Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of cancer worldwide (1),
and develops in patients with chronic liver disease and cirrhosis
(2). The treatment of HCC depends on
its stage of progression (3).
Surgical resection remains the mainstay of potentially curative
therapy, but the majority of patients with HCC have passed the
opportunity for treatment with surgery at the time of diagnosis
(4,5).
Transcatheter arterial chemoembolization (TACE) is
an essential treatment option for patients with HCC who cannot be
treated with other potentially more effective therapies, including
surgical resection or local ablative therapies, and is the standard
of care for patients with noninvasive multinodular tumors at an
intermediate stage (6,7). However, the long-term outcomes of
patients treated with TACE are not entirely satisfactory, mainly
due to the low tumor necrosis rates (8) and high recurrence rates (6).
Radiofrequency (RF) ablation (RFA) represents a safe
and effective first-line locoregional treatment for HCCs with three
tumors or fewer, of ≤3 cm in size (3,9). Despite
the high complete necrosis rate of RFA, early local or distant
tumor recurrence within 1 year may still occur (10). TACE combined with RFA can enhance the
advantages of each individual treatment (8) and increase their cooperative effect,
demonstrating the potential benefits of a multidisciplinary
approach for advanced HCC (11,12).
Furthermore, the combination therapy of RFA and TACE has been shown
to be superior to TACE or RFA alone in increasing locoregional
control and improving the curative effect and survival time in
patients with advanced HCC (13,14).
However, the risk factors for tumor recurrence following treatment
of HCC have not yet been clarified in detail.
The aims of the present study were to identify the
characteristics of HCC associated with recurrence following
successful initial treatment with TACE, and to compare the
recurrence patterns between patients with HCC who received TACE
alone and those treated with TACE combined with RFA treatment.
Methods and materials
Study design
The present study was a retrospective cohort study
performed at a single center. The medical records of 357 patients
treated with TACE between June 2009 and June 2013 at Nara Medical
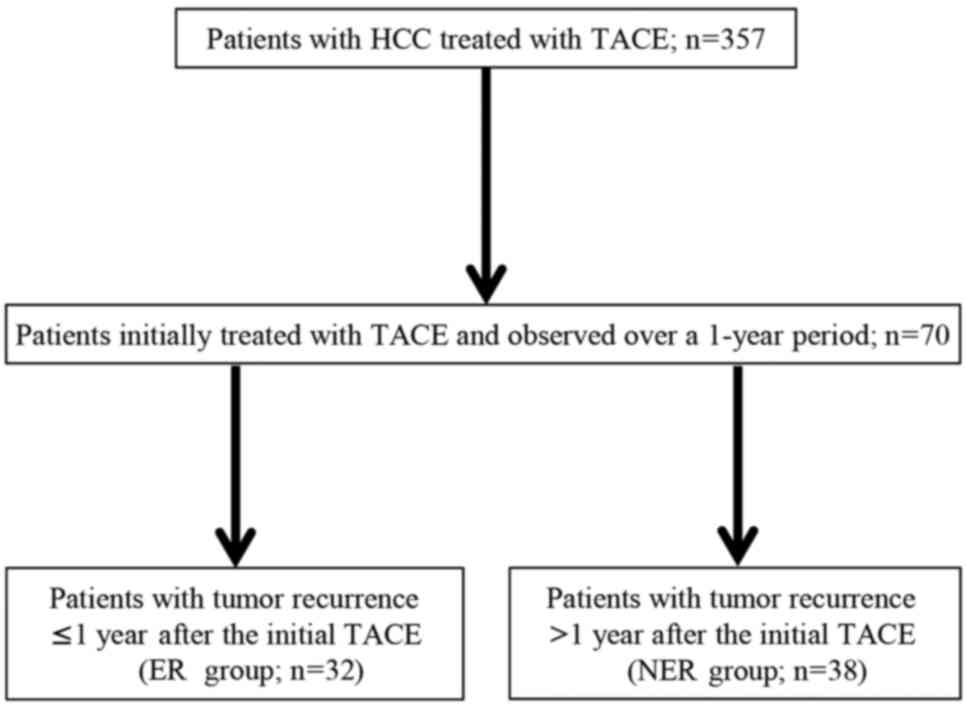
University (Kashihara, Japan) were reviewed (Fig. 1). Of these, 70 patients were initially
treated with TACE and subsequently observed over a 1-year period.
These patients were divided into two groups according to DFS status
at 1 year: The early recurrence (ER) group (recurrence within 1
year after initial TACE; n=32) and the non-early recurrence (NER)
group (no recurrence within 1 year after initial TACE; n=38). Of
the 32 patients in the ER group, 5 did not achieve a complete
remission (CR), with 2 succumbing to HCC. Of the 38 patients in the
NER group, 1 succumbed to HCC, while 15 did not experience a
recurrence of HCC for >1 year after the initial TACE. The degree
of lipiodol retention in the tumor within 1 week of TACE was
routinely evaluated by multi-detector row computed tomography
(MDCT). RFA was administered if MDCT and contract-enhanced
ultrasonography (CE-US) detected a residual viable tumor. The study
was approved by the local ethics committee of Nara Medical
University (Nara, Japan) and written informed consent was obtained
from all patients prior to treatment.
Diagnosis of HCC
The diagnosis of HCC was confirmed without biopsy in
patients with chronic liver disease and cirrhosis who had a tumor
that exhibited a typical vascular pattern on dynamic imaging
modalities [such as contrast-enhanced MDCT (CE-MDCT) and gadolinium
ethoxybenzyl diethylenetriamine pentaacetic acid
(Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI)], in
accordance with the practice guidelines of the Japan Society of
Hepatology (15). However, if the
vascular profile on imaging was not characteristic, or if a nodule
was detected in a healthy normal liver, supplementary tests,
including Gd-EOB-DTPA-enhanced MRI, CE-US, computed tomography (CT)
angiography and liver tumor biopsy, were considered. All patients
were examined by CE-MDCT or Gd-EOB-DTPA-enhanced MRI every 2 or 3
months after initial TACE.
TACE
The procedure for TACE has previously been described
in detail (16). Briefly, a single
femoral approach was used, following Seldinger's technique
(17), by inserting a 4-Fr catheter
(RH-6SP0061i; Terumo Medical Corporation, Tokyo, Japan) over a 5-Fr
introducer sheath into the celiac artery, using superior mesenteric
artery angiography as well as selective hepatic arteriography to
identify tumor feeders. The artery was selectively catheterized
with a microcatheter/microguidewire system and embolized with a
mixture of epirubicin with iodized oil (lipiodol; Laboratoire Andre
Guerbet, Aulnay-sous-Bois, France). The feeders were then embolized
with gelatin sponge pledgets (Cutanplast; Mascia Brunelli S.p.A,
Milan, Italy) until complete stasis of the blood flow was detected
by angiography. Collateral artery embolization was performed if
branches such as the phrenic artery and internal thoracic artery
were engaged in the tumor blood supply. Post-TACE cone-beam CT was
performed to assess the extent of lipiodol uptake in the tumor at
the end of TACE.
Percutaneous RFA
RFA of HCC was performed using the Cool-tip™ RF
system (Integra Burlington MA, Inc., MA, USA). RFA with ultrasound
guidance was conducted under general and local anesthesia, using a
3.5-MHz probe with an incorporated guide and a 17-gauge cooled-tip
electrode (Cool-tip; Valleylab, Burlington, MA, USA) with a 2- or
3-cm exposed portion. This system consisted of an RF generator to
produce a current of 480 kHz at a maximal power of 200 W, a
single-electrode RFA, a water-pumping machine and return grounding
pads. The RFA started at a low power (40 or 60 W) and increased by
10 W/min, and the delivery of RF energy was automatically modulated
according to the tissue impedance around the electrode. Tumor
ablation continued at maximum power until the tissue impedance
increased to the point at which the power output fell rapidly (the
‘break’). The treatment response was assessed based on CE-MDCT or
CE-US performed within 1 week of RFA treatment.
Assessment and follow-up
The Response Evaluation Criteria in Solid Tumors
(RECIST) criteria (18) are
recommended for evaluating treatment efficacy in clinical trials
and practice. The response to treatment was assessed by CE-MDCT
according to the RECIST criteria at 4 weeks post-TACE or within 1
week of RFA, and additional RFA was performed until no residual
viable tumor was detectable. Following the final treatment session,
patients were evaluated every 3 months for 2 years and followed up
every 6 months thereafter by CE-MDCT or CE-MRI. The primary
end-point was the HCC DFS period following initial TACE.
Statistical analysis
Categorical variables were analyzed using the
Mantel-Haenszel test. Bivariate analyses of nominal parameters were
performed using the χ2 test. Univariate and multivariate
logistic regression analyses were conducted to assess the effect of
different parameters on HCC recurrence following initial TACE. Data
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using IBM SPSS statistics
version 22 (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics of
patients
Baseline characteristics of the enrolled 70 patients
are summarized in Table I. The
patient group included 51 males (73%) and 19 females (27%), and the
mean age was 70.5±8.9 years. A total of 54 patients (77%) were
classified as having Child-Pugh class (19) A cirrhosis, and 16 (23%) patients were
classified as having class B cirrhosis. At the initial diagnosis,
the mean tumor number was 2.3±2.1, and 8 patients (11%) had
multiple lesions. The mean serum aspartate aminotransferase (AST)
and alanine aminotransferase (ALT) levels were 53.3±43.4 and
40.3±36.4 IU/l, respectively. The mean α-fetoprotein (AFP) level
was 1,450.0±7,430.0 ng/ml.
 | Table I.Baseline characteristics of the
patients (n=70). |
Table I.
Baseline characteristics of the
patients (n=70).
| Variable | Value |
|---|
| Age,
yearsa | 70.5±8.9
(49–83) |
| Sex, n |
|
|
Male | 51 |
|
Female | 19 |
| Etiology, n |
|
|
HBV | 12 |
|
HCV | 37 |
|
Others | 21 |
| Child-Pugh
classification, n |
|
| A | 54 |
| B | 16 |
| TNM stage, n |
|
| I | 20 |
| II | 29 |
|
III | 19 |
| IV | 2 |
| Tumor size,
cma | 3.4±3.1
(0.7–20.0) |
| Tumor
numbera | 2.3±2.1 (1–9) |
| Aspartate
transaminase, IU/la | 53.3±43.4
(12–285) |
| Alanine
aminotransferase, IU/la | 40.3±36.4
(11–236) |
| α-fetoprotein,
ng/mla |
1,450.0±7,430.0 |
|
| (1.7–56,831.6) |
| Protein induced by
vitamin K |
3,342.0±10,537.0 |
|
absence/antagonist-II, mAU/mla | (7.0–66,753.0) |
Risk factors associated with HCC
recurrence
Univariate and multivariate analyses were performed
to identify predictive risk factors for HCC recurrence through
comparisons between the ER and NER groups. Univariate analysis
revealed that the levels of AST, ALT and AFP, as well as tumor
number, were associated with early HCC recurrence following TACE
(Table II). The ER and NER groups
did not differ significantly in terms of mean age, sex ratio, tumor
etiology, Child-Pugh classification, tumor stage, mean tumor size
or levels of protein induced by vitamin K absence/antagonist-II.
Multivariate logistic regression analysis indicated that AST levels
[odds ratio (OR), 1.069; P=0.009] and tumor number (OR, 1.661;
P=0.038) were independent risk factors associated with HCC
recurrence following initial TACE (Table III).
 | Table II.Baseline characteristics of the
patients. |
Table II.
Baseline characteristics of the
patients.
| Variable | Early recurrence
group (n=32) | Non-early
recurrence group (n=38) |
P-valuea |
|---|
| Age,
yearsb | 69.5±9.0
(50–83) | 71.3±8.8
(49–82) | 0.354 |
| Sex, n |
|
| 0.865 |
|
Male | 23 | 28 |
|
|
Female | 9 | 10 |
|
| Etiology, n |
|
| 0.871 |
|
HBV | 5 | 7 |
|
|
HCV | 18 | 19 |
|
|
Others | 9 | 12 |
|
| Child-Pugh
classification, n |
|
| 0.695 |
| A | 24 | 30 |
|
| B | 8 | 8 |
|
| TNM stage, n |
|
| 0.555 |
| I | 7 | 13 |
|
| II | 13 | 16 |
|
|
III | 11 | 8 |
|
| IV | 1 | 1 |
|
| Tumor size,
cmb | 4.4±4.2
(1.7–20.0) | 2.6±1.3
(0.7–6.6) | 0.153 |
| Tumor
numberb | 3.0±2.7 (1–9) | 1.6±0.9 (1–4) | 0.005 |
| Aspartate
transaminase, IU/lb | 69.8±57.4
(24–285) | 39.4±18.1
(12–96) | 0.003 |
| Alanine
aminotransferase, IU/lb | 50.2±46.2
(11–236) | 32.1±22.9
(13–126) | 0.027 |
| α-fetoprotein,
ng/mlb | 3144.6±10,836.0
(2.3–56,831.6) | 22.7±35.2
(1.7–144.2) | 0.002 |
| Protein induced by
vitamin K absence/antagonist-II, mAU/mlb | 6,396.5±14,871.0
(7.0–66,753.0) | 646.9±1,291.0
(8.0–5,845.0) | 0.053 |
 | Table III.Multiple logistic regression analysis
of risk factors for recurrence within 1 year of initial
transcatheter arterial chemoembolization for hepatocellular
carcinoma. |
Table III.
Multiple logistic regression analysis
of risk factors for recurrence within 1 year of initial
transcatheter arterial chemoembolization for hepatocellular
carcinoma.
| Variable | P-value | Odds ratio | 95% confidence
interval |
|---|
| Aspartate
transaminase | 0.009 | 1.069 | 1.017–1.125 |
| Alanine
aminotransferase | 0.220 | 0.916 | 0.270–1.001 |
| α-fetoprotein | 0.142 | 2.961 | 0.740–6.372 |
| Tumor number | 0.038 | 1.661 | 1.029–2.680 |
Association between tumor number and
recurrence patterns with DFS period following initial TACE
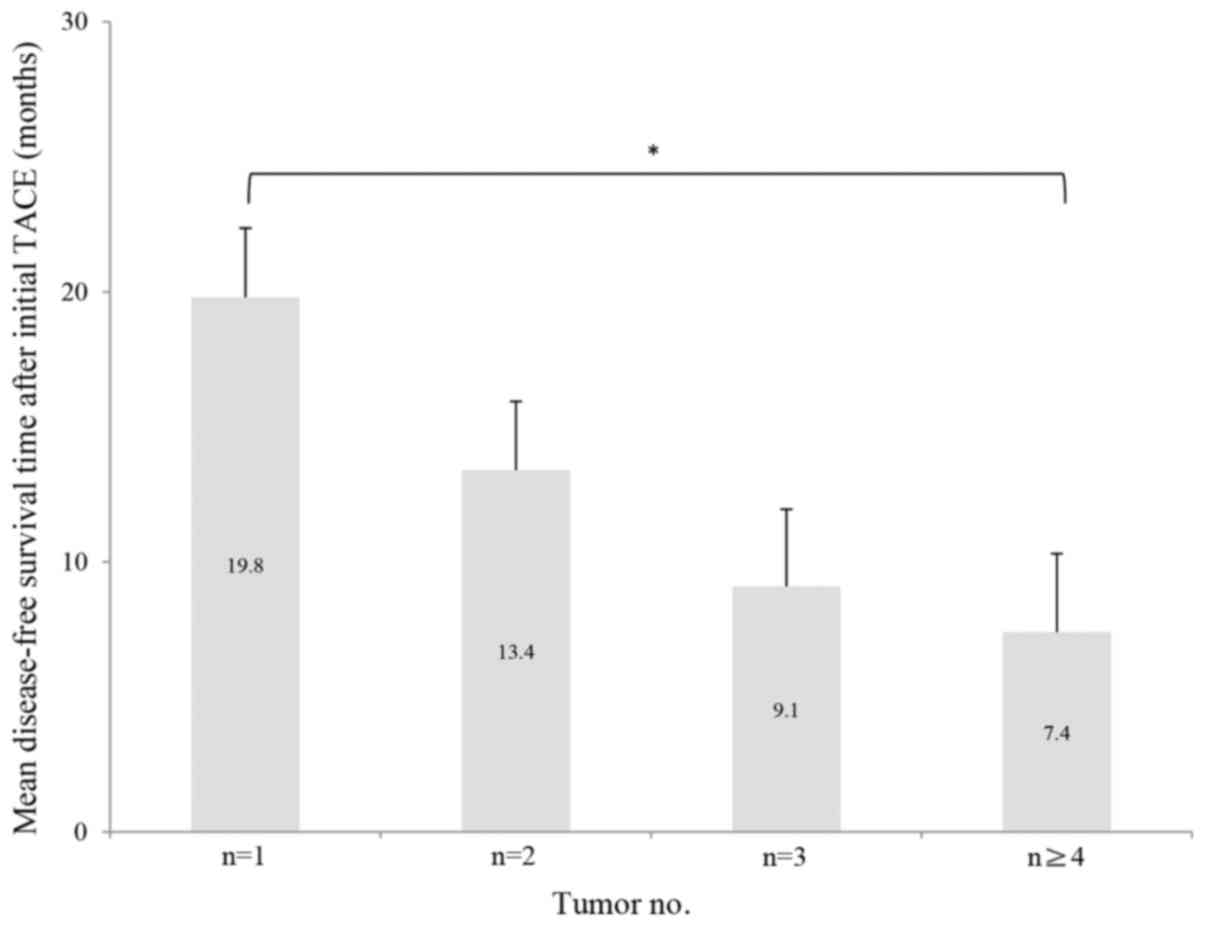
Of the 70 patients, 32 had a single nodule, 20 had
two nodules, 7 had three nodules and 11 had four or more nodules.
The mean HCC DFS periods for patients with one, two, three and four
or more nodules were 19.8, 13.4, 9.1 and 7.4 months, respectively.
The χ2 test for trend demonstrated an inverse
association between tumor number and DFS period in these patients
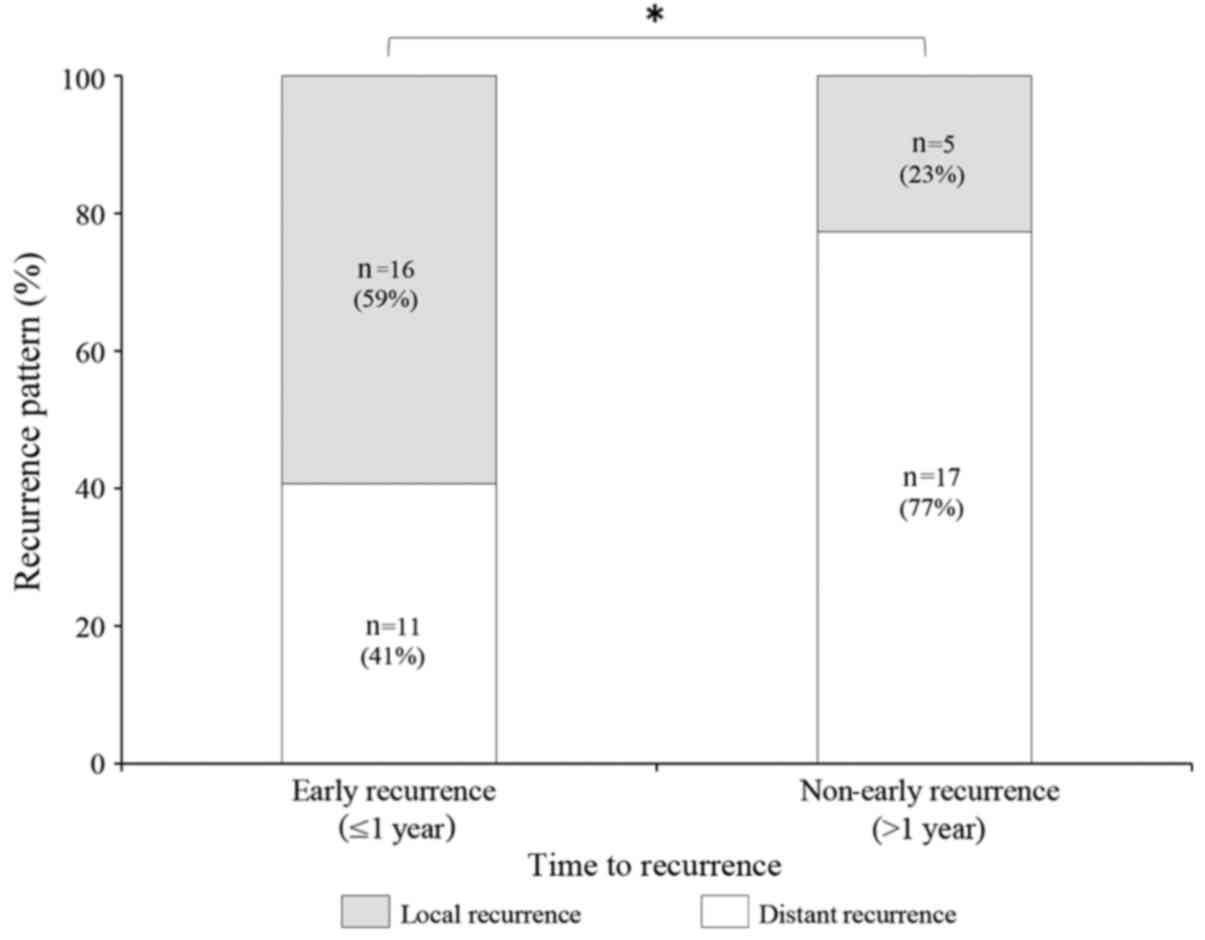
(Fig. 2). Recurrence within 1 year of
TACE was documented in 27 patients, including distant recurrence in
11 (41%) of these patients, and locoregional recurrence in 16 (59%)
patients. Recurrence after 1 year was documented in 22 patients,
including distant recurrence in 17 (77%) of these patients and
locoregional recurrence in 5 (23%) patients. The association
between recurrence patterns of HCC and DFS following initial TACE
was also analyzed. A χ2 test revealed that, among all
patients with recurrence, the proportions of locoregional and
distant recurrence differed significantly between the ER group and
the NER group (P<0.05), with a greater proportion of regional
recurrence observed in the ER group (Fig.
3).
Comparison of HCC recurrence patterns
between combined TACE and RFA treatment and TACE alone
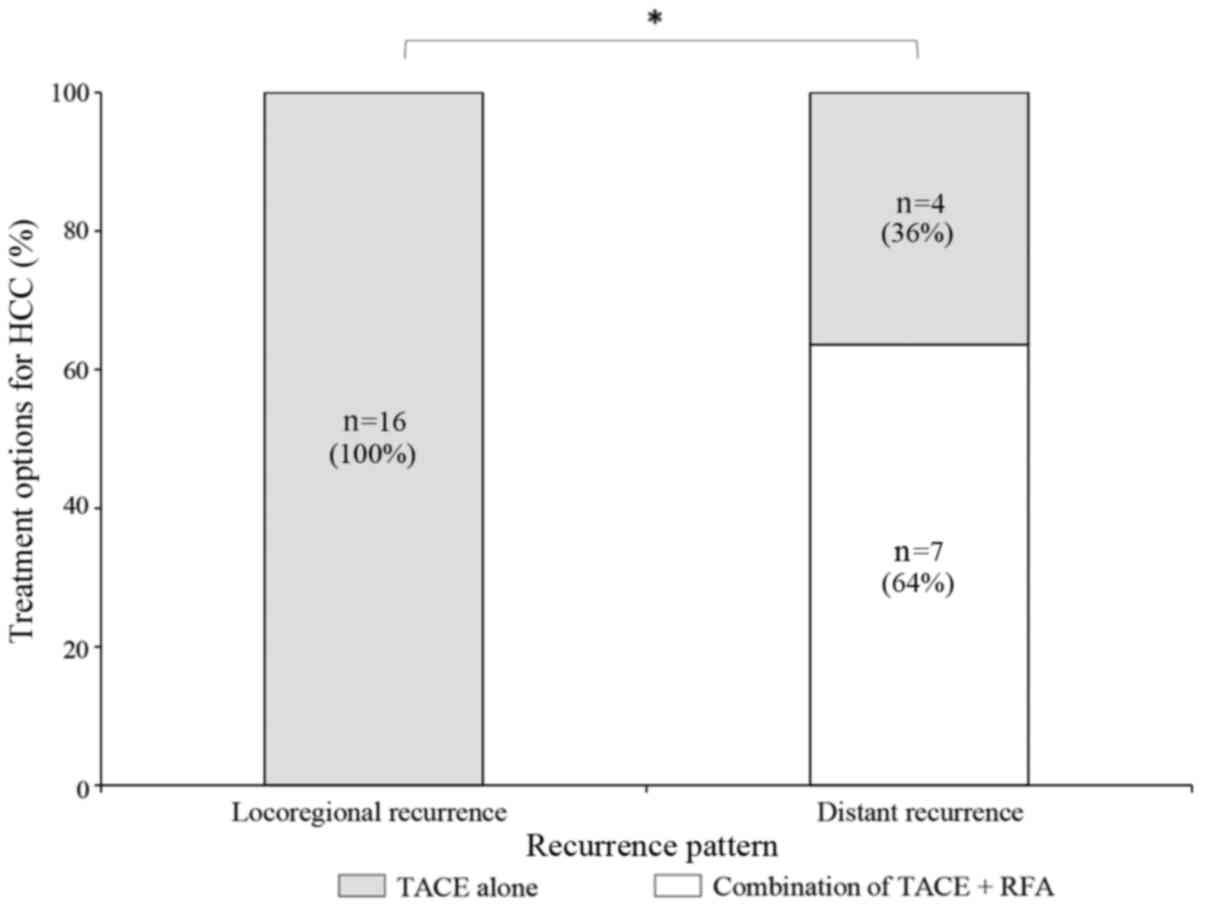
In the ER group (n=32), 5 patients underwent TACE
and RFA and the remaining 27 patients received TACE alone. In the
NER group, all 38 patients received TACE alone. No locoregional
recurrence was observed following treatment with a combination of
TACE and RFA. Of the 11 patients who experienced distant recurrence
in the ER group, 7 (64%) patients had undergone a combination of
TACE and RFA and 4 (36%) patients received TACE alone. A
χ2 test revealed that the combination treatment of TACE
and RFA was associated with a significantly lower locoregional
recurrence rate compared with TACE treatment alone in patients of
the ER group (P<0.05; Fig. 4).
Patients treated with TACE alone had a higher incidence of distant
recurrence compared with those treated with combination
therapy.
Discussion
TACE is a well-established procedure that offers a
palliative survival benefit for patients with HCC that is
unresectable or not suitable for local ablative treatment (20). Several case-control and retrospective
studies have revealed a benefit of TACE for patient survival when
comparing TACE-treated patients with untreated or historical
controls (the patients with unresectable HCC received conservative
treatment) (9,20). It has been a challenge for patients
with advanced HCC to maintain a CR following TACE due to
extracapsular invasion of HCC and residual viable cancer cells
around the fibrous capsules subsequent to TACE (21). Complications associated with TACE,
including an impaired hepatic functional reserve, support the use
of RFA rather than repeated TACE treatments (22,23).
Emerging evidence suggests that a combination of TACE and RFA
exerts a synergistic anticancer activity against HCC, particularly
for larger lesions that do not respond sufficiently to either TACE
or RFA treatments alone (24–26). An analysis of the factors that carry a
high risk of early recurrence following TACE may improve the
selection of patients suited to a combination of TACE with RFA.
In the present study, the clinical courses of 70
patients with HCC treated with TACE, and the different risk factors
associated with HCC recurrence following initial remission after
TACE, were examined. To the best of our knowledge, this is the
first study to show that tumor number is the most important risk
factor for recurrence within 1 year of initial TACE. The findings
of the present study demonstrated an inverse association between
tumor number and DFS time following initial TACE. Consistent with
the present study, several previous studies have identified tumor
number as a predictor of intrahepatic recurrence following initial
TACE for HCC (27–31). Recurrence following initial remission
by TACE has been more often reported in patients with
multinodular-type HCC and with portal vein thrombosis (32). By contrast, Matsuda et al
(33) conjectured that tumor
multiplicity was not associated with 1-year HCC recurrence status.
It has also been reported that the risk factors for early HCC
recurrence subsequent to achieving CR by TACE included large tumor
size, non-compact lipiodol uptake and an AFP concentration >20
ng/ml, but not tumor number (34).
This discrepancy may be explained in part by the different
treatment modalities and the baseline characteristics of the
patients with HCC between studies. HCC often consists of different
cell types, including moderately differentiated and
undifferentiated carcinoma (35,36), which
is not eligible for TACE due to its hypovascularity. Early
recurrence following a CR achieved by TACE may be mainly attributed
to residual tumors that were undetectable on angiography. The
distribution of lipiodol uptake determined by tumor differentiation
and the blood vessel network have been shown to affect the local
recurrence rate and long-term outcome in patients with HCC
(37,38). Furthermore, consistent with the
present study, a previous study showed that local recurrence
developed more frequently in patients with early recurrence (≤1
year) compared with those with late recurrence (>1 year)
(27). These results indicated that
early recurrence is associated with local recurrence arising from
limitations of the radiological evaluation of tumor response, and
remnant tumors can develop and be recognizable by imaging
modalities over time.
In combined treatment with TACE and RFA, the main
roles of TACE are to counteract the heat-sink effect of hepatic
blood flow by hepatic artery embolization (39) and to reduce the portal venous flow by
filling the peripheral portal vein around the HCC (40). TACE can also lead to ischemic edema,
which may enlarge the area of tumor necrosis induced by RFA
(24). The combination of TACE and
RFA generally results in complete tumor remission if the liver
function reserve is sufficiently maintained post-TACE (12). No local HCC recurrence was observed in
patients treated with a combination of TACE and RFA in the present
study. The combination of TACE and RFA has significant advantages
in terms of local tumor control and longer patient survival
compared with TACE alone (41,42). These
findings reinforce the notion that the combination of TACE and RFA
resulted in a more efficient microscopic local tumor response
compared with TACE alone.
The present study had a number of limitations.
First, it was conducted in a single center and the sample size of
patients with HCC recurrence was small. Second, there may have been
unexpected factors that effected the probability of therapeutic
response and recurrence. Third, patients who were lost to follow-up
within 1 year and those who succumbed to the disease were excluded
from the analysis, since the presence of early recurrence could not
be confirmed; this may have led to selection bias.
In conclusion, multinodularity of HCC is an
independent risk factor for 1-year recurrence of HCC in patients
with initial remission following TACE. In particular, in patients
who developed early recurrence, intrahepatic local recurrence more
frequently occurred in HCC patients who received TACE alone
compared with those treated with TACE combined with RFA treatment,
which indicated that the combination treatment of TACE and RFA may
be beneficial in preventing early recurrence of HCC.
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simonetti RG, Cammà C, Fiorello F, Politi
F, D'Amico G and Pagliaro L: Hepatocellular carcinoma. A worldwide
problem and the major risk factors. Dig Dis Sci. 36:962–972. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hwang S, Lee SG, Ko GY, Kim BS, Sung KB,
Kim MH, Lee SK and Hong HN: Sequential preoperative ipsilateral
hepatic vein embolization after portal vein embolization to induce
further liver regeneration in patients with hepatobiliary
malignancy. Ann Surg. 249:608–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ribero D, Abdalla EK, Madoff DC, Donadon
M, Loyer EM and Vauthey JN: Portal vein embolization before major
hepatectomy and its effects on regeneration, resectability and
outcome. Br J Surg. 94:1386–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lencioni R: Loco-regional treatment of
hepatocellular carcinoma. Hepatology. 52:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Real MI, Montana X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: A
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu HC, Shan EB, Zhou L, Jin H, Cui PY,
Tan Y and Lu YM: Combination of percutaneous radiofrequency
ablation with transarterial chemoembolization for hepatocellular
carcinoma: Observation of clinical effects. Chin J Cancer Res.
26:471–477. 2014.PubMed/NCBI
|
|
9
|
European Association For The Study Of The
Liver1; European Organisation For Research And Treatment Of Cancer,
. EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu HC, Cheng JS, Lai KH, Lin CP, Lo GH,
Lin CK, Hsu PI, Chan HH, Lo CC, Tsai WL and Chen WC: Factors for
early tumor recurrence of single small hepatocellular carcinoma
after percutaneous radiofrequency ablation therapy. World J
Gastroenterol. 11:1439–1444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao GS, Yu CY, Shih ML, Chan DC, Liu YC,
Yu JC, Chen TW and Hsieh CB: Radiofrequency ablation after
transarterial embolization as therapy for patients with
unresectable hepatocellular carcinoma. Eur J Surg Oncol. 34:61–66.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan S, Xu D and Sun B: Combination of
radiofrequency ablation with transarterial chemoembolization for
hepatocellular carcinoma: A meta-analysis. Dig Dis Sci.
58:2107–2113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koh PS, Chan AC, Cheung TT, Chok KS, Dai
WC, Poon RT and Lo CM: Efficacy of radiofrequency ablation compared
with transarterial chemoembolization for the treatment of recurrent
hepatocellular carcinoma: A comparative survival analysis. HPB
(Oxford). Oct 16–2015.(Epub ahead of print). View Article : Google Scholar
|
|
14
|
Tang C, Shen J, Feng W, Bao Y, Dong X, Dai
Y, Zheng Y and Zhang J: Combination therapy of radiofrequency
ablation and transarterial chemoembolization for unresectable
hepatocellular carcinoma: A retrospective study. Medicine
(Baltimore). 95:e37542016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kokudo N, Hasegawa K, Akahane M, Igaki H,
Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, et al:
Evidence-based clinical practice guidelines for hepatocellular
carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC
Guidelines). Hepatol Res. 45:2015. View Article : Google Scholar
|
|
16
|
Nishiofuku H, Tanaka T, Matsuoka M, Otsuji
T, Anai H, Sueyoshi S, Inaba Y, Koyama F, Sho M, Nakajima Y and
Kichikawa K: Transcatheter arterial chemoembolization using
cisplatin powder mixed with degradable starch microspheres for
colorectal liver metastases after FOLFOX failure: Results of a
phase I/II study. J Vasc Interv Radiol. 24:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bilehjani E and Fakhari S: Using central
venous catheter for suprapubic catheterization in cardiac surgery.
Res Rep Urol. 9:1–4. 2017.PubMed/NCBI
|
|
18
|
Padhani AR and Ollivier L: The RECIST
(Response Evaluation Criteria in Solid Tumors) criteria:
Implications for diagnostic radiologists. Br J Radiol. 74:983–986.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pagliaro L: MELD: The end of Child-Pugh
classification? J Hepatol. 36:141–142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jansen MC, van Hillegersberg R, Chamuleau
RA, van Delden OM, Gouma DJ and van Gulik TM: Outcome of regional
and local ablative therapies for hepatocellular carcinoma: A
collective review. Eur J Surg Oncol. 31:331–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamashita Y, Torashima M, Oguni T,
Yamamoto A, Harada M, Miyazaki T and Takahashi M: Liver parenchymal
changes after transcatheter arterial embolization therapy for
hepatoma: CT evaluation. Abdom Imaging. 18:352–356. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Groupe d'Etude et de Traitement du
Carcinome Hépatocellulaire: A comparison of lipiodol
chemoembolization and conservative treatment for unresectable
hepatocellular carcinoma. N Engl J Med. 332:1256–1261. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Hu Y, Ren M, Lu X, Lu G and He S:
Efficacy and safety of radiofrequency ablation combined with
transcatheter arterial chemoembolization for hepatocellular
carcinomas compared with radiofrequency ablation alone: A
time-to-event meta-analysis. Korean J Radiol. 17:93–102. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo W, He X, Li Z and Li Y: Combination of
transarterial chemoembolization (TACE) and radiofrequency ablation
(RFA) vs. surgical resection (SR) on survival outcome of early
hepatocellular carcinoma: A meta-analysis. Hepatogastroenterology.
62:710–714. 2015.PubMed/NCBI
|
|
26
|
Chen QW, Ying HF, Gao S, Shen YH, Meng ZQ,
Chen H, Chen Z and Teng WJ: Radiofrequency ablation plus
chemoembolization versus radiofrequency ablation alone for
hepatocellular carcinoma: A systematic review and meta-analysis.
Clin Res Hepatol Gastroenterol. 40:309–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin YJ, Chung YH, Kim JA, Park W, Lee D,
Shim JH, Lee D, Kim KM, Lim YS, Lee HC, et al: Predisposing factors
of hepatocellular carcinoma recurrence following complete remission
in response to transarterial chemoembolization. Dig Dis Sci.
58:1758–1765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kinugasa H, Nouso K, Takeuchi Y, Yasunaka
T, Onishi H, Nakamura S, Shiraha H, Kuwaki K, Hagihara H, Ikeda F,
et al: Risk factors for recurrence after transarterial
chemoembolization for early-stage hepatocellular carcinoma. J
Gastroenterol. 47:421–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeong SO, Kim EB, Jeong SW, Jang JY, Lee
SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, et al: Predictive
factors for complete response and recurrence after transarterial
chemoembolization in hepatocellular carcinoma. Gut Liver.
11:409–416. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park W, Chung YH, Kim JA, Jin YJ, Lee D,
Shim JH, Lee D, Kim KM, Lim YS, Lee HC, et al: Recurrences of
hepatocellular carcinoma following complete remission by
transarterial chemoembolization or radiofrequency therapy: Focused
on the recurrence patterns. Hepatol Res. 43:1304–1312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Colecchia A, Schiumerini R, Cucchetti A,
Cescon M, Taddia M, Marasco G and Festi D: Prognostic factors for
hepatocellular carcinoma recurrence. World J Gastroenterol.
20:5935–5950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JK, Chung YH, Song BC, Shin JW, Choi
WB, Yang SH, Yoon HK, Sung KB, Lee YS and Suh DJ: Recurrences of
hepatocellular carcinoma following initial remission by
transcatheter arterial chemoembolization. J Gastroenterol Hepatol.
17:52–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuda M, Omata F, Fuwa S, Saida Y,
Suzuki S, Uemura M, Ishii N, Iizuka Y, Fukuda K and Fujita Y:
Prognosis of patients with hepatocellular carcinoma treated solely
with transcatheter arterial chemoembolization: Risk factors for
one-year recurrence and two-year mortality (preliminary data).
Intern Med. 52:847–853. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rou WS, Lee BS, Moon HS, Lee ES, Kim SH
and Lee HY: Risk factors and therapeutic results of early local
recurrence after transcatheter arterial chemoembolization. World J
Gastroenterol. 20:6995–7004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsumura H, Nirei K, Nakamura H, Higuchi
T, Arakawa Y, Ogawa M, Tanaka N and Moriyama M: Histopathology of
type C liver disease for determining hepatocellular carcinoma risk
factors. World J Gastroenterol. 19:4887–4896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paradis V: Histopathology of
hepatocellular carcinoma. Recent Results Cancer Res. 190:21–32.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stefanini GF, Amorati P, Biselli M, Mucci
F, Celi A, Arienti V, Roversi R, Rossi C, Re G and Gasbarrini G:
Efficacy of transarterial targeted treatments on survival of
patients with hepatocellular carcinoma. An Italian experience.
Cancer. 75:2427–2434. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwan SW, Fidelman N, Ma E, Kerlan RK Jr
and Yao FY: Imaging predictors of the response to transarterial
chemoembolization in patients with hepatocellular carcinoma: A
radiological-pathological correlation. Liver Transpl. 18:727–736.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sugimori K, Nozawa A, Morimoto M, Shirato
K, Kokawa A, Saito T, Numata K and Tanaka K: Extension of
radiofrequency ablation of the liver by transcatheter arterial
embolization with iodized oil and gelatin sponge: Results in a pig
model. J Vasc Interv Radiol. 16:849–856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seki T, Tamai T, Nakagawa T, Imamura M,
Nishimura A, Yamashiki N, Ikeda K and Inoue K: Combination therapy
with transcatheter arterial chemoembolization and percutaneous
microwave coagulation therapy for hepatocellular carcinoma. Cancer.
89:1245–1251. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang NK, Shin SS, Kim JW, Kim HJ, Jeong
YY, Heo SH, Kim JK and Kang HK: Effect of ultrasound-guided
radiofrequency ablation in incompletely treated hepatocellular
carcinoma after transcatheter arterial chemoembolization. Korean J
Radiol. 13 Suppl 1:S104–S111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie H, Wang H, An W, Ma W, Qi R, Yang B,
Liu C, Gao Y, Xu B and Wang W: The efficacy of radiofrequency
ablation combined with transcatheter arterial chemoembolization for
primary hepatocellular carcinoma in a cohort of 487 patients. PLoS
One. 9:e890812014. View Article : Google Scholar : PubMed/NCBI
|