Introduction
In addition to numerous types of oxygen-centered
free radicals, various physiological and biochemical processes in
the human body may produce other reactive oxygen species (ROS)
(1). High concentrations of these
free radicals may induce oxidative damage to cell structures
including lipids and membranes, proteins and nucleic acids; this
damage is often referred to as oxidative stress and eventually
leads to numerous chronic diseases, including atherosclerosis,
cancer, diabetes and other degenerative diseases in humans
(2,3).
ROS have previously been revealed to be involved in cancer
initiation and promotion, and patients with neoplasms demonstrated
elevated malondialdehyde (MDA) concentrations (4). Furthermore, evidence indicating that
antioxidants inhibit free radical damage suggests that treatment
with a combination of antioxidants may be a potent adjunctive
preventive treatment for cancer (5).
Phytochemicals are still widely used worldwide and
across the major groups of human medicine, presently, they comprise
~50% of the total pharmaceutical market (6). A number of countries and studies are
extracting and investigating potent and nontoxic antioxidants from
natural sources, in particular those identified in edible or
medicinal plants, in order to prevent free radical-associated human
disorders and to replace synthetic compounds, which likely possess
carcinogenic activity or activity that is harmful to the lungs and
liver (7). For ~5,000 years,
thousands of plant species in Bangladesh have demonstrated
medicinal value, and various sections of several medicinal plants
have been used for the prevention and treatment of complex diseases
(6).
Aponogeton undulatus belongs to the family
Aponogetonaceae and may be found growing in India, Sri Lanka,
Myanmar, Bangladesh and China (8).
The rootstock of the plant is an important source of food that may
be useful as a nutrient supplement in numerous areas of the world,
where purchasing power is limited due to low incomes (9). The rootstock of Aponogeton
undulatus may provide an adequate supply of carbohydrates
(42.8/100 g), protein (8.3/100 g), fats (0.7/100 g), iron (18.2/100
g), calcium (37.2/100 g) and various additional minerals (9). Additionally, in Ayurvedic medicine, leaf
pastes from the plant combined with hot water have been used to
treat cuts and wounds; the plant has been claimed to be effective
against coughs, tuberculosis, acne, cancer, diarrhea, dysentery and
jaundice, among other medical problems (10). As reported previously, the primary
constituents of Aponogeton undulatus are tannins and
alkaloids, which possess thrombolytic and broad-spectrum
antibacterial activity, in addition to potential toxicity (11). The present study aimed to evaluate the
in vitro antioxidative and in vivo anticancer
activity of Aponogeton undulatus leaf extracts, in addition
to its organic fractions. To the best of our knowledge, this is the
first report investigating the in vivo anticancer activity
of Aponogeton undulatus extract against Ehrlich ascites
carcinoma (EAC) cells in mice.
Materials and methods
Plant materials
Aponogeton undulatus leaves were collected
from the village Kamatpara in the Panchagarh district of
Bangladesh, during August 2009. The leaves were identified by a
taxonomist at the National Herbarium of Bangladesh (Mirpur, Dhaka),
and its voucher specimen number (32095) has been maintained in our
laboratory for future reference.
Chemicals
Folin-chiocaltu phenol reagent was purchased from
Merck KGaA (Darmstadt, Germany). In addition,
1,1-diphenyl-2-picryl-hydrazyl (DPPH), ascorbic acid, quercetin,
5,5′-dithiobis (2-nitrobenzoic acid; DTNB),
L-2-amino-3-mercapto-3-methylbutanoic acid (L-penicillamine),
diethylene triamine pentaacetic acid (DTPA) and potassium
ferricyanide, bleomycin, and trichloroacetic acid (TCA) were
purchased from Sigma (St. Louis, MO, USA).
6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was
purchased from Aldrich Chemical Co. (Milwaukee, WI, USA).
Thiobarbituric acid (TBA) and nitroblue tetrazolium chloride (NBT)
were purchased from Loba Chemie Pvt., Ltd. (Mumbai, India), and
5,5′-dithio bis-2-nitro benzoic acid (DTNB), phenazine
methosulfate (PMS) and nicotinamide adenine dinucleotide (NADH)
were supplied by Sisco Research Laboratories Pvt., Ltd. (Mumbai,
India). All chemicals and reagents were of the highest analytical
grade.
Preparation of crude plant
extract
The plant material was shade-dried with occasional
shifting, prior to being ground to a powder using a mechanical
grinder, passed through sieve no. 40 (particle/mesh; size, 0.420
mm) and stored in an airtight container. A total of ~500 g of the
dried, powdered plant material was added to an amber colored
extraction bottle (capacity, 2.5 l) and the material was soaked
with methanol (extraction performed 3 times; volume, 1.0 l each
time). The sealed bottle containing plant material and methanol was
stored for seven days at room temperature (RT; 37°C) with
occasional shaking and stirring. The combined extracts were
filtered through cotton and subsequently Whatman No. 1 filter
papers, prior to being concentrated with a rotary evaporator under
reduced pressure at 45°C until dry in vacuo, to provide 40 g
crude methanol extract of Aponogeton undulatus leaves (MAU).
The extract was suspended with 100 ml of water at RT for 2 h
(fractionation for 2 h at RT), followed by fractionated by
chloroform, and ethyl acetate (in each 100 ml) to obtain the
chloroform fraction (CAU; 5.08 g), ethyl acetate fraction (EAU;
10.18 g) and finally aqueous fraction (WAU; 9.15 g).
In vitro antioxidant activity
Determination of total phenol
Total phenolic content of the extracts was
determined by the modified Folin-Ciocalteu method previously
described by Yu et al (12).
An aliquot of the extracts/standard was mixed with 2 ml
Folin-Ciocalteu reagent (Sigma-Aldrich; Merck KGaA; previously
diluted with water 1:10 v/v) and 2 ml (75 g/l) sodium carbonate.
Tubes were vortexed at 1,000 × g for 15 sec and allowed to stand
for 20 min at 25°C, for color development. Absorbance was
subsequently determined at 760 nm with a UV-spectrophotometer
(Shimadzu Corporation, Kyoto, Japan). Samples of extracts/standard
were evaluated at a final concentration of 0.1 mg/ml. Total
phenolic content was expressed in terms of the gallic acid
equivalent (GAE; standard curve equation: y=0.011x+0.066,
R2=0.998), mg GAE/g of dry extract.
Determination of total flavonoid content
Total flavonoid content was determined using the
method as previously described by Chang et al (13). To a total of 0.5 ml of
samples/standard, 1.5 ml methanol, 100 µl 10% aluminum chloride,
100 µl 1 M potassium acetate solution and 2.8 ml distilled water
were added. Following a 90-min incubation at RT, the absorbance was
evaluated at 420 nm using UV-spectrophotometer (Shimadzu
Corporation). The samples and standard were observed at a final
concentration of 0.1 mg/ml. Total flavonoid content was expressed
in terms of the quercetin equivalent (QAE; standard curve equation:
y=0.003x+0.022, R2=0.997), mg of QAE/g of dry
extract.
Determination of total flavonol content
Total flavonol content in the plant extracts was
determined using the method previously described by Kumaran and
Karunakaran (14). To 2.0 ml
sample/standard, 2.0 ml 2% AlCl3 ethanol and 3.0 ml (50
g/l) sodium acetate solutions were mixed together and stand for
complete the reaction at 20°C for 1 h, followed by the absorption
was measured at 440 nm using a spectrophotometer (Shimadzu, Tokyo,
Japan). Extracts and the standard were evaluated at a final
concentration of 0.1 mg/ml. The total content of flavonols was
expressed in terms of QAE (standard curve equation:
y=0.0255x+0.0069, R2=0.9995), mg of QAE/g of dry
extract.
Determination of total antioxidant capacity
(TAC)
The TAC of samples/standard was determined using the
method previously described by Prieto et al (15), with partial modifications as follows.
A total of 0.5 ml of the extract/fractions (200 µg) and the
standard at various concentrations (0, 25, 50, 75, 100, 125, 150
and 200 µg/ml) were mixed with 3 ml reaction mixture in test tubes,
which contained, 0.6 M sulfuric acid, 28 mM sodium phosphate and 1%
ammonium molybdate. The test tubes were incubated at 95°C for 10
min in order to complete the reaction. Following cooling at RT (30
min), the absorbance was determined at 695 nm using a
spectrophotometer (Shimadzu, Tokyo, Japan) against a blank.
Ascorbic acid was used as the standard. A typical blank solution
contained 3 ml reaction mixture and the appropriate volume of the
same solvent used for the samples/standard, and it was incubated at
95°C for 10 min prior to the absorbance being evaluated at 695 nm.
Increased absorbance of the reaction mixture indicated an increase
in the total antioxidant capacity of Aponogeton
undulatus.
DPPH free radical scavenging assay
A solution of 0.1 mM DPPH in methanol was prepared
and 2.4 ml of the solution was mixed with 1.6 ml of each
extract/fractions at various concentrations (10–160 µg/ml). The
reaction mixture was vortexed thoroughly (1,000 × g) and left in
the dark at RT for 30 min. The absorbance of the mixture was
evaluated spectrophotometrically at 517 nm. Ascorbic acid was used
as a reference standard. Percentage of DPPH radical scavenging
activity was calculated by the following equation:
%DPPHradicalscavengingactivity=[(A0–A1)/A0]x100
A0 is the absorbance of the control and
A1 is the absorbance of the extractives/standard.
Subsequently, the percentage of inhibition was plotted against
concentration, and from the graph the IC50 was
determined (16).
Lipid peroxidation inhibition assay
The lipid peroxidation inhibition assay was
determined according to the method previously described by Liu and
Ng (17). Briefly, excised rat liver
(surgically removed from healthy rats) was homogenized in PBS and
centrifuged at 20,000 × g for 15 min at 4°C to obtain liposomes. In
total, 0.5 ml supernatant, 100 µl 10 mM FeSO4, 100 µl
0.1 mM AA and 0.3 ml extractives/standard at various concentrations
(10, 20, 40, 80 and 160 µg/ml) were mixed together to make the
final volume of 1 ml. The reaction mixture was incubated at 37°C
for 20 min. A total of 1 ml (28%) TCA and 1.5 ml (1%) TBA were
added immediately subsequent to heating (100°C). Finally, the
reaction mixture was heated again at 100°C for 15 min and cooled at
RT for 30 min. Following cooling, the absorbance was determined at
532 nm. Percentage of inhibition of lipid peroxidation was
determined using the following equation:
%lipidperoxidationinhibition=[(A0–A1)/A0]x100
Subsequently, the percent of inhibition was plotted
against concentration, and using the graph, the IC50 was
evaluated.
Determination of ferrous reducing antioxidant
capacity
The reducing power of Aponogeton undulatus
leaf extract was determined according to the previously described
study by Oyaizu (18). Briefly, 0.25
ml samples/standard solution at different concentrations (10, 20,
40, 80 and 160 µg/ml) 0.625 ml potassium buffer (0.2 M) and 0.625
ml 1% potassium ferricyanide [K3Fe(CN)6]
solution was added to the test tubes. The reaction mixture was
incubated for 20 min at 50°C to complete the reaction.
Subsequently, 0.625 ml 10% TCA solution was added to the test
tubes. The total mixture was centrifuged at 3,000 × g for 10 min at
RT. Following this, 1.8 ml supernatant was withdrawn from the test
tubes and was mixed with 1.8 ml distilled water and 0.36 ml 0.1%
FeCl3 solution. The absorbance of the solution was
evaluated at 700 nm using a spectrophotometer (Shimadzu
Corporation) against a blank. A typical blank solution containing
the same solution mixture without plant extracts/standard was also
incubated under the same conditions, and the absorbance of the
blank solution was evaluated at 700 nm. Increased absorbance of the
reaction mixture indicates increased reducing capacity.
In vivo anticancer activity
Animals
A total of 60, 6–7-week-old, Swiss albino mice
(weight range, 25–30 g) of both genders (Samtako Bio Korea, Osan,
Korea), were divided into six groups, each consisting of 12
animals, and were used to assess biological activity. Animals were
maintained in an air-conditioned room at a temperature of 22±1°C
and a humidity of 55±1% with a 12 h light/dark cycle. They were fed
a standard commercial rodent pellet diet (Samtako Bio Korea), and
had ad libitum access to water. The animals were allowed to
acclimate to the environment for seven days prior to the
experimental session. All animal experiments were performed in
accordance with the guidelines of the Institutional Animal Ethics
Committee, Atish Dipankar University of Science & Technology
(Dhaka, Bangladesh). Animal treatment and maintenance for acute
toxicity and effects were strictly followed in accordance with the
Principle of Laboratory Animal Care (19) and the Animal Care and Use Guidelines
of Atish Dipankar University of Science & Technology.
Acute toxicity study
A total of 30, 6–7-week-old, Swiss albino mice
(weight range, 25–30 g) of both genders (Samtako Bio Korea), were
divided into five groups (n=6), and were used to assess acute
toxicity activity. The test was performed using increasing oral
doses of EAU in distilled water (50, 100, 200, 500 and 1,000 mg/kg
body weight) that were administered orally at 20 ml/kg to each test
group. The normal control group received distilled water (1 ml).
Following treatment, mice were allowed to feed ad libitum
and observed for 48 h for any mortality or behavioral changes
(20).
Transplantation of cancer cells
EAC cells were acquired from Professor M. Ekramul
Haque (Department of Pharmacy, Rajshahi University, Rajshahi,
Bangladesh). The EAC cells were maintained in vivo in Swiss
albino mice by intraperitoneal transplantation of 2×106
cells suspended in PBS per mouse every 10 days. Ascitic fluid was
drawn from EAC cell-bearing mice at the log phase (days 7–8 of
tumor bearing) of the cancer cell growth, and each test animal
received 0.1 ml cancer cell suspension containing 2×106
cells intraperitoneally (i.p.) (21).
Treatment schedule
Mice were divided into six groups (n=12) and
provided with food and water ad libitum. All animals in each
group received EAC cells (2×106 cells/mouse, i.p.)
except group I, which served as the normal saline control (5 ml/kg
body weight i.p.). Group II served as the EAC control. Following a
24-h period post-EAC transplantation, group VI received bleomycin
0.3 (i.p.) mg/kg body weight (positive control), and groups III, IV
and V were treated with 50, 100 and 200 mg/kg body weight (p.o.)
EAU, respectively, for nine consecutive days. Following 24 h from
the last dose, the animals were fasted for 18 h; six animals in
each group were subsequently sacrificed by cardiac puncture to
estimate hematological parameters, as well as to determine
anticancer activity. The remaining mice were provided with food and
water ad libitum and observed to determine if there were any
changes in lifespan. The anticancer activity of the extract was
evaluated in EAC animals.
Determination of tumor and packed cell volume
(PVC)
Mice were dissected and ascetic fluid was collected
from the peritoneal cavity. Volume was determined using a graduated
conical centrifuge tube, following which the PCV was determined by
centrifuging the fluid at 1,000 × g at 4°C for 5 min.
Viable and nonviable cancer cell count
Ascetic fluid was collected in a white blood cell
(WBC) pipette and diluted 100 times using PBS. A drop of the
diluted suspension was subsequently placed on a Neubauer counting
chamber, and the cells were stained with trypan blue (0.4% in
normal saline). Cells that did not take up the dye were considered
viable, whereas those that did were considered non-viable. These
viable and non-viable cells were counted using the following
equation:
Cell count = (number of cells × dilution
factor)/(area × thickness of liquid film)
Determination of median survival time and
percentage increase in lifespan
Mortality was monitored by recording the percentage
increase in lifespan (% ILS) and the median survival time (MST),
according to the following formula (22):
%ILS=(MeansurvivaltimeofthetreatedgroupMeansurvivaltimeofthecontrolgroup–1)x100
Where mean survival time* = (day of first mortality
+ day of last mortality)/2; (*time is denoted by the number of
days).
Estimation of hematological parameters
Collected blood was used to estimate the hemoglobin
(Hb) content, red blood cell (RBC) and WBC count. Differential
counts of WBC were performed using Leishman stained blood smears
(23).
Statistical analysis
All values are expressed as the mean ± standard
error of the mean of three replicated experiments. The analysis was
performed using SPSS statistical package for WINDOWS (version 16.0;
SPSS, Inc., Chicago, IL, USA). Results associated with the reducing
power activities were statistically analyzed by applying the
Student's t-test, and P<0.001 was considered to indicate a
statistically significant difference. All in vivo data were
subjected to analysis of variance followed by Dunnett's test, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
In vitro antioxidant activity
Total phenolic, flavonoid and flavonol
contents
As presented in Table
I, the total extractable phenolic content of the Aponogeton
undulatus fractions was calculated to be 95.24±0.42 in the MAU,
24.57±1.02 in the CAU, 134.29±1.12 in the EAU and 41.29±0.22 in the
WAU, all of which are expressed as GAE (mg/g of each extract). The
flavonoid content in the fractions was determined to be 129.40±0.21
in the MAU, 28.40±0.61 in the CAU, 205.40±0.31 in the EAU and
70.20±0.41 in the WAU, all of which are expressed as QAE (mg/g of
each extract). Total flavonol contents were 46.40±0.11 in the MAU,
71.47±0.31 in the CAU, 57.80±0.51 in the EAU and 16.00±0.21 in the
WAU, all of which are expressed in terms of QAE. There was an
association between the extract phenolic content, the DPPH radical
scavenging activity and lipid peroxidation inhibition activity with
linear correlation coefficients. R2=0.9559 in MAU,
R2=0.9633 in CAU, R2=0.8474 in EAU and
R2=0.8991 in WAU for phenolic content and DPPH-radical
scavenging activity (Fig. 1A) and
R2=0.8419 in MAU, R2=0.9455 in CAU,
R2=0.8467 in EAU and R2=0.9108 in WAU for
phenolic content and lipid peroxidation inhibition activity
(Fig. 1B).
 | Table I.Total quantity of phenols, flavonoids
and flavonols and the total antioxidant capacity of various organic
soluble fractions of Aponogeton undulatus leaves. |
Table I.
Total quantity of phenols, flavonoids
and flavonols and the total antioxidant capacity of various organic
soluble fractions of Aponogeton undulatus leaves.
| Sample | aTotal phenols mg/g plant extract (in
GAE) | bTotal flavonoids mg/g plant extract
(in QAE) | bTotal flavonols mg/g plant extract
(in QAE) | cTotal antioxidant capacity mg/g
plant extract (in ASC) |
|---|
| MAU | 95.24±0.42 | 129.40±0.21 | 46.40±0.11 | 109.20±0.41 |
| CAU | 24.57±1.02 | 28.40±0.61 | 71.47±0.31 | 32.80±0.11 |
| EAU | 134.29±1.12 | 205.40±0.31 | 57.80±0.51 | 175.80±0.41 |
| WAU | 41.29±0.22 | 70.20±0.41 | 16.00±0.21 | 54.20±0.81 |
Total antioxidant capacity
The total antioxidant capacity of various organic
soluble fractions of Aponogeton undulatus leaf extract were
expressed as the number of equivalents of ascorbic acid (Table I). The total antioxidant capacity of
EAU was the highest of all the fractions and was revealed to be
175.80±0.41 mg/g, followed by MAU, WAU and CAU, which displayed
antioxidant capacities of 109.20±0.41, 54.20±0.81 and 32.80±0.11
mg/g equivalent of ascorbic acid, respectively.
DPPH free radical scavenging assay
All Aponogeton undulatus fractions exhibited
H-donor activity. EAU demonstrated the highest DPPH scavenging
activity with an IC50 value of 38.84±0.02 µg/ml,
followed by MAU and WAU, which had IC50 values of
75.24±0.56 and 137.63±0.12 µg/ml, respectively (Table II). CAU exhibited no activity within
the experimental concentration range.
 | Table II.Scavenging/inhibitory effects of
various organic soluble fractions of Aponogeton undulatus
leaves against DPPH scavenging and lipid peroxide inhibition. |
Table II.
Scavenging/inhibitory effects of
various organic soluble fractions of Aponogeton undulatus
leaves against DPPH scavenging and lipid peroxide inhibition.
| Sample | DPPH
IC50 (µg/ml) | Lipid peroxide
IC50 (µg/ml) |
|---|
| MAU | 75.24±0.56 | 49.11±0.41 |
| CAU | >160 | 138.38±0.61 |
| EAU | 38.84±0.02 | 42.52±0.32 |
| WAU | 137.63±0.12 | 113.98±0.33 |
| Ascorbic acid | 18.84±0.02 | 38.52±0.12 |
Lipid peroxidation inhibition assay
The lipid peroxide scavenging activity of the
Aponogeton undulatus fractions was investigated and compared
to the standard ascorbic acid. The IC50 values of MAU,
CAU, EAU and WAU were 49.11±0.41, 138.38±0.61, 42.52±0.32 and
113.98±0.33 µg/ml, respectively (Table
II). By contrast, the IC50 value of standard
ascorbic acid was 38.52±0.12 µg/ml.
Ferrous reducing antioxidant capacity
Reductive ability was determined by evaluating the
transformation of Fe3+ to Fe2+ in the
presence of Aponogeton undulatus leaf extract and its
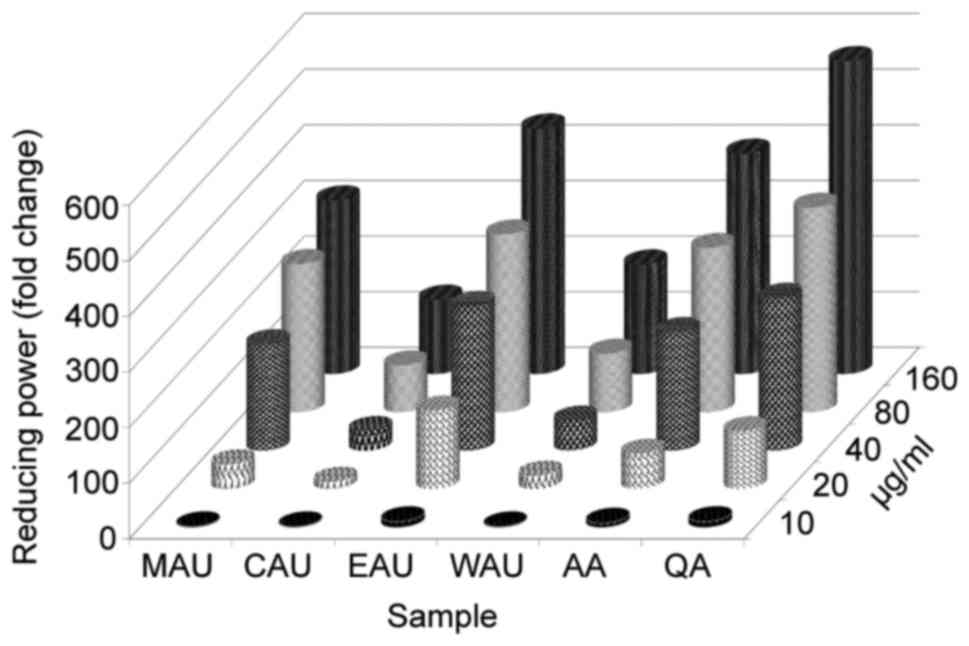
various organic fractions. Fig. 2
depicts the reductive capabilities of the Aponogeton
undulatus leaf extract fractions, which exhibited
dose-dependent activity compared with the commercial ascorbic acid
and quercetin.
In vivo anticancer activity
Acute toxicity studies
Acute toxicity studies primarily aim to establish
the therapeutic index (the ratio between the pharmacologically
effective dose and the lethal dose) within the same strain and
species (20). EAU was safe at doses
as high as 1,000 mg/kg (p.o.) body weight. The behavior of the
animals was closely observed for the first 3 h, then every 4 h
during the next 48-h period. The extract did not induce mortality,
behavioral changes, locomotor ataxia, diarrhea or weight loss in
mice during the 48-h observation period. Furthermore, food and
water intake did not differ among the groups studied.
Cell growth and survival parameters
EAU (200 mg/kg body weight) administration
significantly reduced tumor volume, PCV (Fig. 3) and the viable cancer cell count;
however, it increased the non-viable cancer cell count relative to
the EAC control group (data not presented). MST significantly
(P<0.05) increased to 22.61±0.21 (% ILS=18.22), 32.58±0.31 (%
ILS=45.32) and 36.51±0.19 (% ILS=65.59) upon administration of EAU
at doses of 50, 100 and 200 mg/kg body weight, respectively,
whereas the EAC control (2×106 cells/mouse) and the
reference drug bleomycin demonstrated survival times of 21.11±0.12
and 40.11±0.11 (% ILS=90.00), respectively (Fig. 3). Finally, the animals' changes in
body weight (data not presented) suggest that EAU may have the
potential to inhibit tumor growth.
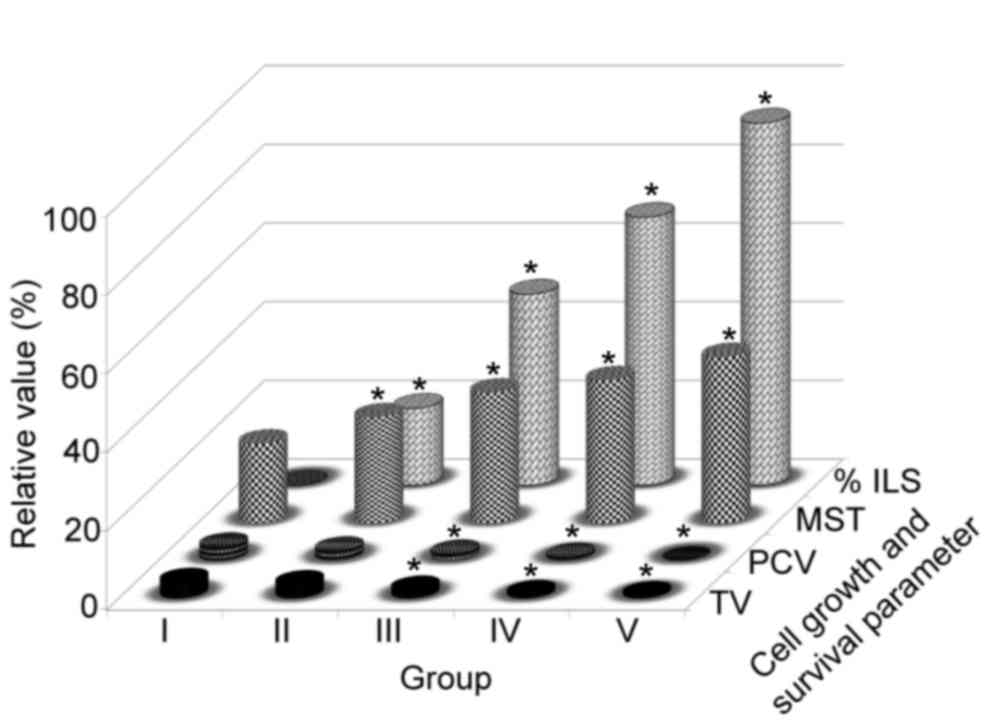 | Figure 3.Effects of Aponogeton
undulatus leaf extracts on TV, PCV, MST and % ILS in
EAC-bearing mice. Each point represents the mean ± standard error
of the mean from triplicate determinations. (n=6 mice per group),
*P<0.05 was considered statistically significant compared to the
EAC control group, Group I animals received EAC control
(2×106 cell/mouse), Group V received bleomycin (0.3
mg/kg body weight), and groups II, III and IV, were treated with
50, 100 and 200 mg/kg body weight (p.o.) of the EAU, respectively.
TV, tumor volume; PCV, packed cell volume; MST, mean survival time;
% ILS, percentage increase in lifespan; EAC, Ehrlich ascites
carcinoma; EAU, ethyl acetate fraction of Aponogeton
undulatus. |
Hematological parameters
The hematological parameters of tumor-bearing mice
were observed to be significantly (P<0.05) altered, relative to
the normal group (Table III). The
total WBC count was revealed to be increased (P<0.05), whereas a
reduction in hemoglobin (Hb) content and RBC count in the EAC
control mice compared with the normal saline group, was observed.
Additionally, neutrophils (Fig. 4A)
and lymphocytes (Fig. 4B) were
observed to decrease, while monocytes (Fig. 4C) increased in the EAC control group,
compared with in the normal saline group. Treatment with EAU (200
mg/kg body weight, p.o.) significantly (P<0.05) changed these
parameters to near normal values (Fig.
4).
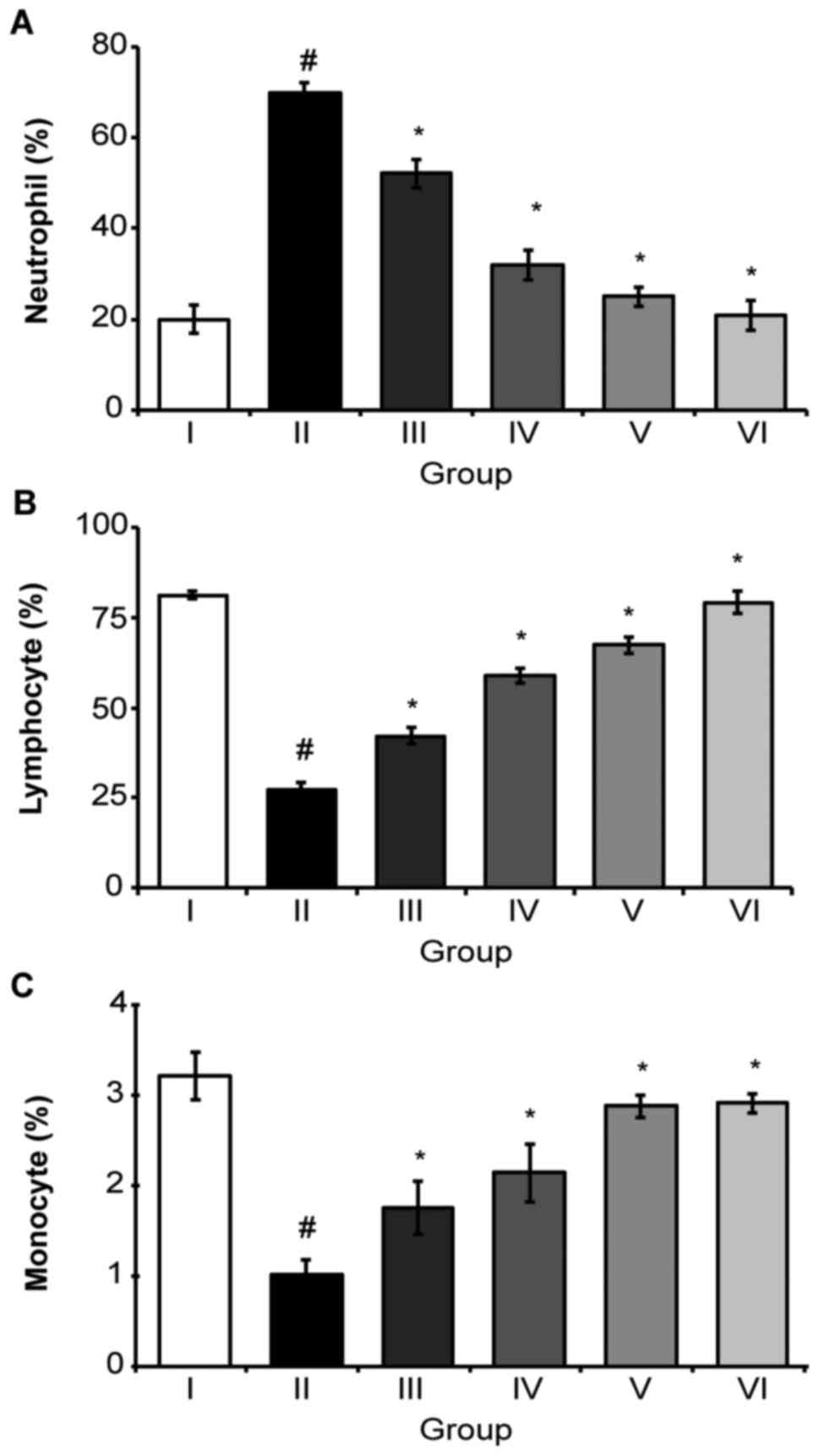 | Figure 4.A classical feature of Aponogeton
undulatus leaf extracts on differential counts of WBC, (A)
neutrophils, (B) lymphocytes and (C) monocytes. Each point
represents the mean ± standard error of the mean from triplicate
determinations. (n=6 mice per group), #P<0.05 was
considered to indicate a statistically significant difference
compared to the normal saline group. *P<0.05 was considered to
indicate a statistically significant difference compared to the EAC
control group. Group I animals received normal saline (5 ml/kg)
whereas group II received EAC control (2×106
cell/mouse), Group VI received bleomycin (0.3 mg/kg body weight),
and groups III, IV and V were treated with 25, 50 and 100 mg/kg
body weight (p.o.) of the EAU, respectively. WBC, white blood cell;
EAC, Ehrlich ascites carcinoma; EAU, ethyl acetate fraction of
Aponogeton undulatus leaves. |
 | Table III.Effect of the EAU on hematological
parameters in EAC-bearing mice. |
Table III.
Effect of the EAU on hematological
parameters in EAC-bearing mice.
| Group | Hb content
(g%) | RBC (cells
×106/mm3) | WBC (cells
×106/mm3) |
|---|
| Normal saline (5
ml/kg) | 11.52±0.76 | 5.22±0.19 | 4.23±0.21 |
| EAC control
(2×106 cell/mouse) |
4.31±0.56a |
2.11±0.26a |
7.02±0.23a |
| EAC + EAU (25
mg/kg) |
5.21±0.13b | 2.32±0.15 |
6.03±0.45b |
| EAC + EAU (50
mg/kg) |
7.12±0.21b |
3.32±0.21b |
5.35±0.52b |
| EAC + EAU (100
mg/kg) |
9.10±0.23b |
4.85±0.22b | 4.62±0.15 |
| Bleomycin (0.3
mg/kg) |
10.82±0.61b |
4.92±0.17b |
4.51±0.24b |
Discussion
Plants contain free radical scavengers, including
phenolic compounds, flavonoids and flavonols, which possess
antioxidant and anticancer activities (5). The active constituents of these
compounds may be isolated and then used as antioxidants for the
prevention and treatment of free radical-associated disorders
(24). In the present study, MAU and
its various organic fractions were investigated for their in
vitro antioxidant activity, and only EAU was evaluated for its
in vivo anticancer activity against EAC cells in Swiss
albino mice.
Phenolic compounds are considered to be secondary
metabolites, which are derived from phenylalanine and tyrosine;
they occur ubiquitously in plants and are widely distributed
(25). However, flavonoids are often
identified as glycosylated forms of polyphenolic compounds,
exhibiting a distinct molecular structure in plants (24). Differentiating the position of the
hydroxyl groups within the phenyl ring significantly alters the
characteristics of solubility of the compounds (14). The present study identified that EAU
demonstrated the highest antioxidant activity, with 175.80±0.41
mg/g plant extract (in ASC) of Aponogeton undulatus. The
total content of phenols, flavonoids and flavonols in the
Aponogeton undulatus fraction isolates were ordered as
follows: EAU> MAU> WAU> CAU (Table I).
EAU demonstrated the highest DPPH scavenging effects
and lipid peroxidation inhibition, and these effects were
dose-dependent (data not presented). The IC50 values of
these effects were 38.84±0.02 and 42.52±0.32 µg/ml, respectively,
while the standard ascorbic acid activity was identified to be
IC50=18.84±0.02 and IC50=38.52±0.12 µg/ml,
respectively (Table II). There was
an association between the extract phenolic content and the DPPH
radical scavenging activity linear correlation coefficient:
R2=0.9559 in MAU, R2=0.9633 in CAU,
R2=0.8474 in EAU and R2=0.8991 in WAU.
Peroxidation of the lipid is a natural phenomenon that occurs on
its exposure to oxygen (14).
Recently, free radical-induced lipid peroxidation has gained
attention due to its involvement in numerous pathological
conditions, including aging, wound healing, oxygen toxicity, liver
disorders and inflammation (1). The
results of the present study revealed that Aponogeton
undulatus extracts inhibit lipid peroxidation in a
dose-dependent manner (data not presented), and produce a strong
and significant correlation between their total phenol content and
lipid peroxidation: R2=0.8419 in MAU,
R2=0.9455 in CAU, R2=0.8467 in EAU and
R2=0.9108 in WAU. Phenolic compounds are commonly
identified in fruits, and have been revealed to scavenge free
radicals through hydrogen or electron donation (26). Previous studies have demonstrated a
positive association between phenolic functional groups and DPPH
free radical scavenging activity (27).
Initially reported as a spontaneous murine mammary
adenocarcinoma, the Ehrlich tumor may be grown in the majority of
mouse strains and is accepted as a transplantable tumor model used
to evaluate the anticancer effects of numerous substances (28). EAC, B-cell lymphoma proliferative
diseases and chronic lymphocytic leukemia diseases are all
characterized by uncontrolled blood cell regulation; they require
treatment with kinase enzyme inhibitors (20,21).
Kinase inhibitors bind to kinase proteins, whereas flavonoids bind
to basic nucleosides in the presence of a nitrogenous base, to form
an intermolecular bond with target protein kinases and inhibit
cells from further growth (23). A
rapid increase in ascetic tumor volume was revealed in EAC
tumor-bearing mice, and treatment with Aponogeton undulatus
extracts reduced the intraperitoneal tumor burden, thereby reducing
tumor volume, tumor weight and viable cell count, in addition to
increasing the lifespan of the tumor-bearing mice. Therefore, the
increased lifespan of EAC-bearing mice in response to EAU treatment
at 200 mg/kg may be due to a decrease in nutritional fluid volume
and a delay in cell division, similar to the results of a previous
study (22). Reductions in viable
cell count and increases in non-viable cell counts towards normal
levels in tumor hosts indicate anticancer effects against EAC cells
in mice. These results suggest that EAU directly associates with
cancer cells at high doses; they directly absorb the drug in the
peritoneal cavity, resulting in cell lysis via a direct and
cytotoxic mechanism (22). Anemia and
myelo-suppression have frequently been observed in ascites
carcinoma due to iron deficiency, either by hemolytic or
myelopathic conditions, which eventually lead to reduced numbers of
RBC (29). Treatment with EAU (100
mg/kg body weight, p.o.) returned the Hb content, RBC and WBC count
to normal levels (Table III). These
results provide support for EAU's hematopoietic protective effect,
which occurs without inducing myelotoxicity, the most common side
effect of cancer chemotherapy (23).
Plant-derived extracts with antioxidant potential
have demonstrated cytotoxicity against cancer cells and anticancer
activity in experimental animals (30). The cytotoxic and anticancer activity
of plant-derived products occurs through the induction of apoptosis
or the inhibition of neovascularization (31). In the present study, higher doses of
EAU reduced cell growth and cancer cell viability, normalized
hematological profiles and increased lifespan, compared with EAC
control mice. EAU's ability to reduce lipid peroxidation and its
free radical scavenging effects and reducing power indicate the
potential use of Aponogeton undulatus extract as an
inhibitor of intracellular free radicals induced by oxidative
stress.
In conclusion, these novel results indicate that the
Aponogeton undulatus leaf extract, in addition to its
various organic fractions, has promising antioxidant properties and
anticancer activity, as revealed by the in vivo studies. The
next aim is to isolate and characterize the lead compounds that may
be responsible for the aforementioned activity of Aponogeton
undulatus. Furthermore, the identification of the ideal
conditions for preserving Aponogeton undulatus samples, to
minimize the damage to its antioxidant and biological properties
following long-term storage, requires further study.
Acknowledgements
The present study was supported by the research fund
from Chosun University, 2016.
References
|
1
|
Ďuračková Z: Some current insights into
oxidative stress. Physiol Res. 59:459–469. 2010.PubMed/NCBI
|
|
2
|
Halliwell B: Free radicals, antioxidants,
and human disease: Curiosity, cause, or consequence? Lancet.
344:721–724. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poli G, Leonarduzzi G, Biasi F and
Chiarpotto E: Oxidative stress and cell signalling. Curr Med Chem.
11:1163–1182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ames BN and Shigenaga MK: Oxidants are a
major contributor to aginga. Ann N Y Acad Sci. 663:85–96. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yeh CC, Hou MF, Tsai SM, Lin SK, Hsiao JK,
Huang JC, Wang LH, Wu SH, Hou LA, Ma H and Tsai LY: Superoxide
anion radical, lipid peroxides and antioxidant status in the blood
of patients with breast cancer. Clin Chim Acta. 361:104–111. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raskin I, Ribnicky DM, Komarnytsky S, Ilic
N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N,
et al: Plants and human health in the twenty-first century. Trends
Biotechnol. 20:522–531. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Branen AL: Toxicology and biochemistry of
butylated hydroxyanisole and butylated hydroxytoluene. J Am Oil
Chem Soc. 52:59–63. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watve A: Aponogeton undulatus. The IUCN
Red List of Threatened Species. 2011.
|
|
9
|
Islam QR: Morphology and nutritional value
of Aponogeton undulatus Roxb. growing in deeply flooded areas in
Bangladesh. Hydrobiologia. 340:3171996. View Article : Google Scholar
|
|
10
|
Biswas SK and Ghosh SE: Bharotio
Bonoushadhi. 6. Calcutta University Press; India: 1977
|
|
11
|
Chowdhury NS, Alam B, Haque ASM Tanbirul,
Zahan R, Mazumder EH and Haque E: In vitro free radical scavenging
and thrombolytic activities of Bangladeshi aquatic plant Aponogeton
undulatus Roxb. Glob J Pharmacol. 5:27–32. 2011.
|
|
12
|
Yu L, Haley S, Perret J, Harris M, Wilson
J and Qian M: Free radical scavenging properties of wheat extracts.
J Agric Food Chem. 50:1619–1624. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang CC, Yang MH, Wen HM and Chern JC:
Estimation of total flavonoid content in propolis by two
complementary colorimetric methods. J food Drug Anal. 10:178–182.
2002.
|
|
14
|
Kumaran A and Karunakaran RJ: In vitro
anti-oxidant activities of methanol extracts of Phyllanthus species
from India. LWT- Food Sci Technol. 40:344–352. 2007. View Article : Google Scholar
|
|
15
|
Prieto P, Pineda M and Aguilar M:
Spectrophotometric quantitation of anti-oxidant capacity through
the formation of a phosphomolybdenum complex: Specific application
to the determination of vitamin E. Anal Biochem. 269:337–341. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi, Hong Ja Yeob, Eun Jhun, Ou Lim
Beong, Ill Min Chung, Suk Hun Kyung and Ki Park Dong: Application
of flow injection-chemiluminescence to the study of radical
scavenging activity in plants. Phytotherapy Research. 14:250–253.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu F and Ng TB: Antioxidative and free
radical scavenging activities of selected medicinal herbs. Life
Sci. 66:725–735. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oyaizu M: Studies on products of browning
reactions. Antioxidative activities of products of browning
reaction prepared from glucoseamine. Jpn J Nutr Diet. 44:3071986.
View Article : Google Scholar
|
|
19
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. National Academies Press;
Washington, DC: 1985
|
|
20
|
Zahan R, Alam B, Islam S M, Sarker GC,
Chowdhury NC, Hosain SB, Mosaddik MA, Jesmin M and Haque E:
Activity of Alangium salvifolium flower in ehrlich ascites
carcinoma bearing mice. Int J Cancer Res. 7:254–262. 2011.
View Article : Google Scholar
|
|
21
|
Alam B, Sajid I, Rashid J, Islam M and
Karmaker BK: Evaluation of antitumor effects of the aerial parts of
polygonum viscosum linn. Glob J Pharmacol. 8:47–52. 2014.
|
|
22
|
Gupta M, Mazumder UK, Kumar RS, Sivakumar
T and Vamsi ML: Antitumor activity and anti-oxidant status of
Caesalpinia bonducella against Ehrlich ascites carcinoma in Swiss
albino mice. J Pharmacol Sci. 94:177–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sur P, Bag SP, Sur B and Khanam JA:
Choroaceto hydroxamic acid as antitumor agent against Ehrlich
ascites carcinoma in mice. Neoplasma. 44:197–201. 1997.PubMed/NCBI
|
|
24
|
Packer L, Rimbach G and Virgili F:
Antioxidant activity and biologic properties of a procyanidin-rich
extract from pine (Pinus maritima) bark, pycnogenol. Free Radic
Biol Med. 27:704–724. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
Implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
26
|
Shahidi F and Wanasundara PK: Phenolic
anti-oxidants. Crit Rev Food Sci Nutr. 32:67–103. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang X, Dai J, Fournier J, Ali AM, Zhang
Q and Frenkel K: Ferrous ion autoxidation and its chelation in
iron-loaded human liver HepG2 cells. Free Radic Biol Med. 32:64–72.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Segura JA, Barbero LG and Márquez J:
Ehrlich ascites tumor unbalances splenic cell populations and
reduced responsiveness of T cells to Stapylococcus aureus
enterotoxin B stimulation. Immunol Lett. 74:111–115. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kennedy D Opare, Kojima A, Hasuma T, Yano
Y, Otani S and Matsui-Yuasa I: Growth inhibitory effect of green
tea extract and (−)-epigallocatechin in Ehrlich ascites tumor cells
involves a cellular thiol-dependent activation of
mitogenic-activated protein kinases. Chem Biol Interact.
134:113–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruby AJ, Kuttan G, Babu KD, Rajasekharan
KN and Kuttan R: Anti-tumor and antioxidant activity of natural
curcuminoids. Cancer Lett. 94:79–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rakshit S, Mandal L, Pal BC, Bagchi J,
Biswas N, Chaudhuri J, Chowdhury AA, Manna A, Chaudhuri U, Konar A,
et al: Involvement of ROS in chlorogenic acid-induced apoptosis of
Ber-Abl+ CML cells. Biochem Pharmacol. 80:1662–1675. 2010.
View Article : Google Scholar : PubMed/NCBI
|