Introduction
The parapharyngeal space (PPS) is located in the
suprahyoid neck between the hyoid bone and the skull base,
alongside the pharynx. It is divided into prestyloid and
poststyloid compartments, separated by the tensor veli palatini
muscle and styloid process (1,2). PPS
tumors are rare and account for ~0.5% of head and neck tumors
(3). Of these tumors, ~80% are benign
(4). The most frequent PPS tumor is a
salivary neoplasm, followed by a neurogenic tumor (5–8). Salivary
neoplasms in the PPS, particularly pleomorphic adenomas, are the
most common lesions in the prestyloid space, whereas paragangliomas
and schwannomas are the most common in the poststyloid space
(5). Malignancy is also most common
in tumors originating in the salivary glands (9).
Pre-operative diagnosis of the PPS tumor assists the
determination of a surgical plan. Computed tomography (CT) and
magnetic resonance imaging (MRI) scans are crucial for the
assessment of PPS tumors. MRI is particularly useful for
determining the tumor localization and distinguishing the tumor
origin. Assignment of the tumor to the prestyloid or poststyloid
compartment depending on its localization provides a potential
diagnosis (1,10–12). If a
paraganglioma is suspected, in which the tumor is located around
the bifurcation of the carotid artery, an angiography or CT
angiography is recommended (6,7,10).
Surgery is the mainstay of treatment for PPS tumors,
and is performed on the basis of information provided by these
diagnostic modalities. A complex structure consisting of major
blood vessels, the cranial nerves (CNs), several multidirectional
muscles, and the jawbone and cranial bones cause the PPS to be
narrow and difficult to approach (10,13,14).
There are several surgical approaches that it is
possible to use for the resection of PPS tumors. These are
predominantly classified into four groups: Transcervical,
transparotid, transoral and transmandibular approaches. These
approaches, or a combined approach, are performed in the surgery of
PPS tumors depending on the characteristics of the tumor (2,10,12,15).
In the present study, the diagnosis, surgical
approach and complications associated with surgical PPS tumor
treatment in our experience are reported on, and previous studies
are discussed, to aid in improving the outcome of this disease.
Materials and methods
The present, retrospective study included 29
patients who underwent surgery for primary PPS tumors between
January 2008 and December 2015 in Nagoya City University Hospital
(Nagoya, Japan). The protocol of the investigation was approved by
the Institutional Review Board of Nagoya City University Hospital
(Nagoya, Japan). Informed consent was obtained from the patients
prior to their inclusion in the study.
In regard to histologic type, there were 16 cases of
neurogenic and 13 of salivary gland origin tumors. The following
data were evaluated: Preoperative symptoms, histological type,
surgical approach and complications Patients were evaluated
following a laboratory examination. Enhanced CT and MRI scans were
used to confirm the location and size of the tumor as a
preoperative diagnosis. In particular, the following aspects were
evaluated: Tumor shape and the tumor margin, the association of the
tumor location with major vasculature, surrounding tissue and the
deep lobe of the parotid gland (DLPG), and whether the tumor
localization was pre- or poststyloid. Preoperative imaging was also
used to estimate the origin of the tumor, the extent of malignancy
and tumor vascularity. For the examination of the histology of the
tumor, fine needle aspiration cytology (FNAC) was performed in the
majority of cases. Fine needle aspiration biopsy (FNAB) or
incisional biopsy were required in certain cases when the
histological results were unclear and there was a suspicion of
malignancy. In the case of malignancy, additional imaging
examination was performed to inform the selection of a treatment
plan. Where tumors exhibited the possibility of paraganglioma,
angiography or CT-angiography was performed to evaluate the
vasculature associated with the carotid artery.
All patients enrolled in the study underwent
surgical treatment. The plan for the surgical approach was selected
according to the tumor location, histological findings, the
relationship to anatomical structures and the suspicion of
malignancy. The approach of wide resection and reconstruction was
selected in cases where malignancy was confirmed. During the
follow-up period, clinical examination was performed each month
following surgical resection and an MRI of the head and neck was
performed at annual intervals for 5 years. Patients with malignant
cases underwent a head and neck CT or MRI every 6 months during the
first year, and head, neck, lung and abdominal CT or MRI scans
annually. If patients exhibited abnormal symptoms (e.g. neck mass
or lower cranial nerve palsy) during the follow-up, an additional
examination was performed, in case of recurrence.
Results
Preoperative symptoms
There were 11 males and 18 females included in the
present study. The mean age at the point of surgery was 43.5±16.3
years (range, 19–73 years) and the mean follow-up duration was
29.3±25.4 months (range, 1.8–88.1 months). Pathological diagnosis
resulted in 16 cases being classified as a neurogenic tumor (55.2%)
and 13 cases as a salivary gland tumor (44.8%; Table I). The most common symptoms of
neurogenic tumors were a neck mass (37.5%), coughing (12.5%),
hoarseness (12.5%) and pharyngeal pain (12.5%; Table IIA). The most common symptoms of
salivary gland tumors were the presence of a mass on the neck
(30.8%) and abnormal sensation of the pharynx (15.4%; Table IIB).
 | Table I.Characteristics of patients according
to tumor origin. |
Table I.
Characteristics of patients according
to tumor origin.
| A, Characteristics of
patients with neurogenic tumors, n=16 |
|---|
|
|---|
| Age | Sex | Approach | Tumor location | Final pathological
diagnosis | Original nerve |
|---|
| 23 | F | Cervical | Poststyloid | Schwannoma | Sympathetic
nerve |
| 28 | M | Cervical | Poststyloid | Schwannoma | Sympathetic
nerve |
| 19 | F | Cervical | Poststyloid | Schwannoma | Sympathetic
nerve |
| 38 | F | Cervical | Poststyloid | Schwannoma | Sympathetic
nerve |
| 38 | F | Cervical | Poststyloid | Schwannoma | Sympathetic
nerve |
| 19 | M | Cervical | Poststyloid | Schwannoma | Sympathetic
nerve |
| 67 | M | Cervical | Poststyloid | Schwannoma | Sympathetic
nerve |
| 64 | M | Cervical | Poststyloid | Schwannoma | Hypoglossus
nerve |
| 28 | F | Cervical | Poststyloid | Paraganglioma | Carotid body |
| 45 | M | Cervical | Poststyloid | Schwannoma | Hypoglossus
nerve |
| 44 | F | Cervical | Poststyloid | Schwannoma | Hypoglossus
nerve |
| 37 | F | Cervical | Poststyloid | Neurofibroma | Sympathetic
nerve |
| 33 | M | Cervical | Poststyloid | Schwannoma | Vagus nerve |
| 34 | M | Cervical | Poststyloid | Schwannoma | Vagus nerve |
| 35 | M | Cervical | Poststyloid | Schwannoma | Vagus nerve |
| 36 | M | Cervical | Poststyloid | Schwannoma | Vagus nerve |
|
| B, Characteristics
of patients with salivary gland tumors, n=13 |
|
| Age | Sex | Approach | Tumor location | Final pathological
diagnosis |
|
| 47 | F | Cervical | Prestyloid | Pleomorphic
adenoma |
| 61 | M | Cervical | Prestyloid | Pleomorphic
adenoma |
| 49 | F | Cervical | Prestyloid | Pleomorphic
adenoma |
| 64 | F | Cervical |
Prestyloid-poststyloid | Pleomorphic
adenoma |
| 54 | F |
Parotid-cervical |
Prestyloid-poststyloid | Pleomorphic
adenoma |
| 73 | M | Cervical | Prestyloid | Pleomorphic
adenoma |
| 66 | M | Cervical |
Prestyloid-poststyloid | Pleomorphic
adenoma |
| 30 | M |
Parotid-cervical | Prestyloid | Pleomorphic
adenoma |
| 54 | F | Cervical | Prestyloid | Pleomorphic
adenoma |
| 53 | M | Cervical | Prestyloid | Pleomorphic
adenoma |
| 48 | F |
Transmandibular | Poststyloid | Carcinoma in
pleomorphic adenoma |
| 25 | F |
Parotid-cervical | Prestyloid | Pleomorphic
adenoma |
| 33 | M |
Parotid-cervical | Prestyloid | Carcinoma in
pleomorphic adenoma |
 | Table II.Incidence of preoperative symptoms in
parapharyngeal space tumors. |
Table II.
Incidence of preoperative symptoms in
parapharyngeal space tumors.
| A, Preoperative
symptoms of neurogenic tumors, n=16 |
|---|
|
|---|
| Symptoms | n (%) |
|---|
| Neck mass | 6 (37.5) |
| Cough | 2 (12.5) |
| Hoarseness | 2 (12.5) |
| Abnormal sensation
of the pharynx | 1 (6.3) |
| Tongue palsy | 1 (6.3) |
| Sleep apnea | 1 (6.3) |
| No symptoms | 1 (6.3) |
|
| B, Preoperative
symptoms of salivary gland tumors, n=13 |
|
| Neck mass | 4 (30.8) |
| No symptoms | 4 (30.8) |
| Abnormal sensation
of the pharynx | 2 (15.4) |
| Pharyngeal
pain | 1 (7.7) |
| Pharyngeal
mass | 1 (7.7) |
| Posterior neck
pain | 1 (7.7) |
Imaging examination
Preoperative CT or MRI imaging was used to evaluate
the location of each tumor. A total of 9 salivary gland tumors were
located in the prestyloid space and 3 cases were located in the
pre- and poststyloid spaces. The remaining case, a malignant
parotid tumor, was difficult to judge as it was localized between
the prestyloid and poststyloid spaces. All 16 schwannoma cases were
in the poststyloid space (Table I).
The size of each tumor was measured with CT or MRI imaging. For
neurogenic tumors, the most frequent range for tumor size was 30–50
mm (7 cases; 43.8%) and the most frequent range for salivary tumor
size was 40–50 mm (5 cases; 38.5%).
Histopathologic examination
A total of 21 of the 29 patients (72.4%) underwent a
preoperative FNAC and 6 patients (27.3%) underwent a preoperative
histopathological evaluation. FNAB was performed in 2 cases (6.9%)
to confirm prestyloid schwannoma following FNAC results from which
there were suspicions of malignancy. The cytological examination
revealed pleomorphic adenoma in 11 cases (84.6%), including
cellular atypia in 4 cases (36.4%) and lymphocytes in 1 case
(9.1%). Of the 10 neurogenic tumors for which it was performed,
FNAC revealed spindle cells in 3 cases (30.0%) and unclear results
in 7 cases (70.0%). FNAC examination only contributed to
preoperative diagnosis in 9 cases (42.9%), whereas FNAB examination
contributed to the surgical plan in the 2 cases where it was
performed.
Surgical method
Tumor resection was performed in all cases. For all
patients with a benign tumor, the transcervical, transparotid or
combined approach was selected. In patients with a malignant tumor,
the mandibular swing approach was used to resect the tissues
surrounding the tumor. The tumor was then exposed through the space
between the submandibular gland and parotid gland. If approaching
the tumor was difficult, the digastric post-berry and stylo-hyoid
muscle was cut. A microscope was used to improve the visibility of
the area surrounding the tumor.
In cases of neurogenic tumors, the transcervical
approach was used. A nerve integrity monitor was used for lower CN
monitoring, including for the VII, IX, X, XI and XII nerves. In
schwannoma cases, tumor enucleation was performed to preserve nerve
functionality. In paraganglioma and neurofibroma cases, an en
bloc resection of the tumor was performed. In tumors of
salivary gland origin, the transcervical or transparotid approach
was selected, according to tumor location. Tumors were performed
with en bloc resection, avoiding tumor spillage. In the
single case of a malignant salivary gland tumor, the mandibular
swing approach was performed and the tumor was resected with a wide
surgical margin as the tumor had invaded the surrounding
tissue.
Postoperative complications
Major complications following surgery are summarized
in Table III. In salivary origin
tumors of the prestyloid space, facial nerve palsy was a
complication observed in 5 cases (38.5%). The most common location
for facial palsy was under the lip (4 cases). Total facial nerve
palsy occurred in 2 cases, although 1 of these patients, who had a
benign tumor, recovered after 4 months. Frey syndrome was not
observed in any case. First bite syndrome (FBS) occurred in 5 cases
(38.5%) and was resolved in 3 cases (60.0%). FBS continues in 2
cases (40.0%), was resolved within 1 year in 2 cases (40.0%) and
was resolved within 1 to 2 years in 1 case (20.0%). In the case of
neurogenic tumors, the tumor was resected with intracapsular
enuclation in order to preserve the original nerve. A total of 10
patients with neurogenic tumors exhibited palsy following surgery.
The paralysis was resolved in 12–18 months for 4 patients (40.0%),
whereas the remaining patients (60.0%) continue to exhibit
paralysis (Table IV). FBS occurred
in 5 cases (31.3%), continues in 4 cases (80.0%), and was resolved
within 1 year in 1 case (20.0%). In a case of paraganglioma, the
patient had transient XII nerve palsy. It was resolved after 3
months. Tumor recurrence was observed in 1 plexiform schwannoma
case following resection. Surgery was performed a short time
following the diagnosis of tumor recurrence. In malignant tumor
cases, VII nerve palsy occurred due to the necessary resection of
the nerve. Facial nerve mandibular branch (FNMB) palsy was also
exhibited in one case where surgery was performed on the lower
cheek flap. No patients presented with free flap issues following
the cheek surgery.
 | Table III.Incidence of postoperative
complications in salivary gland tumors. |
Table III.
Incidence of postoperative
complications in salivary gland tumors.
| Symptoms | n (%) |
|---|
| First bite
syndrome | 6 (46.2) |
| Lower lip
palsy | 4 (30.8) |
| Total facial nerve
palsy | 1 (7.7) |
| Abnormal feeling of
neck | 1 (7.7) |
| Pharyngeal
pain | 1 (7.7) |
 | Table IV.Incidence of nerve deficiency as a
postoperative symptom, according to the original nerve. |
Table IV.
Incidence of nerve deficiency as a
postoperative symptom, according to the original nerve.
| Origin | Symptom of nerve
deficiency | Frequency, n
(%) | Symptom
continuation, n (%) |
|---|
| Synpathetic nerve,
n=10 | Horner
syndrome | 5 (50.0) | 4 (40.0) |
|
| Tongue palsy | 1 (10.0) | 1 (10.0) |
| Vagus nerve,
n=4 | Vocal cord
palsy | 2 (50.0) | 0 (0.0) |
|
| Tongue palsy | 2 (50.0) | 1 (25.0) |
|
|
Pharyngoparalysis | 2 (50.0) | 1 (25.0) |
| Hypoglossal nerve,
n=3 | Tongue palsy | 3 (100.0) | 2 (66.7) |
|
| Vocal cord
palsy | 2 (66.7) | 2 (33.3) |
| Carotid body tumor,
n=1 | Tongue palsy | 1 (100.0) | 0 (0.0) |
|
|
Pharyngoparalysis | 1 (100.0) | 0 (0.0) |
Discussion
PPS tumors occur deep within the neck, and this
location results in difficulties in diagnosis and surgical
treatment. The purpose of the present study was to evaluate the PPS
treatment process and characteristics from diagnosis to surgical
treatment and thereafter. There was also the opportunity to
evaluate the utility of functional preservation surgery with lower
CN monitoring. According to previous reports, 70–90% of PPS tumors
are benign (6,7,9–11,14,16,17).
Only 2 cases (8%) of the cohort examined in the present study were
malignant, which was less than in these other studies. Previous
studies have reported that the most common subtype is pleomorphic
adenoma originating from the deep lobe of the salivary gland
(4,16), whereas Carrau et al (6) reported that 57% of PPS neoplasms were
neurogenic tumors. In the present study, >50% of the cases were
schwannoma. The most frequent origin nerves of the schwannomas were
the sympathetic and vagus nerves. Liu et al (18) corroborated this observation, reporting
that the sympathetic and vagus nerves were the most common nerves
of origin in head and neck schwannomas (18). However, no previous study has
described the nerves of origin of PPS schwannomas. A study by
Tryggvason et al (19)
revealed that schwannomas arise in nerves with a sensory component
and are associated with sensory ganglia. In addition, it was
reported that the majority of sympathetic chain schwannomas are
associated with the superior cervical ganglion (19). It is thus logical that these nerves
are common origins for PPS schwannomas.
The most frequent symptom in the cohort of the
present study was a neck mass, followed by pharyngeal mass; this is
comparable with other studies (6,15).
Dysphasia and pain have also been reported to be common symptoms
(6,10,15). No
patients presented with these symptoms in the present study. A
total of 2 patients with neurogenic tumors exhibited preoperative
neural deficits in the present study. The tumor mass resulted in a
deficit caused by injury to the nerve of origin (XII) in 1 patient,
and in the remaining case, the symptoms were due to injury of the
adjacent nerve (X) from a tumor of the XII nerve. In particular,
the vagus nerve contacts with the sublingual nerve. A previous
study has reported that, for malignant tumors, the most frequent
symptoms are a rapidly growing neck mass, pain, trismus, otalgia
and cranial nerve deficits (2). A
malignant tumor in the present study presented with the symptom of
a neck mass.
Preoperative imaging analysis of the PPS is
performed with the aim of providing information as follows: i) Size
of tumor; ii) location of tumor (pre- or poststyloid, relation to
parotid gland) and iii) whether there is extension of the tumor to
the adjacent area and major vasculature. MRI imaging is often able
to provide this information for soft tissue. A previous study
reported the superiority of MRI vs. CT as it permits the improved
discrimination of soft tissue types (20). Enhanced CT is useful for analysis of
tumor location and to preoperatively establish a method of approach
for surgery (10). Three
dimensional-CT angiography is indicated when the tumor is suspected
to be a carotid body tumor from its location, in order to evaluate
its vascularity and confirm the diagnosis (21).
It is also necessary to distinguish between benign
and malignant tumors prior to surgery. In a previous study, CN
palsy and pain were identified as symptoms likely to be associated
with malignancy (2). However, in the
present study, just one patient with PPS schwannomas exhibited
preoperative CN palsy; therefore, CN palsy is not a specific
symptom for malignant PPS tumors. Previous reports have stated that
the radiological signs of malignancy include irregular tumor
margins, spread into surrounding tissues and fat planes as
determined by CT or MRI, and evidence of enlarged, necrotic lymph
nodes in the retropharyngeal and cervical area (2,8,10).
Histological evaluation, particularly when there are
suspicions of malignancy, is desirable. Performing an incisional
biopsy carries the risk of tumor dissemination; however, it is
suggested when there is suspicion of a malignant neoplasm (6). The PPS is an unusual target for FNAB and
is associated with certain disadvantages, including difficulty of
access (2). FNAC of PSS tumors is
performed for lesions of the prestyloid PPS and may allow for the
differentiation of salivary gland malignancies. Needle biopsy of
the poststyloid PPS carries the risk of vascular complications
(22), and its use is also limited in
cases of the diagnosis of hemorrhagic specimens in paragangliomas
and hypocellular specimens in schwannomas (23,24). A
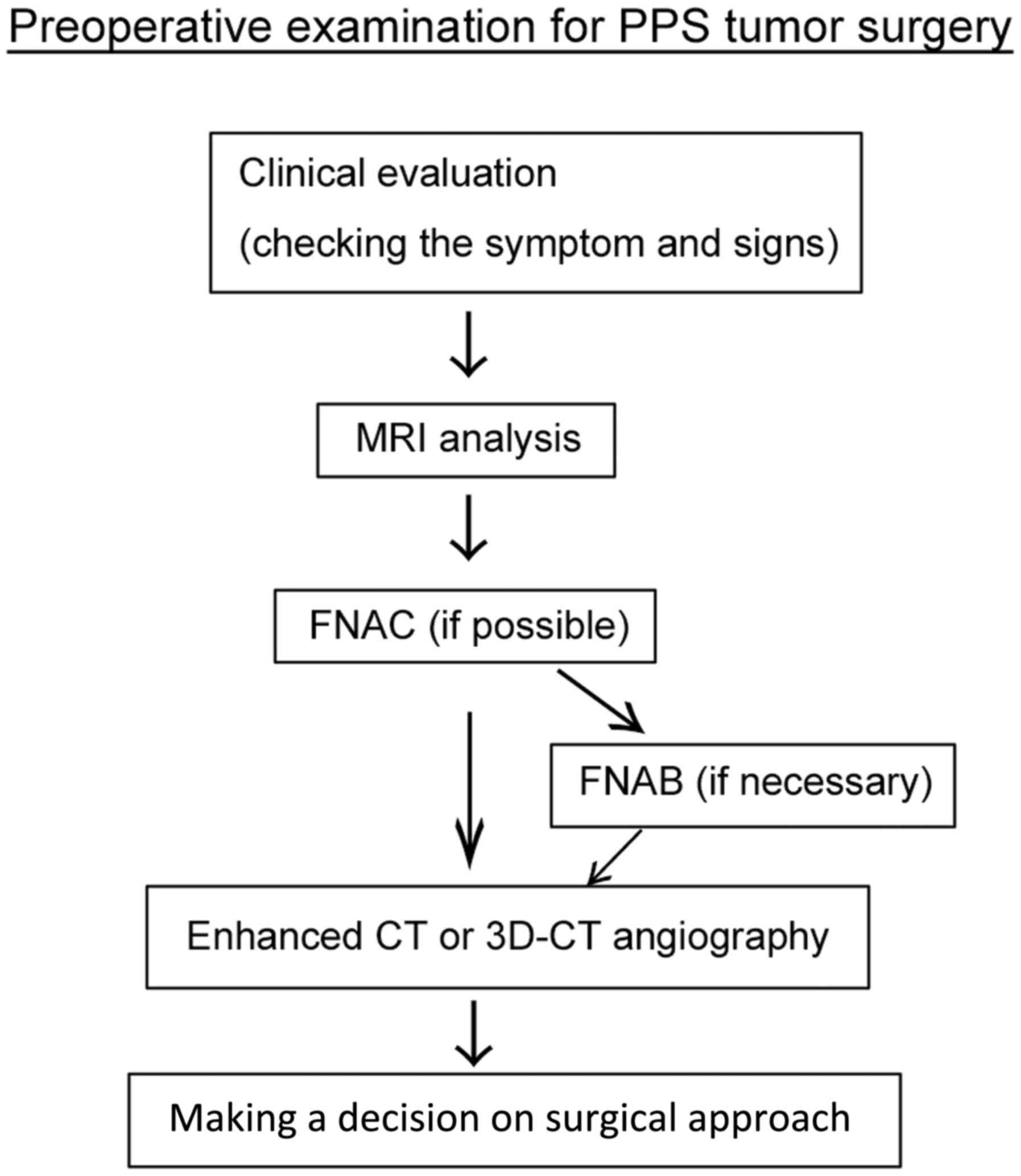
flow chart of the complete preoperative examination process is
presented in Fig. 1.
A number of authors have described the indications
for a transcervical, transoral, transparotid-transcervical or
transmandibular approach to surgery. The transcervical approach is
most frequently used to access PPS tumors (6,7,15), and was performed in the present study
for all neurogenic tumors and 61.5% of salivary gland tumors
(Table I). This approach allows
direct access to the PPS. The skin is incised from below the
submandibular gland to below the parotid gland. At the deeper
layer, it is possible to treat the digastric, stylohyoid and
stylomandibular ligaments (10). If
the vertical extension of the tumor causes suspicions of
intracranial extension, it may be difficult to use a transcervical
approach to dissect the tumor from the surrounding tissues,
particularly when it is in the vicinity of the cranial base
(2). In cases of malignant tumors,
difficulties in obtaining clear margins may arise from the
restricted visibility of this approach (15). The transparotid-transcervical approach
is suitable for prestyloid tumors arising in the DLPG that extend
into the PPS, requiring the identification and preservation of each
branch of the facial nerves (10).
The transoral approach presents a direct route to tumors though the
oropharynx, but provides no control of the large blood vessels
(6). This approach may be useful for
small benign avascular tumors that present in the oropharynx and do
not extend to the styloid process (14,19,25,26).
However, other authors have reported that this approach for PPS
tumor was associated with a 25% recurrence rate within 5 years
(27). Furthermore, a number of
authors avoid using this approach due to the risk of damaging the
blood vessels and the potential for incomplete tumor extirpation
(6,7,10). Robotic
transoral surgery of the PPS tumor was previously reviewed, and the
authors determined that the risk of violation of the capsule of a
pleomorphic adenoma was most severe when the capsule was ruptured
and tumor spillage occurred (28). In
our opinion, this would produce an unacceptable risk to prognosis;
tumor localization, size and suspicions of malignancy should be
carefully reviewed prior to considering this approach. In the
present study, the indications for the transmandibular approach in
PPS surgery were malignant neoplasms. Large recurrent neoplasms,
large benign neoplasms and highly vascular neoplasms where improved
vascular control is required also indicate this approach (9,29). This
approach allows the safe resection of lesions invading the skull
base that need further exposure (7).
The use of infratemporal fossa approaches, including the
orbito-zygomatic-middle fossa approach, is limited to PPS tumors
surrounding the temporal, lateral skull base and the infratemporal
fossa. This approach is used in cases of trigeminal schwannoma
(30).
Tracheostomy is not ordinarily necessary for the
surgery of a benign PPS tumor. If a defect following tumor
resection requires reconstruction with a musculocutanous flap that
may cause narrowing of the upper respiratory tract, tracheostomy is
performed at the end of the surgery, as previously reported
(10). Particular cases where
tracheostomy was indicated in a previous study included cases of
tumors >10 cm, or cerebral infarction (2).
In previous studies, the most frequently reported
complication of PPS tumor surgery was FNMB palsy (10,15). These
studies predominantly enrolled patients with a pleomorphic adenoma
of the PPS. In the cohort of the present study, FNMB palsy
typically occurred following surgery to remove tumors of salivary
origin, as the surgery was performed close to the mandible. The
second most common complication in these previous studies was the
presentation of FBS. FBS also occurred in the present study,
following prestyloid tumor surgery. Frey syndrome was reported only
in the deep lobe of tumors treated with the
transcervical-transparotid approach, but the cause was not
described in these previous reports.
The most serious complication of PPS tumor resection
is CN paralysis. The associated CNs are VII, IX, X, XI and XII
(31). The original nerve of the
tumor tends to be injured following the resection of neurogenic
tumors, including schwannomas and paragangliomas. CN X palsy is the
most common form (32), and may be
severe following PPS surgery. A previous study (4) revealed that neurogenic tumors,
particularly vagal paragangliomas, are particularly at risk for
developing postoperative sequelae. Horner syndrome may also be
presented postoperatively in cases of sympathetic PPS schwannomas
(4). The tumor enucleation of PPS
schwannomas may allow the preservation of the function of the
original nerves. Mapping the nerve fiber with an electromyographic
(EMG) system may help to decide the incision line on the tumor
capsule (33,34). In the present study, an EMG system was
used when there was suspicion of a neurogenic tumor, particularly
schwannomas, in order to lessen damage to the nerve fibers as much
as possible during tumor enucleation. When a nerve bundle is
visible, the tumor should be incised where it is separated from the
nerve fiber. When the nerve bundle is not visible, the tumor should
be incised where it swells (33,34). An
EMG system is particularly useful to map the nerve fibers of motor
nerves.
The strategy used during PPS surgery should be
dependent on the status of the tumor. In the present study, this
was determined with information obtained via clinical evaluation,
imaging analysis and histological analysis. The most common
histologic types were schwannoma or pleomorphic adenoma. In
schwannoma cases, tumors should be enucleated to preserve the
function of the lower CNs. When pleomorphic adenomas are resected,
tumor spillage should be avoided to prevent relapse.
The major goals of PPS tumor resection are to
completely remove the tumor and reduce the number of postoperative
complications. This may be achieved by improving the methods of
examination, carefully selecting the approach for surgery and with
accurate management during surgery.
References
|
1
|
Curtin HD: Separation of the masticator
space from the parapharyngeal space. Radiology. 163:195–204. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Basaran B, Polat B, Unsaler S, Ulusan M,
Aslan I and Hafiz G: Parapharyngeal space tumours: The efficiency
of a transcervical approach without mandibulotomy through review of
44 cases. Acta Otorhinolaryngol Ital. 34:310–316. 2014.PubMed/NCBI
|
|
3
|
Stell PM, Mansfield AO and Stoney PJ:
Surgical approaches to tumors of the parapharyngeal space. Am J
Otolaryngol. 6:92–97. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen SM, Burkey BB and Netterville JL:
Surgical management of parapharyngeal space masses. Head Neck.
27:669–675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batsakis JG and Sneige N: Parapharyngeal
and retropharyngeal space diseases. Ann Otol Rhinol Laryngol.
98:320–321. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carrau RL, Myers EN and Johnson JT:
Management of tumors arising in the parapharyngeal space.
Laryngoscope. 100:583–589. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khafif A, Segev Y, Kaplan DM, Gil Z and
Fliss DM: Surgical management of parapharyngeal space tumors: A
10-year review. Otolaryngol Head Neck Surg. 132:1–406. 2005.
View Article : Google Scholar
|
|
8
|
Work WP and Hybels RL: A study of tumors
of the parapharyngeal space. Laryngoscope. 84:1–1755. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shahab R, Heliwell T and Jones AS: How we
do it: A series of 114 primary pharyngeal space neoplasms. Clin
Otolaryngol. 30:364–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dimitrijevic MV, Jesic SD, Mikic AA,
Arsovic NA and Tomanovic NR: Parapharyngeal space tumors: 61 case
reviews. Int J Oral Maxillofac Surg. 39:983–989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allison RS, Van der Waal I and Snow GB:
Parapharyngeal tumours: A review of 23 cases. Clin Otolaryngol
Allied Sci. 14:199–203. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuet ML, Kasbekar AV, Masterson L and Jani
P: Management of tumors arising from the parapharyngeal space: A
systematic review of 1,293 cases reported over 25 years.
Laryngoscope. 125:1372–1381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horowitz G, Ben-Ari O, Wasserzug O,
Weizman N, Yehuda M and Fliss DM: The transcervical approach for
parapharyngeal space pleomorphic adenomas: Indications and
technique. PLoS One. 9:e902102014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olsen KD: Tumors and surgery of the
parapharyngeal space. Laryngoscope. 104 5 Pt 2 Suppl 63:S1–S28.
1994. View Article : Google Scholar
|
|
15
|
Papadogeorgakis N, Petsinis V, Goutzanis
L, Kostakis G and Alexandridis C: Parapharyngeal space tumors:
Surgical approaches in a series of 13 cases. Int J Oral Maxillofac
Surg. 39:243–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eneroth CM: Histological and clinical
aspects of parotid tumours. Acta Otolaryngol Suppl. 188 Suppl
191:S1–S99. 1964.
|
|
17
|
Hughes KV III, Olsen KD and McCaffrey TV:
Parapharyngeal space neoplasms. Head Neck. 17:124–130. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu HL, Yu SY, Li GK and Wei WI:
Extracranial head and neck Schwannomas: A study of the nerve of
origin. Eur Arch Otorhinolaryngol. 268:1343–1347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tryggvason G, Barnett A, Kim J, Soken H,
Maley J and Hansen MR: Radiographic association of schwannomas with
sensory ganglia. Otol Neurotol. 33:1276–1282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Som PM, Sacher M, Stollman AL, Biller HF
and Lawson W: Common tumors of the parapharyngeal space: Refined
imaging diagnosis. Radiology. 169:81–85. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caldarelli C, Bucolo S, Spisni R and
Destito D: Primary parapharyngeal tumours: A review of 21 cases.
Oral Maxillofac Surg. 18:283–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arnason T, Hart RD, Taylor SM, Trites JR,
Nasser JG and Bullock MJ: Diagnostic accuracy and safety of
fine-needle aspiration biopsy of the parapharyngeal space. Diagn
Cytopathol. 40:118–123. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zaharopoulos P: Diagnostic challenges in
the fine-needle aspiration diagnosis of carotid body
paragangliomas: Report of two cases. Diagn Cytopathol. 23:202–207.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu GH, Sack MJ, Baloch Z and Gupta PK:
Difficulties in the fine needle aspiration (FNA) diagnosis of
schwannoma. Cytopathology. 10:186–194. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bass RM: Approaches to the diagnosis and
treatment of tumors of the parapharyngeal space. Head Neck Surg.
4:281–289. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luna-Ortiz K, Navarrete-Alemán JE,
Granados-García M and Herrera-Gómez A: Primary parapharyngeal space
tumors in a Mexican cancer center. Otolaryngol Head Neck Surg.
132:587–591. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goodwin WJ Jr and Chandler JR: Transoral
excision of lateral parapharyngeal space tumors presenting
intraorally. Laryngoscope. 98:266–269. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan JY, Tsang RK, Eisele DW and Richmon
JD: Transoral robotic surgery of the parapharyngeal space: A case
series and systematic review. Head Neck. 37:293–298. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kolokythas A, Eisele DW, El-Sayed I and
Schmidt BL: Mandibular osteotomies for access to select
parapharyngeal space neoplasms. Head Neck. 31:102–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krishnamurthy S, Holmes B and Power SK:
Schwannomas limited to the infratemporal fossa: Report of two
cases. J Neurooncol. 36:269–277. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Presutti L, Molteni G, Malvè L, Marchioni
D, Ghidini A, Tassi S, Chiarini L and Alicandri-Ciufelli M:
Parapharyngeal space tumors without mandibulotomy: Our experience.
Eur Arch Otorhinolaryngol. 269:265–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yasumatsu R, Nakashima T, Miyazaki R,
Segawa Y and Komune S: Diagnosis and management of extracranial
head and neck schwannomas: A review of 27 cases. Int J Otolaryngol.
2013:9730452013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Behuria S, Rout TK and Pattanayak S:
Diagnosis and management of schwannomas originating from the
cervical vagus nerve. Ann R Coll Surg Engl. 97:92–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boufettal M, Azouz M, Rhanim A, Abouzahir
M, Mahfoud M, Bardouni AE, Berrada MS and Yaacoubi ME: Schwannoma
of the median nerve: Diagnosis sometimes delayed. Clin Med Insights
Case Rep. 7:71–73. 2014. View Article : Google Scholar : PubMed/NCBI
|