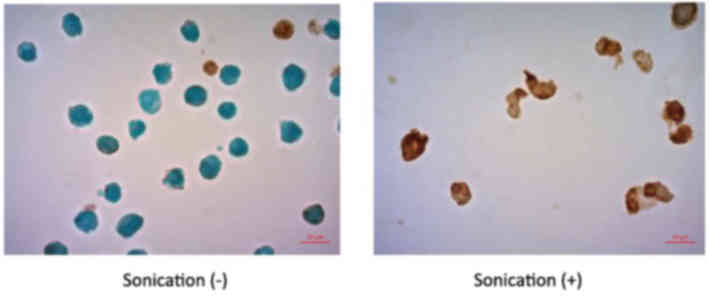
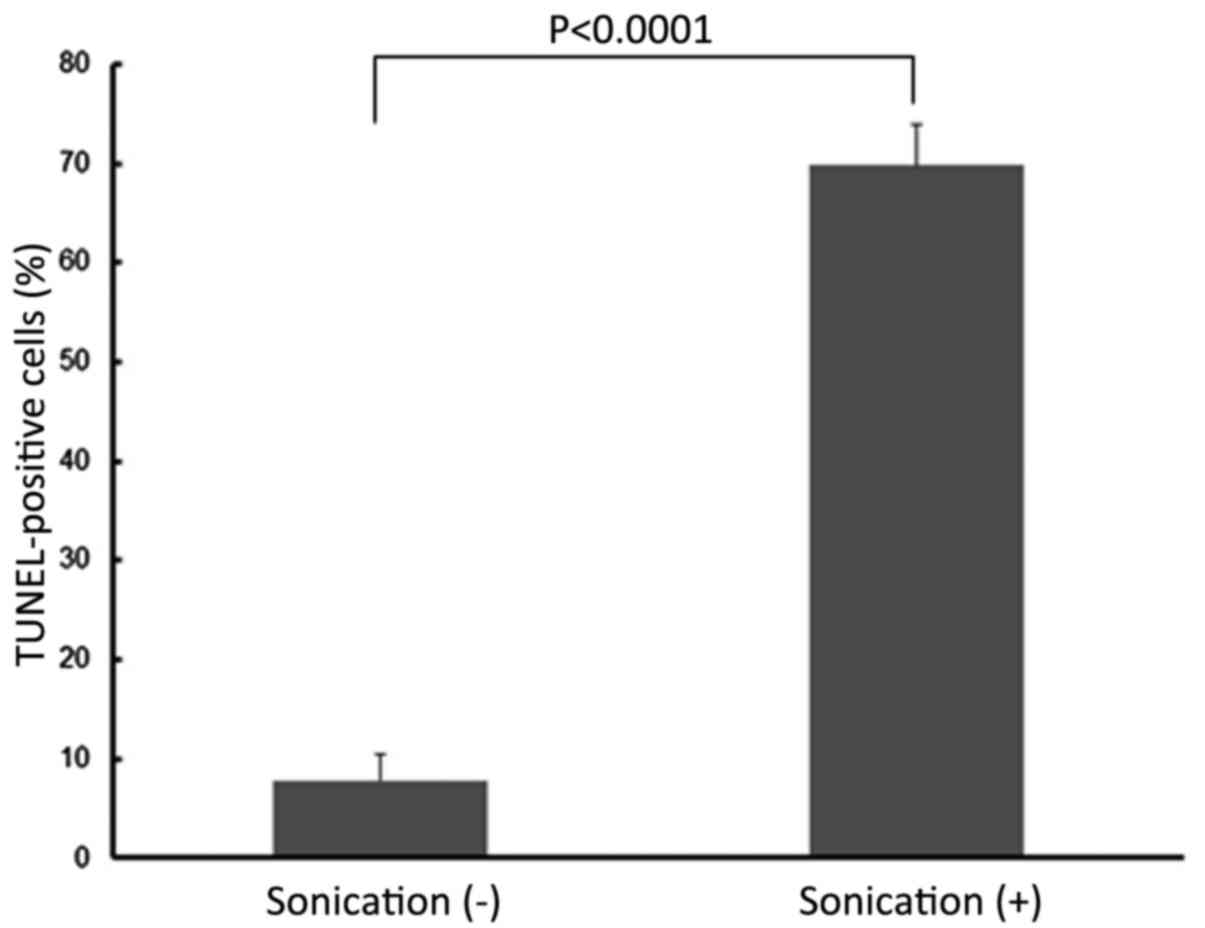
TUNEL staining analysis indicated that the LM8 cells treated with
LIPUS had significantly higher numbers of apoptotic cells compared
with cells without LIPUS treatment (P<0.0001; Figs. 6 and 7).
Analysis of intracellular signaling
molecules
As determined with a stress and apoptosis signaling
array (Fig. 8), LIPUS treatment of
LM8 cells for 24 h significantly enhanced the levels of
phosphorylated (p)-mitogen-activated protein kinase 3/1 (ERK1/2),
p-Akt and NF-κB inhibitor α (IκBα; p-ERK1/2 P<0.0001; p-Akt;
P<0.0001; IκBα: P<0.0001) and reduced the levels of
p-mitogen-activated protein kinase 7 (TAK1) and p-checkpoint kinase
1 (Chk1; TAK1, P<0.0001; Chk1, P=0.0006). However, there were no
effects on the levels of p-Bcl-2-associated agonist of cell death
(Bad) (P=0.6926; Fig. 9). LIPUS
treatment of the LM8 cells for 48 h significantly increased p-Akt
and IκBα levels (Akt, P<0.0001; IκBα, P=0.0001) and reduced
p-TAK1 and p-Chk1 levels (p-TAK1, P<0.0001; p-Chk1, P=0.0008).
However, there were no effects on the levels of p-ERK1/2 or p-Bad
(p-ERK1/2, P=0.2437; Bad, P=0.9837; Fig.
10).
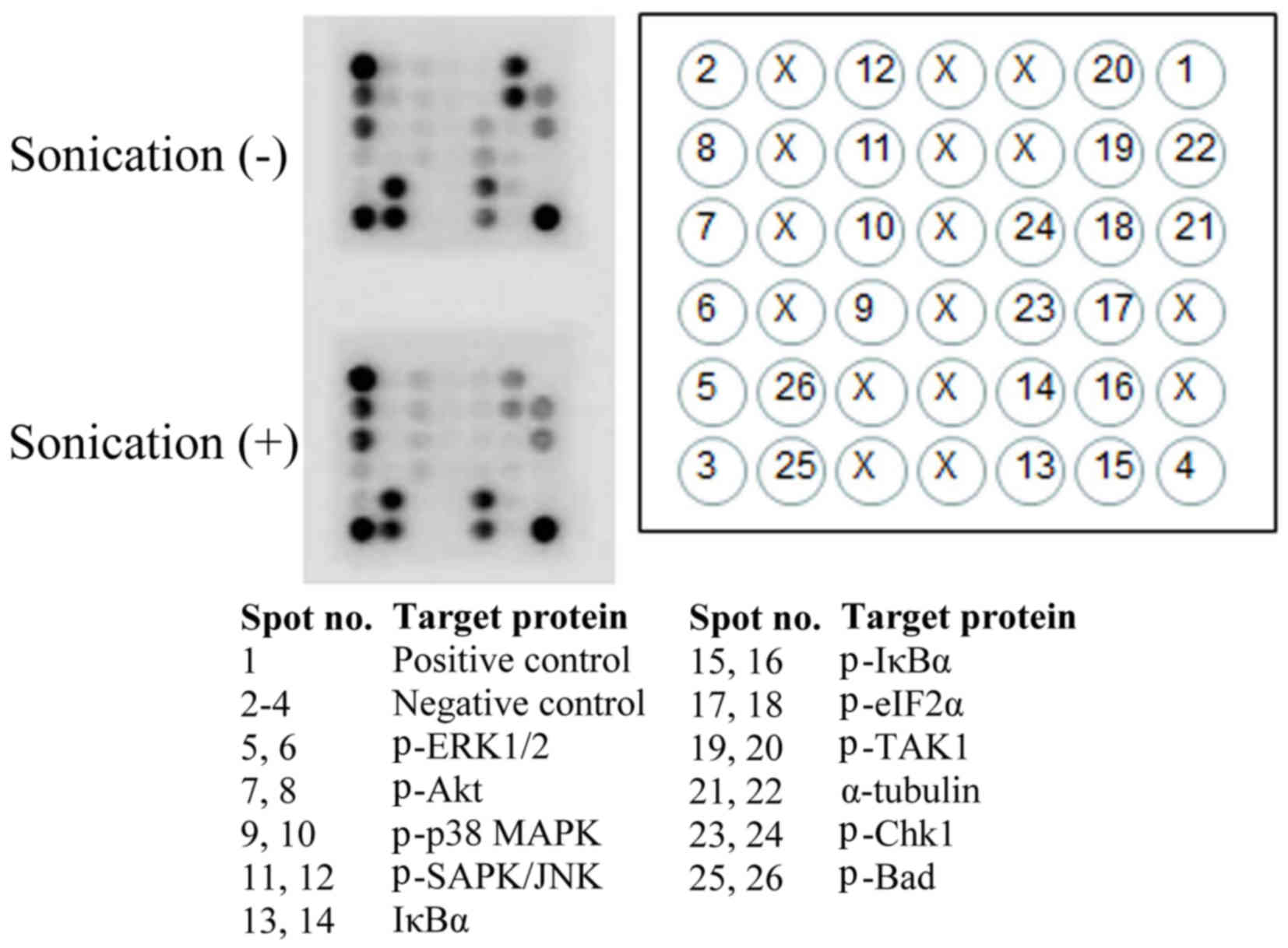 | Figure 8.Representative image of a stress and
apoptosis signaling protein array following the treatment of LM8
cells with or without low-intensity pulsed ultrasound for 48 h. X,
blank spot; p-, phosphorylated; ERK, mitogen-activated protein
kinase; MAPK, mitogen-activated protein kinase; SAPK/JNK,
mitogen-activated protein kinase 8; IκBα, NFκB inhibitor α; eIF2α,
eukaryotic translation initiation factor 2α; TAK1,
mitogen-activated protein kinase 7; Chk1, checkpoint kinase 1; Bad,
Bcl-2-associated agonist of cell death. |
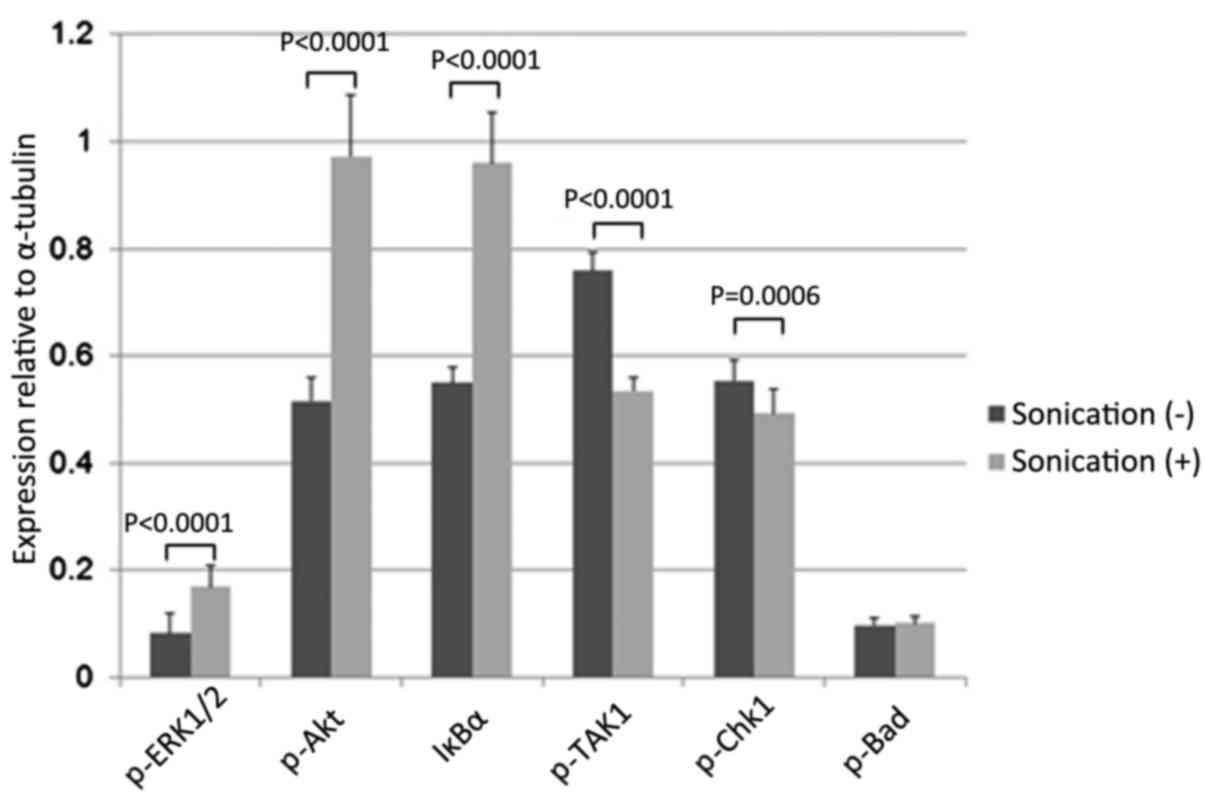 | Figure 9.Effects on comprehensive intracellular
signaling following LIPUS treatment for 24 h. LM8 cells were
treated with LIPUS for 24 h, resulting in a significant increase in
p-ERK1/2, p-Akt, and IκBα levels (p-ERK1/2, P<0.0001; p-Akt,
P<0.0001; IκBα, P<0.0001) and a decrease in p-TAK1 and p-Chk1
levels (p-TAK1, P<0.0001; p-Chk1, P=0.0006). There was no effect
on p-Bad levels (P=0.6926). LIPUS, low-intensity pulsed ultrasound;
ERK1/2, mitogen-activated protein kinase 3/1; IκBα, NFκB inhibitor
α; TAK1, mitogen-activated protein kinase 7; Chk1, checkpoint
kinase 1; Bad, Bcl-2-associated agonist of cell death. |
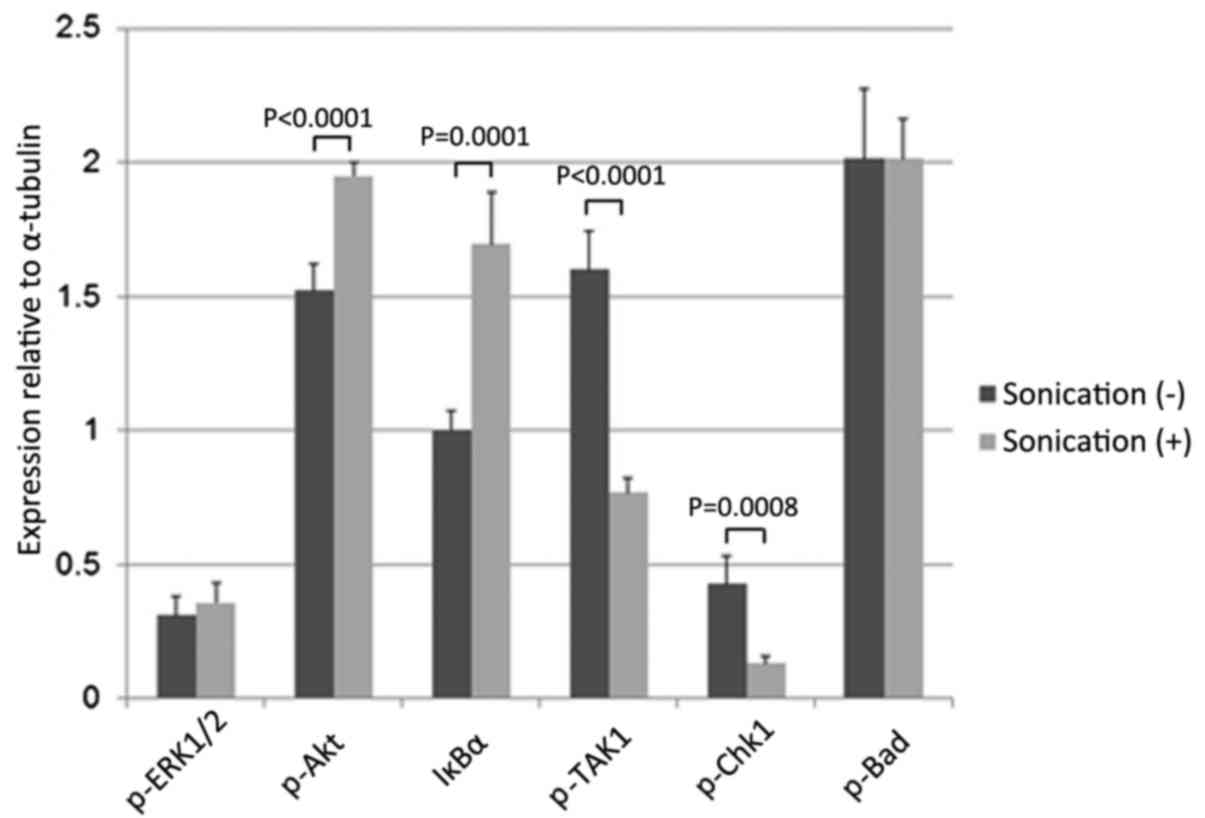 | Figure 10.Effects on intracellular signaling
following LIPUS treatment for 48 h. LM8 cells were treated with
LIPUS for 48 h, resulting in a significant increase in p-Akt and
IκBα levels (p-Akt, P<0.0001; IκBα, P=0.0001) and a decrease in
p-TAK1 and p-Chk1 levels (p-TAK1, P<0.0001; p-Chk1, P=0.0008).
There were no effects on p-ERK1/2 or p-Bad levels (p-ERK1/2,
P=0.2437; Bad, P=0.9837). LIPUS, low-intensity pulsed ultrasound;
IκBα, NFκB inhibitor α; TAK1, mitogen-activated protein kinase 7;
Chk1, checkpoint kinase 1; ERK, mitogen-activated protein kinase;
Bad, Bcl-2-associated agonist of cell death. |
Discussion
LIPUS therapy may promote bone formation in cases of
fracture (6,7) and accelerate bone maturation in cases of
distraction osteogenesis (8) and
delayed fracture union (9) by
positively affecting all phases of fracture repair (14), as well as eliciting effects on
cyclooxygenase 2 (15,16) and prostaglandins (17,18). There
have been several studies reporting that LIPUS has antitumor
effects via the induction of apoptosis in cancer cells (19–23). For
example, LIPUS was previously demonstrated to induce apoptosis by
causing membrane damage in human leukemia cells (19), affecting the
Ca2+/mitochondrial pathway in human hepatocellular
carcinoma cells (23) and enhancing
adjuvant chemotherapy in cases of lymphoma (20). However, there is currently limited
data regarding the antitumor effects of LIPUS on osteosarcoma
cells, although the treatment of osteosarcoma in children and
adolescents remains a serious challenge.
In previous studies, the mechanical stress caused by
LIPUS stimulation of osteoblasts was demonstrated to induce the
expression of chemokines and the receptors of nuclear factor κB
ligand, an essential osteoblast differentiation factor (12). Furthermore, LIPUS stimulates the
ability of osteoblasts to differentiate, mature and form bone, with
elevated bone sialoprotein expression observed during late
osteoblast differentiation (13). As
osteosarcoma is characterized by malignant osteoblastic
differentiation and osteoid matrix production (11), cell growth was investigated in the
present study to determine whether LIPUS may be used to inhibit
osteosarcoma cell growth. The results revealed that treatment with
LIPUS for 18 or 24 h (P=0.0133 and P=0.0022, respectively)
significantly inhibited LM8 cell growth, whereas no effects were
observed on MC3T3-E1 cell growth at the same time points.
Additionally, the LM8 cells undergoing LIPUS treatment for 48 h
exhibited a significantly lower mitochondrial membrane potential
compared with untreated cells (P=0.0019), whereas no significant
effects were observed in the MC3T3-E1 cells, with or without
treatment. These results indicate that mechanical stimulation of
osteosarcoma cells by LIPUS damages intracellular mitochondria,
decreases mitochondrial membrane potential and inhibits viability.
In addition, flow cytometry analysis revealed that LM8 cells
subjected to LIPUS treatment had significantly higher numbers of
apoptotic and necrotic cells compared with untreated cells
(apoptotic cells, P<0.0001; necrotic cells, P=0.0091), and TUNEL
staining analysis demonstrated that LM8 cells treated with LIPUS
had significantly higher numbers of apoptotic cells compared with
cells without treatment (P<0.0001). These results demonstrate
that LIPUS treatment effectively inhibits cell viability and
induces apoptosis in osteosarcoma cells.
Previous reports have revealed that LIPUS stimulated
the phosphorylation of ERK1/2 and Akt in osteosarcoma cell lines
(21) as well as the activation of
ERK1/2 in osteosarcoma cells, which have been induced to undergo
apoptosis and autophagy (24). In
addition, Akt may induce apoptosis via inhibition of Chk1 (25). In the present study, the levels of
p-ERK1/2 and p-Akt were increased and the level of p-Chk1 was
decreased in apoptotic LM8 cells following LIPUS treatment.
TAK1, a common upstream regulator of NF-κB, prevents
apoptosis via NF-κB-induced downregulation of IκBα (26,27). This
mechanism may partially explain why LIPUS treatment significantly
reduced the levels of p-TAK1 (P<0.0001) and enhanced the levels
of IκBα (P=0.0001) as a result of the induction of apoptosis in LM8
cells.
The use of adjuvant chemotherapy has proven
effective in osteosarcoma (2,4). Despite treatment advances and
significant improvements in survival rate, the prognosis for
patients with osteosarcoma remains poor (4). To improve the prognosis of patients with
osteosarcoma, it is necessary to develop novel effective adjuvant
treatments. LIPUS, in combination with chemotherapy or radiation,
is a promising therapy for osteosarcoma patients.
In conclusion, it has been demonstrated that LIPUS
treatment may effectively inhibits cell growth and induces
apoptosis in osteosarcoma cells. As a non-invasive approach, LIPUS
promises a range of clinical advantages, including brief, targeted
applications to the tumor site.
Acknowledgements
The authors wish to express sincere appreciation for
technical assistance to Mrs Mayu Takagi from WDB Co.
References
|
1
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: A
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosen G, Marcove RC, Caparros B, Nirenberg
A, Kosloff C and Huvos AG: Primary osteogenic sarcoma: The
rationale for preoperative chemotherapy and delayed surgery.
Cancer. 43:2163–2177. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Enneking WF and Dunham WK: Resection and
reconstruction for primary neoplasms involving the innominate bone.
J Bone Joint Surg Am. 60:731–746. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ham SJ, Koops H Schraffordt, van der Graaf
WT, van Horn JR, Postma L and Hoekstra HJ: Historical, current and
future aspects of osteosarcoma treatment. Eur J Surg Oncol.
24:584–600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Durfee RA, Mohammed M and Luu HH: Review
of osteosarcoma and current management. Rheumatol Ther. 3:221–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heckman JD, Ryaby JP, McCabe J, Frey JJ
and Kilcoyne RF: Acceleration of tibial fracture-healing by
non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg
Am. 76:26–34. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harrison A, Lin S, Pounder N and
Mikuni-Takagaki Y: Mode and mechanism of low intensity pulsed
ultrasound (LIPUS) in fracture repair. Ultrasonics. 70:45–52. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Mowafi H and Mohsen M: The effect of
low-intensity pulsed ultrasound on callus maturation in tibial
distraction osteogenesis. Int Orthop. 29:121–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rutten S, Nolte PA, Guit GL, Bouman DE and
Albers GH: Use of low-intensity pulsed ultrasound for posttraumatic
nonunions of the tibia: A review of patients treated in the
Netherlands. J Trauma. 62:902–908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xin Z, Lin G, Lei H, Lue TF and Guo Y:
Clinical applications of low-intensity pulsed ultrasound and its
potential role in urology. Transl Androl Urol. 5:255–266. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Czerniak B: OsteosarcomaBone Tumors. 2nd.
Elsevier; Philadelphia: pp. 200–355. 2016
|
|
12
|
Bandow K, Nishikawa Y, Ohnishi T, Kakimoto
K, Soejima K, Iwabuchi S, Kuroe K and Matsuguchi T: Low-intensity
pulsed ultrasound (LIPUS) induces RANKL MCP-1 and MIP-1beta
expression in osteoblasts through the angiotensin II type 1
receptor. J Cell Physiol. 211:392–398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki A, Takayama T, Suzuki N, Sato M,
Fukuda T and Ito K: Daily low-intensity pulsed ultrasound-mediated
osteogenic differentiation in rat osteoblasts. Acta Biochim Biophys
Sin (Shanghai). 41:108–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azuma Y, Ito M, Harada Y, Takagi H, Ohta T
and Jingushi S: Low-intensity pulsed ultrasound accelerates rat
femoral fracture healing by acting on the various cellular
reactions in the fracture callus. J Bone Miner Res. 16:671–680.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naruse K, Miyauchi A, Itoman M and
Mikuni-Takagaki Y: Distinct anabolic response of osteoblast to
low-intensity pulsed ultrasound. J Bone Miner Res. 18:360–369.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang CH, Yang RS, Huang TH, Lu DY, Chuang
WJ, Huang TF and Fu WM: Ultrasound stimulates cyclooxygenase-2
expression and increases bone formation through integrin, focal
adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in
osteoblasts. Mol Pharmacol. 69:2047–2057. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giannoudis PV, MacDonald DA, Matthews SJ,
Smith RM, Furlong AJ and De Boer P: Nonunion of the femoral
diaphysis. The influence of reaming and non-steroidal
anti-inflammatory drugs. J Bone Joint Surg Br. 82:655–658. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simon AM, Manigrasso MB and O'Connor JP:
Cyclo-oxygenase 2 function is essential for bone fracture healing.
J Bone Miner Res. 17:963–976. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feril LB Jr, Kondo T, Cui ZG, Tabuchi Y,
Zhao QL, Ando H, Misaki T, Yoshikawa H and Umemura S: Apoptosis
induced by the sonomechanical effects of low intensity pulsed
ultrasound in a human leukemia cell line. Cancer Lett. 221:145–152.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kondo T, Yoshida T, Ogawa R, Hassan MA,
Furusawa Y, Zhao QL, Watanabe A, Morii A, Feril LB Jr, Tachibana K,
et al: Low-intensity ultrasound adjuvant therapy: Enhancement of
doxorubicin-induced cytotoxicity and the acoustic mechanisms
involved. J Med Ultrason (2001). 36:612009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sawai Y, Murata H, Koto K, Matsui T, Horie
N, Ashihara E, Maekawa T, Fushiki S and Kubo T: Effects of
low-intensity pulsed ultrasound on osteosarcoma and cancer cells.
Oncol Rep. 28:481–486. 2012.PubMed/NCBI
|
|
22
|
Cochran M and Wheatley MA: In vitro gene
delivery with ultrasound-triggered polymer microbubbles. Ultrasound
Med Biol. 39:1102–1119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi M, Liu B, Liu G, Wang P, Yang M, Li Y
and Zhou J: Low intensity-pulsed ultrasound induced apoptosis of
human hepatocellular carcinoma cells in vitro. Ultrasonics.
64:43–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duan L, Perez RE, Hansen M, Gitelis S and
Maki CG: Increasing cisplatin sensitivity by schedule-dependent
inhibition of AKT and Chk1. Cancer Biol Ther. 15:1600–1612. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Waiwut P, Shin MS, Inujima A, Zhou Y,
Koizumi K, Saiki I and Sakurai H: Gomisin N enhances TNF-α-induced
apoptosis via inhibition of the NF-κB and EGFR survival pathways.
Mol Cell Biochem. 350:165–175. 2011. View Article : Google Scholar
|
|
27
|
Muramatsu Y, Matsui T, Deie M and Sato K:
Pulsed electromagnetic field stimulation promotes anti-cell
proliferative activity in doxorubicin-treated mouse osteosarcoma
cells. In Vivo. 31:61–68. 2017. View Article : Google Scholar : PubMed/NCBI
|