Introduction
Colorectal cancer (CRC) is the third most common
cause of death due to cancer in Japan. The adenoma-carcinoma
sequence is thought to be the primary route involved in the
development of CRC (1), and
neoplastic lesions found during colonoscopy are associated with a
future risk of neoplasms. It has been demonstrated that colonoscopy
with polypectomy reduces the risk of recurrent CRC and mortality
(2,3);
the colonoscopy surveillance interval is based on risk
stratification according to the number of adenomas, the maximum
size of polyps and the histopathological findings of all resected
lesions (4,5). This is the primary screening method used
in the US and several European countries (6,7). On the
other hand, the most suitable colonoscopy interval was described
briefly in the Japanese guidelines as follows: ‘<3 years
surveillance colonoscopy is recommended after polypectomy’
(8). Additionally, Japanese
guidelines indicate diminutive neoplastic lesions without
carcinomatous findings can be left untreated and followed up (i.e.,
semi-clean colon). The vast majority of polyps removed during a
colonoscopy are diminutive (≤5 mm in size), and approximately half
of the diminutive polyps are adenomas (9–11).
Furthermore, only a small percentage of diminutive adenomatous
polyps (DAPs) contain advanced histological features (12). Indeed, some endoscopists in Japan
leave DAPs unresected after a detailed observation and a close
follow-up. This is because povlypectomy may be unnecessary because
of the low prevalence of advanced features in these polyps, and it
may be unnecessary to expose patients to added risks during
colonoscopy (13).
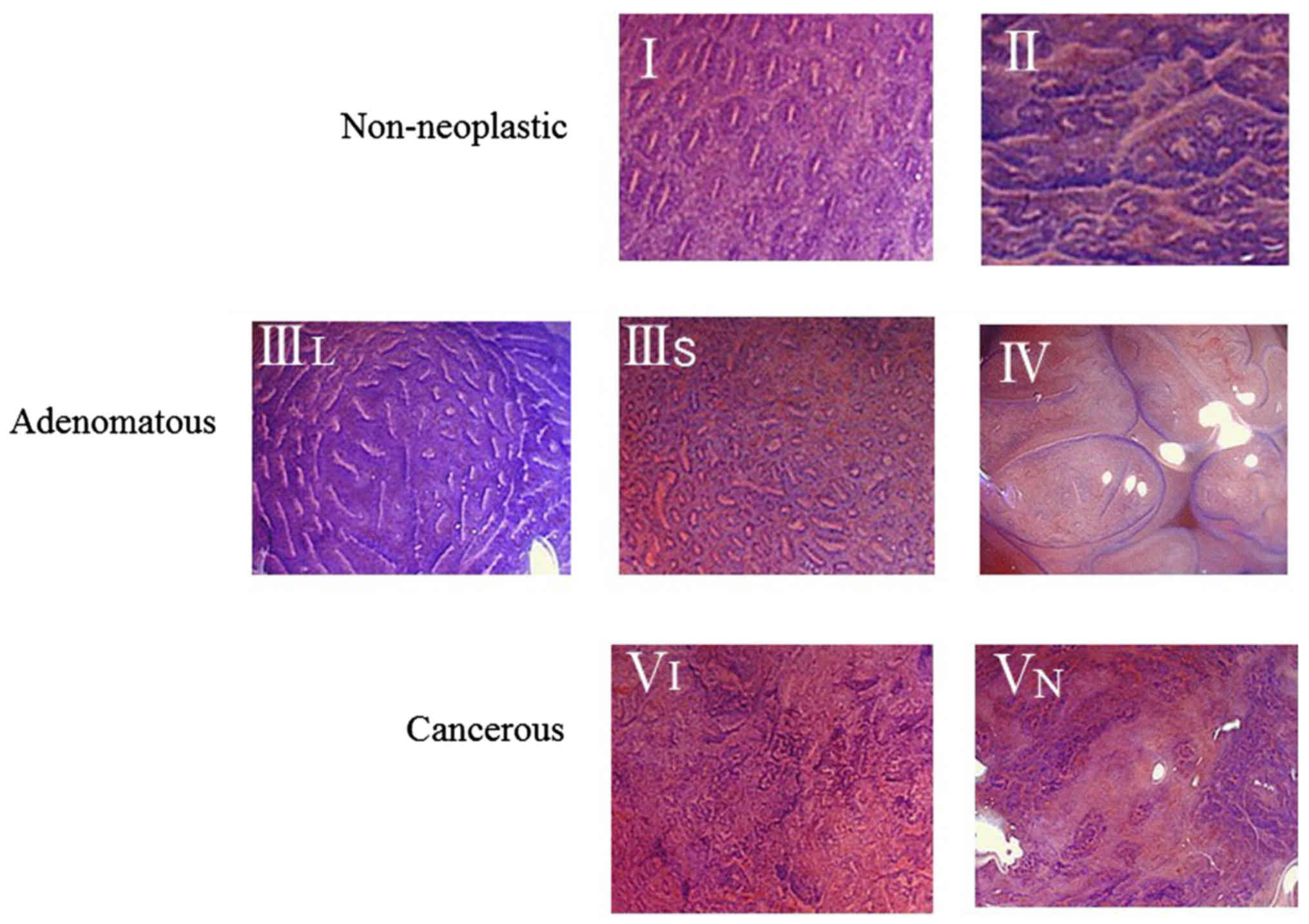
In the PIT classification system described by Kudo
et al (14) (Fig. 1), type I and type II lesions are
defined as having non-neoplastic patterns; type IIIL, type IIIs and
type IV are adenomatous; and type VI and type VN are cancerous.
Concerning type IIIL, a previous study reported that lesions in
this category exhibited no invasive characteristics (15). However, type III is a standard pit
pattern observed in depressed-types of early cancer, and type IV
lesions often contain characteristics of advanced neoplasia (e.g.,
high-grade adenomas or villous components).
Magnifying a chromoendoscopy with PIT assessment
increases the diagnostic accuracy for both distinguishing adenomas
from hyperplastic polyps (85–96%) as well as diagnosing massively
invasive submucosal colorectal cancer which has the possibility of
metastasising (76.4–89.3%) (16–18).
Moreover, this classification system has a good-to-excellent
inter-observer agreement (0.78–0.96) and could be used to
accurately diagnose patients before treatment (19,20).
Recently, the number of patients taking antiplatelet
or anticlotting medications to prevent cerebral vascular disease or
myocardial infarction has increased. For patients who continue to
take their antiplatelet or anticlotting medications, or those who
have a large number of polyps with difficulty resecting at a time,
we often recommend leaving type IIIL PIT DAPs untreated and
conducting a follow-up without an immediate re-examination
(semi-clean colon strategy). However, the risk and adequate
surveillance intervals of the semi-clean colon are not clear.
Therefore, this retrospective case-control study was
designed to investigate the feasibility of a semi-clean colon.
Materials and methods
Patients and study design
In this retrospective case-control study, we
acquired data from a database of colonoscopy examinations that had
been prospectively recorded in Showa University Northern Yokohama
Hospital (Yokohama, Japan), a tertiary referral centre in Japan.
The study subjects consisted of patients over 30 years of age who
were referred for an initial total colonoscopy and were followed up
for >3 years from April 2001 to March 2014.
Patients who met the following criteria were
excluded from the study: Those that were not caecal intubation;
those with lesions >5 mm in size and/or classified as type IIIS,
IV, or V PIT that were initially left untreated; those that had a
detected number of adenomas polyps ≥10; and those with a history of
familial adenomatous polyposis, Lynch syndrome, advanced colorectal
cancer, inflammatory bowel disease, or a colectomy. All
participants provided written informed consent, and the study was
conducted according to the Declaration of Helsinki.
Participants were classified into three groups
according to histological findings and treatment at the time of the
initial colonoscopy: Group A, patients with type IIIL DAPs that
were left untreated (semi-clean colon group); group B, patients in
whom all neoplastic polyps, including DAPs were resected (clean
colon group); and group C, patients without any adenomatous polyps
(internal control group). Furthermore, groups A and B were
classified as high- and low-risk patients, based on the number of
adenomas, maximum size of the polyps and the histopathological
findings according to the US guidelines as follows: Low-risk, 1–2
tubular adenomas <10 mm; high-risk, 3–10 adenomas or ≥1 advanced
adenoma (6).
We retrospectively reviewed the database and medical
records. The study protocol was approved by the ethics committee of
Showa University Northern Yokohama Hospital (no. 1,410-05) and
registered in the UMIN clinical trial registry (UMIN000016367).
Endoscopic procedures
Prior to the examination, patients underwent a bowel
preparation with 2–3 liters of a polyethylene glycol solution.
Diazepam and butyl scopolamine were used intravenously for sedation
and prevention of peristalsis. All patients underwent total
colonoscopies with magnifying endoscopes (CF-240ZI, CF-H260AZI,
PCF-240ZI; Olympus Corp., Tokyo, Japan) using approximately 80- to
100-fold magnification. During the magnifying observation, on-site
endoscopists first sprayed the target lesion with indigo carmine,
and if necessary, they then stained the lesion with crystal violet.
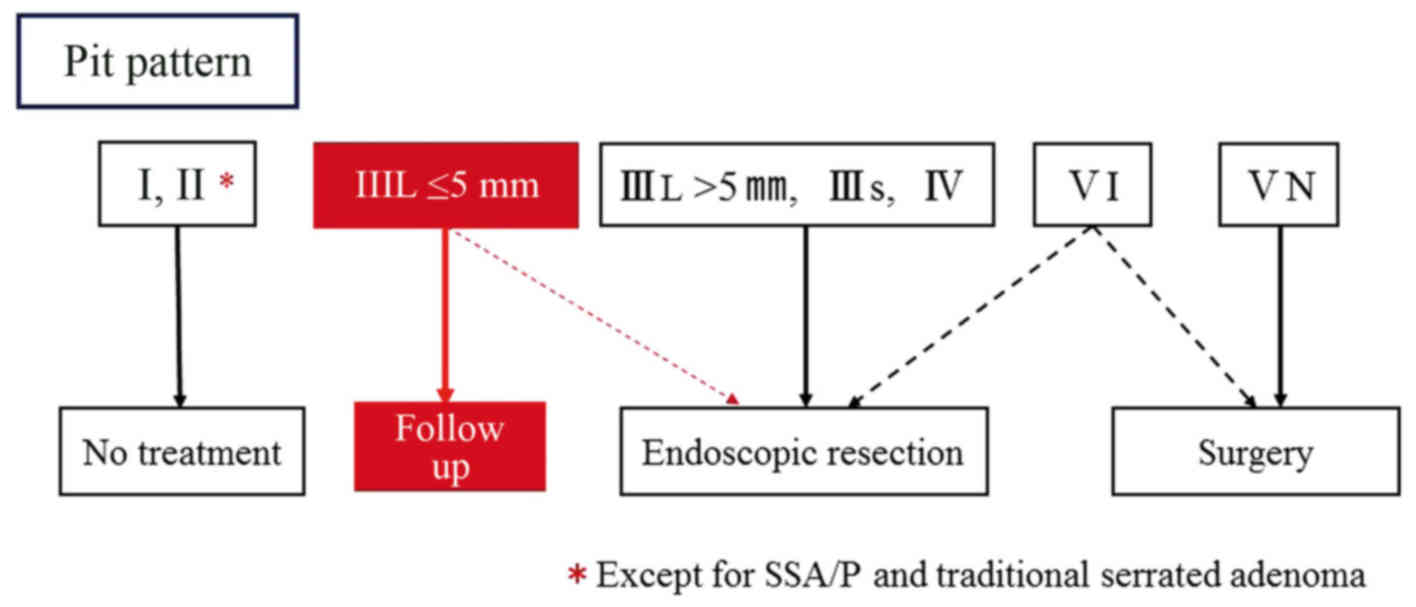
All detected lesions were diagnosed on the basis of the PIT
classification system described by Kudo et al (14). Each diagnosis was recorded in the
database just after the colonoscopy. Lesion size, location and
shape [Paris classification 10 (21)]
were also measured and recorded. The lesions diagnosed as
non-neoplastic were left untreated. Conversely, all neoplastic
lesions, except for type IIIL PIT DAPs, were completely removed.
Type IIIL PIT DAPs were left untreated at the discretion of the
on-site endoscopists as Fig. 2.
Histopathological evaluation
All resected specimens were fixed in 10% formalin,
embedded in paraffin, serially sectioned, and stained using
haematoxylin and eosin. Experienced gastrointestinal pathologists
evaluated all pathological specimens. Histopathological diagnoses
were determined based on the World Health Organization criteria
(22).
Outcome measures
As a primary outcome measure, the cumulative
incidence of index lesions (ILs) for the follow-up colonoscopy was
analysed among the three groups. The ILs diagnosed during the
follow-up colonoscopy were defined as follows: Large adenomatous
polyps ≥10 mm, high-grade dysplasia (intramucosal cancer) and
invasive cancers. We also evaluated the incidence of invasive and
interval cancers. In this study, interval cancer was defined as
invasive cancer diagnosed within the 36 months following a baseline
colonoscopy. This definition has been used in multiple previous
studies (23,24).
We also compare the ILs incidence between untreated
DAPs (group A) and resected DAPs group in group B.
Statistical analysis
For the statistical analyses, a computerised
database was designed using R (v. 2.13.0; The R Foundation for
Statistical Computing, Vienna, Austria). Quantitative data were
expressed as the mean and standard deviation values. The cumulative
incidence rates over the maximum follow-up period among the three
groups were compared using the Grey test. Statistical significance
was evaluated using the Chi-squared test and P<0.05 was
considered to indicate a statistically significant difference. The
Bonferroni method was used to counteract the problem of multiple
comparisons. With regard to multivariable analysis, logistic
regression analysis was conducted and P<0.05 was considered to
indicate a statistically significant difference. All authors had
access to the study data and reviewed and approved the final
manuscript.
Results
Subjects and outlines of the initial
colonoscopy
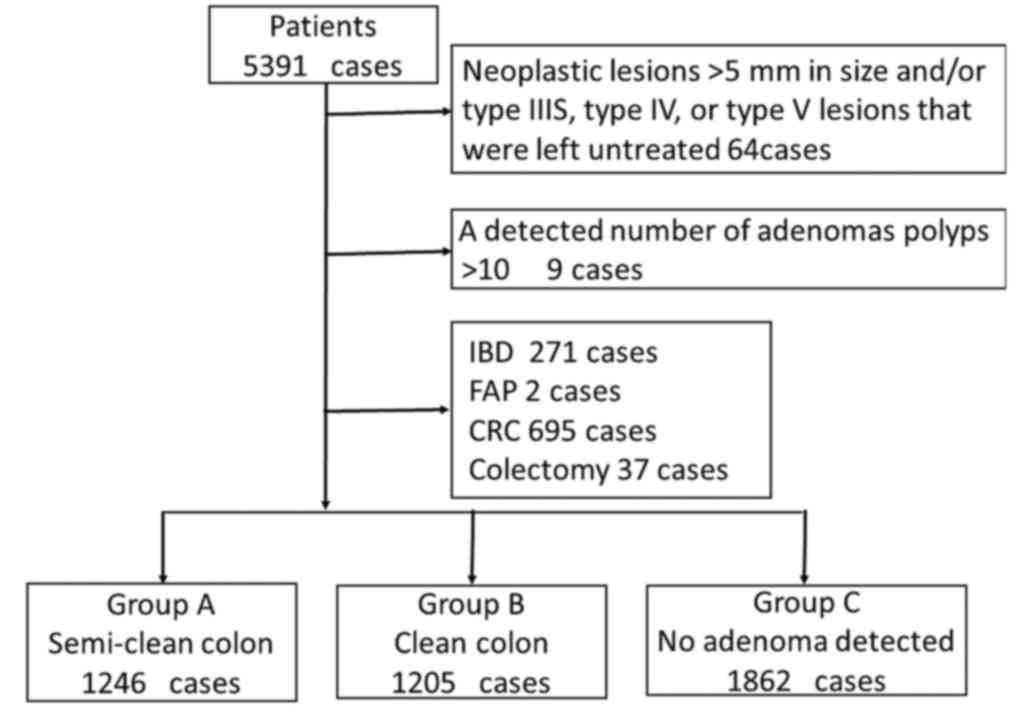
A total of 5,391 patients were analysed and 1,078
patients were excluded in accordance with the exclusion criteria. A
total of 4,313 patients, including 2,631 (61.0%) male patients,
were enrolled in this study, as listed in Table I. Eligible patients were classified
into three groups as follows: Group A, 1246 patients (28.9%); group
B, 1205 patients (27.9%); and group C, 1862 patients (43.2%) as
Fig. 3. The mean ages were 62.4±18.7,
60.6±20.4 and 56.1±19.3 years in groups A, B and C, respectively.
The rates of the high-risk group detected during the baseline
colonoscopy were 36.1% (450 patients) and 37.2% (448 patients) in
groups A and B, respectively. The average number of lesions
diagnosed as neoplastic were 2.2 and 1.5 lesions in groups A and B,
respectively.
 | Table I.Characteristics of patients and ILs
diagnosed by a follow-up colonoscopy. |
Table I.
Characteristics of patients and ILs
diagnosed by a follow-up colonoscopy.
| Characteristics | Group A | Group B | Group C | Total |
|---|
| Patients (no) | 1,246 | 1,205 | 1,862 | 4,313 |
| Male sex [no.
(%)] | 723 (58.0) | 736 (61.1) | 1,172 (62.9) | 2,631 (61.0) |
| Age (years ± SD) | 62.4±18.7 | 60.6±20.4 | 56.1±19.3 | 57.2±19.5 |
| Risk
stratification |
|
|
|
|
| Low
risk | 796 (63.9%) | 757 (62.8%) | – | – |
| High
risk | 450 (36.1%) | 448 (37.2%) | – | – |
| Number of
neoplastic lesions | 2.2 (1–9) | 1.5 (1–7) | – | – |
| Follow-up period
(years ± SD) | 5.1±1.8 | 5.0±1.9 | 5.2±1.7 | 5.1±1.8 |
| Average number of
TCS | 3.1 | 2.9 | 2.1 | 2.6 |
Follow-up colonoscopy
Overall, the median follow-up period and the
frequency of colonoscopy were 5.1 years and 2.9 times,
respectively. There were no significant differences in the
follow-up period among the groups. Moreover, the average number of
follow-up colonoscopies was 3.5, 3.2 and 2.1 in groups A-C,
respectively.
Incidence of ILs
A total of 259 ILs in 233 patients were newly
diagnosed during the follow-up colonoscopies. The incidence rates
of ILs in each group were as follows: Group A, 8.0% (n=100); group
B, 8.6% (n=104); and group C, 1.6% (n=29). No significant
difference was found between groups A and B in the incidence of ILs
(Table II). There were 13 (5.0%), 57
(22.0%), 59 (22.8%), 20 (7.7%), 94 (36.3%), and 18 (6.9%) ILs
located in the cecum, ascending colon, transverse colon, descending
colon, sigmoid, and rectum, respectively. Of these ILs, the
macroscopic types were 119 (45.9%) polypoid, 112 (43.2%) flat, and
10 (3.8%) depressed lesions. Histopathologically, 159 (61.3%) ILs
were adenoma (≥10 mm), 75 (29.0%) were high grade dysplasia, 11
(4.2%) were submucosal invasive cancer, and 14 (5.4%) were advanced
cancer (Table III).
 | Table II.The incidence of index lesions and
invasive cancers. |
Table II.
The incidence of index lesions and
invasive cancers.
|
Characteristics | Group A | Group B |
P-valuea | Group C |
P-valueb | Total |
|---|
| Patients, no. | 1,246 | 1,205 |
| 1,862 |
| 4,313 |
| ILs, no. |
116 |
110 |
| 33 |
|
259 |
| Patients with ILs,
no. (%) | 100 (8.0) | 104 (8.6) | 0.609 | 29 (1.6) | <0.01 | 233 (5.4) |
| ILs within 36
months, no. | 38 | 36 |
| 12 |
| 86 |
| Patients with ILs
within 36 months, no. (%) | 32 (2.6) | 30 (2.5) | 0.818 | 11 (0.6) | <0.01 | 73 (1.7) |
| ICs, no. | 9 | 8 |
| 8 |
| 25 |
| Patient with ICs,
no. (%) | 8 (0.6) | 7 (0.6) | 1.000 | 8 (0.4) | 0.579 | 23 (0.5) |
| ICs within 36
months, no. | 2 | 3 |
| 4 |
| 9 |
| Patients with ICs,
no. (%) | 2 (0.2) | 3 (0.2) | 1.000 | 4 (0.2) | 0.721 | 9 (0.2) |
 | Table III.Clinicopathological characteristics
of ILs diagnosed by follow-up colonoscopy. |
Table III.
Clinicopathological characteristics
of ILs diagnosed by follow-up colonoscopy.
|
Characteristics | Group A | Group B | Group C | Total |
|---|
| Number of patients
with ILs | 100 | 104 | 29 | 233 |
| Number of ILs | 116 | 110 | 33 | 259 |
| Location, no.
(%) |
|
|
|
|
|
Cecum | 4 (3.4) | 9 (8.2) | 0 | 13 (5.0) |
|
Ascending | 25 (21.6) | 23 (20.9) | 9 (27.3) | 57 (22.0) |
|
Transverse | 23 (19.8) | 31 (28.2) | 5 (15.2) | 59 (22.8) |
|
Descending | 12 (10.3) | 8 (7.3) | 0 | 20 (7.7) |
|
Sigmoid | 47 (41.6) | 32 (29.1) | 15 (45.5) | 94 (36.3) |
|
Rectum | 5 (4.3) | 7 (6.4) | 6
(18.2) | 18 (6.9) |
| Macroscopic type,
no. (%) |
|
|
|
|
| Adenoma
and early cancer | 112 (96.9) | 100 (90.6) | 29 (81.8) | 241 (93.1) |
|
Polypoid | 65 (56.0) | 39 (35.5) | 15 (45.5) | 119 (45.9) |
|
Flat | 43 (37.1) | 58 (52.7) | 11 (33.3) | 112 (43.2) |
|
Depressed | 4 (3.4) | 3 (2.7) | 3 (9.1) | 10 (3.8) |
| Histopathology, no.
(%) |
|
|
|
|
| Adenoma
(≥10 mm) | 68 (58.6) | 75 (68.2) | 16 (48.5) | 159 (61.3) |
| High
grade dysplasia | 39 (33.6) | 27 (24.5) | 9 (27.3) | 75 (29.0) |
| SM
invasive cancer | 5 (4.3) | 2 (1.8) | 4 (12.1) | 11 (4.2) |
|
Advanced cancer | 4 (3.4) | 6 (5.5) | 4 (12.1) | 14 (5.4) |
The cumulative incidence of ILs at 3 and 5 years
were 2.6/4.3, 2.5/4.9, and 0.6/1.1% in groups A-C, respectively
(Table IV).
 | Table IV.The cumulative incidence of index
lesions. |
Table IV.
The cumulative incidence of index
lesions.
|
| Cumulative
incidence of ILs (%) |
|
|
|---|
|
|
|
|
|
|---|
| Group | 3-year | 5-year | Maximum follow-up
period | N | Patients with ILs
(N) |
|---|
| Group A | 2.6 | 4.3 | 8.0 | 1,246 | 100 |
| Group B | 2.5 | 4.9 | 8.6 | 1,205 | 104 |
| Group C | 0.6 | 1.1 | 1.6 | 1,862 | 29 |
The cumulative incidence rates of the ILs are
presented in Fig. 4. No significant
difference was found between groups A and B for the cumulative
incidence of the ILs. The incidence of ILs in group C was lower
than that in both groups A and B (P<0.01 and P<0.01,
respectively).
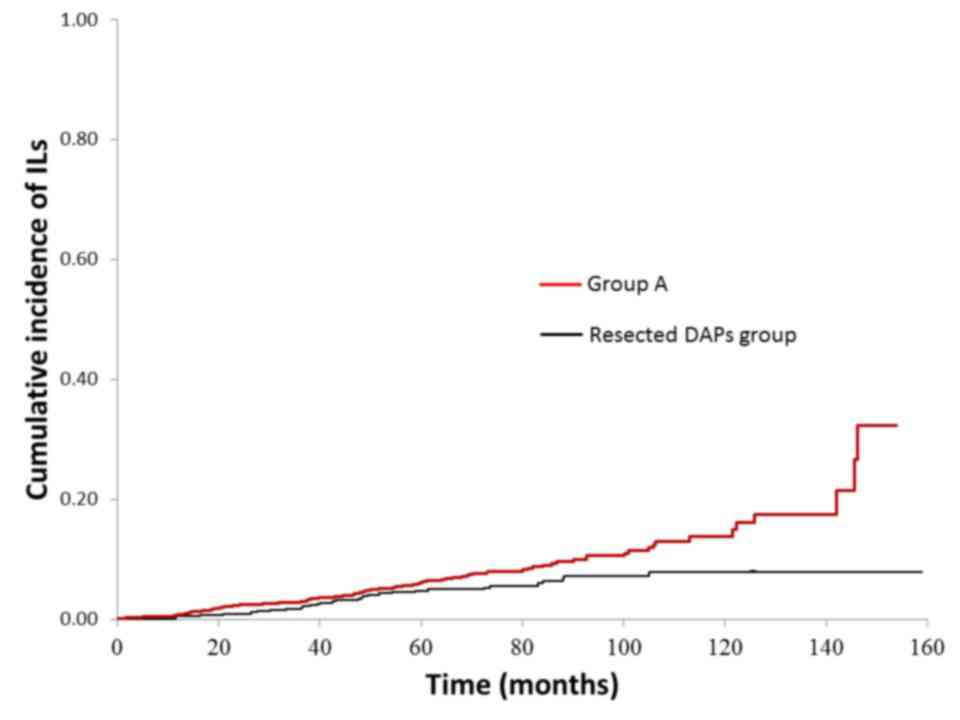
In group B, resected DAPs group are significantly
higher than group A in terms of the cumulative incidence rates of
the ILs (P=0.012) (Fig. 5).
Incidence of invasive and interval
cancers
A total of 25 invasive cancers in 23 patients were
newly diagnosed during the follow-up colonoscopies (Table II). The incidence rates of invasive
cancer in each group were as follows: Group A, 0.6% (n=9); group B,
0.6% (n=8); and group C, 0.4% (n=8). The incidence of invasive
cancer in Group A was similar to that of group B, as shown in
Table II.
In particular, a total of 9 invasive cancers in 9
patients were diagnosed within 36 months following the baseline
colonoscopy, as shown in Table II.
There was no significant difference in the incidence of invasive
cancers diagnosed within 36 months between groups A and B.
The risk factors in future detection
of ILs among group B
‘The number of neoplastic lesions ≥3′ and, ‘Follow
up periods ≥5 years’ showed significant high odds rate for future
detection of ILs in multivariate logistic regression analyses
(Table V).
 | Table V.The risk factors in future detection
of index lesions among clean colon group. |
Table V.
The risk factors in future detection
of index lesions among clean colon group.
| Factors | OR | 95% CI | P-value |
|---|
| ILs at initial
CS | 1.13 | 0.25–5.11 | 0.86 |
| Neoplastic lesion
≥10 mm at initial CS | 1.89 | 0.45–7.98 | 0.39 |
| SM invasive cancers
at initial CS | 0.39 | 0.05–3.12 | 0.38 |
| High grade
dysplasia at initial CS | 0.87 | 0.42–1.77 | 0.69 |
| Villous component
at initial CS | 1.73 | 0.88–3.42 | 0.11 |
| The number of
neoplastic lesions ≥3 | 2.50 | 1.48–4.20 | <0.001 |
| Follow up periods
≥5 years | 3.70 | 2.38–5.88 | <0.001 |
Discussion
This study was a retrospective case-control study
that analysed the concept of ‘semi-clean colon’ using Kudo's PIT
classification assessed by magnifying the chromoendoscopy for the
baseline colonoscopy. The results indicate that there was no
significant difference in the cumulative incidence of ILs and
invasive cancer among patients with type IIIL PIT DAPs that were
left untreated and those in whom all neoplastic lesions were
removed. Colonoscopy combined with the removal of all adenomatous
polyps has been reported to reduce the risk of recurrent CRC and
CRC-related mortality in the National Polyp Study, which has been
conducted since 1980 (2,3). Although the Japanese guidelines indicate
that some neoplastic lesions can be left untreated, diminutive
polypoid lesions should be followed up (8). To our knowledge, this was the first
study to analyse the semi-clean colon strategy and compare it to
the clean colon strategy.
In addition, a previous study reported that advanced
adenomas typically manifest with measurable interval growth,
whereas non-advanced adenomas tend to demonstrate intermediate
behaviour. Furthermore, the majority of other benign small polyps
tend to remain stable or regress over time (25), and a follow-up of unresected
colorectal polyps up to 9 mm is safe (14). However, some DAPs may have advanced
histological features and may even develop into invasive cancer
(26). PIT diagnosis by magnifying
the chromoendoscopy enables the distinction of neoplasia from
non-neoplasia in daily practice. While emerging virtual
chromoendoscopy systems [e.g., narrow-band imaging or Fuji
intelligent chromoendoscopy (17,18)] are
very convenient tools, they do not replace PIT diagnosis in terms
of diagnostic ability. Magnifying the chromo-observation is
necessary to identify diminutive invasive cancer, even among DAPs.
It is essential to perform magnifying chromo-observation to
determine the feasibility of leaving diminutive adenomatous lesions
in place.
In this study, 39 high grade dysplasia, 5 invasive
cancer, 4 advanced cancer were detected in ‘semi-clean group’.
However, Is it not possible to confirm definitely the relationship
between untreated DAPs and these cancer lesions.
A previous study reported that the neoplastic
lesions found during a colonoscopy are associated with the future
risk of incident neoplasms (3),
similar to the results of our study. In Japan, Matsuda, et
al reported that patients with any adenomatous polyps >6 mm
or intra-mucosal cancer at the time of the initial colonoscopy have
a higher risk of advanced lesions than those with no initial
neoplasia or small adenomas do (27).
In this present study, the clean colon and the semi-clean colon
group had a higher incidence of ILs than the internal control group
did. This suggested that patients with high-risk polyps were at a
future risk of incident neoplasms, regardless of whether their
low-risk polyps were removed.
Another issue is that important lesions may be
overlooked during the initial colonoscopy but detected during a
follow-up colonoscopy. However, in the present study, all
examinations were performed under the supervision of experts. It
has been reported that one in 13 (7.9%) cases of CRC may be missed
on the index colonoscopy (23). In
our study, 12 invasive cancers, including eight advanced cancers,
were diagnosed with interval cancers within 36 months. Many of
these cases were likely because of the presence of lesions that
were missed at the time of baseline colonoscopy. In our study, the
ascending and sigmoid colon were the most common sites of interval
cancer. Conversely, a previous report found that a history of prior
colonoscopy with polypectomy is one of the strongest risk factors
for interval cancer (i.e., early/missed cancer) (23). One reason for this may be that a
polypectomy requires more time to perform, and it may be difficult
to then set aside an adequate amount of time for observation. One
strength of the semi-clean colon strategy is that it may save
time.
The ‘resect and discard’ strategy, as proposed by
Ignjatovic et al (28), has
the potential to change the standard management of DAPs and reduce
the cost of screening and surveillance colonoscopy. The primary
benefit of this strategy is the cost savings that can be achieved
by reducing the number of polyps that are sent out for
histopathological examination, as low-risk lesions are not examined
(29). Our strategy for DAPs using
PIT classification also has the potential to lower costs by
reducing both the number of lesions examined histopathologically,
as well as the number of lesions that are treated. The resect and
discard strategy for diminutive colorectal lesions was shown to be
safe in earlier reports (28).
However, the application of this strategy for all diminutive
colorectal lesions is unsatisfactory since the evidence from
earlier reports was primarily based on sessile lesions (30).
Recently, the number of patients who receive
antiplatelet or anticoagulant drugs has increased. Discontinuation
of these medications before the polypectomy increases the risk of
certain adverse events (e.g., cerebral vascular disease, myocardial
infarction, or even death) (31,32).
Heparinization, as an alternative therapy, necessitates
hospitalisation, increases cost, and is time-consuming for
patients. The present study presents the semi-clean colon strategy
for colorectal DAPs as a new treatment option.
Although the cumulative incidence of ILs between the
semi-clean and clean colon groups was not significantly different
in this study, the Kaplan-Meier curve of the semi-clean colon group
exhibited a large increase over the last decade. Thus, we cannot
exclude the future potential of DAP to be a malignancy.
However, a surveillance colonoscopy is recommended
<3 years after endoscopic resection as per the Japanese
guidelines (8). In addition to after
endosopic resection, the semi-clean colon may be acceptable with a
surveillance colonoscopy performed <3 years after the initial
procedure.
There are several limitations to this study. First,
we could not investigate the main indication for the initial
colonoscopy. Patients were not limited strictly to asymptomatic
cases. However, in this study, outcomes were evaluated based on the
findings of the follow-up colonoscopy. Second, this study was a
single-centre study. Both expert endoscopists and trainees were
involved, but the trainees were always accompanied by experts when
determining an on-site PIT diagnosis. Although the validity of the
PIT classification was feasible among the expert endoscopists, the
diagnostic ability of trainees and their learning curves should be
evaluated for a more practical assessment of PIT. Third, this study
was a retrospective study and thus might contain some
methodological bias, thereby compromising the generalisation of the
study results. To compensate for this defect in the study design,
we accumulated a relatively large number of samples. However,
further multi-centre randomised controlled studies are required to
validate these results.
In conclusion, DAPs with type IIIL pit patterns may
be left untreated and subsequently observed. The management of
colorectal DAPs using pit pattern classification with magnifying
chromoendoscopy has the potential to reduce cost, time and risk of
polypectomy and repeated colonoscopy. To assess the clinical
efficacy of the concept of ‘semi-clean colon’, further multi-centre
prospective clinical trials involving a larger number patients and
direct comparisons with a long-term clinical prognosis are
required.
Acknowledgements
The authors thank Enago for editing the
manuscript.
References
|
1
|
Morson B: President's address. The
polyp-cancer sequence in the large bowel. Proc R Soc Med. 67:pp.
451–457. 1974; PubMed/NCBI
|
|
2
|
Zauber AG, Winawer SJ, O'Brien MJ,
Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH,
Schapiro M, Panish JF, et al: Colonoscopic polypectomy and
long-term prevention of colorectal-cancer deaths. N Engl J Med.
366:687–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winawer SJ, Zauber AG, Ho MN, O'Brien MJ,
Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF,
et al: Prevention of colorectal cancer by colonoscopic polypectomy.
The national polyp study workgroup. N Engl J Med. 329:1977–1981.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines: Colorectal Cancer Screening. Version 3.
2013.
|
|
5
|
Davila RE, Rajan E, Baron TH, Adler DG,
Egan JV, Faigel DO, Gan SI, Hirota WK, Leighton JA, Lichtenstein D,
et al: ASGE guideline: Colorectal cancer screening and
surveillance. Gastrointest Endosc. 63:546–557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lieberman DA, Rex DK, Winawer SJ,
Giardiello FM, Johnson DA and Levin TR: United States Multi-Society
Task Force on Colorectal Cancer: Guidelines for colonoscopy
surveillance after screening and polypectomy: A consensus update by
the US multi-society task force on colorectal cancer.
Gastroenterology. 143:844–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Atkin WS, Valori R, Kuipers EJ, Hoff G,
Senore C, Segnan N, Jover R, Schmiegel W, Lambert R and Pox C:
International Agency for Research on Cancer: European guidelines
for quality assurance in colorectal cancer screening and diagnosis.
First Edition-Colonoscopic surveillance following adenoma removal.
Endoscopy. 44 Suppl 3:SE151–SE163. 2012.PubMed/NCBI
|
|
8
|
Tanaka S, Saitoh Y, Matsuda T, Igarashi M,
Matsumoto T, Iwao Y, Suzuki Y, Nishida H, Watanabe T, Sugai T, et
al: Evidence-based clinical practice guidelines for management of
colorectal polyps. J Gastroenterol. 50:252–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Butterly LF, Chaes MP, Pohl H and Fiarman
GS: Prevalence of clinically important histology in small adenomas.
Clin Gastroenterol Hepatol. 4:343–348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rex DK, Overhiser AJ, Chen SC, Cummings OW
and Ulbright TM: Estimation of impact of American college of
radiology recommendations on CT colonography reporting for
resection of high-risk adenoma findings. Am J Gastroenterol.
104:149–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su MY, Ho YP, Chen PC, Chiu CT, Wu CS, Hsu
CM and Tung SY: Magnifying endoscopy with indigo carmine contrast
for differential diagnosis of neoplastic and nonneoplastic colonic
polyps. Dig Dis Sci. 49:1123–1127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta N, Bansal A, Rao D, Early DS,
Jonnalagadda S, Wani SB, Edmundowicz SA, Sharma P and Rastogi A:
Prevalence of advanced histological features in diminutive and
small colon polyps. Gastrointest Endosc. 75:1022–1030. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuda T, Kawano H, Hisabe T, Ikematsu H,
Kobayashi N, Mizuno K, Oka S, Takeuchi Y, Tamai N, Uraoka T, et al:
Current status and future perspectives of endoscopic diagnosis and
treatment of diminutive colorectal polyps. Dig Endosc. 26 Suppl
2:S104–S108. 2014. View Article : Google Scholar
|
|
14
|
Kudo S, Tamura S, Nakajima T, Yamano H,
Kusaka H and Watanabe H: Diagnosis of colorectal tumorous lesions
by magnifying endoscopy. Gastrointest Endosc. 44:8–14. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kudo S, Rubio CA, Teixeira CR, Kashida H
and Kogure E: Pit pattern in colorectal neoplasia: Endoscopic
magnifying view. Endoscopy. 33:367–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi Y, Kudo SE, Miyachi H, Hosoya T,
Ikehara N, Ohtsuka K, Kashida H, Hamatani S, Hinotsu S and Kawakami
K: Clinical usefulness of pit patterns for detecting colonic
lesions requiring surgical treatment. Int J Colorectal Dis.
26:1531–1540. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wada Y, Kashida H, Kudo SE, Misawa M,
Ikehara N and Hamatani S: Diagnostic accuracy of pit pattern and
vascular pattern analyses in colorectal lesions. Dig Endosc.
22:192–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakamoto T, Saito Y, Nakajima T and
Matsuda T: Comparison of magnifying chromoendoscopy and narrow-band
imaging in estimation of early colorectal cancer invasion depth: A
pilot study. Dig Endosc. 23:118–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ and
Chiu CT: Comparative study of conventional colonoscopy,
chromoendoscopy, and narrow-band imaging systems in differential
diagnosis of neoplastic and nonneoplastic colonic polyps. Am J
Gastroenterol. 101:2711–2716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Q, Fukami N, Kashida H, Takeuchi T,
Kogure E, Kurahashi T, Stahl E, Kudo Y, Kimata H and Kudo SE:
Interobserver and intra-observer consistency in the endoscopic
assessment of colonic pit patterns. Gastrointest Endosc.
60:520–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
The Paris endoscopic classification of
superficial neoplastic lesions: Esophagus, stomach, and colon:
November 30 to December 1, 2002. Gastrointest Endosc. 58 6
Suppl:S3–S43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamilton SR and Aaltonen LA: WHO
Classification of Tumors: Pathology and Genetics of Tumors of the
Digestive System. IARC Press; Lyon: 2000
|
|
23
|
Singh H, Nugent Z, Demers AA and Bernstein
CN: Rate and predictors of early/missed colorectal cancers after
colonoscopy in Manitoba: A population-based study. Am J
Gastroenterol. 105:2588–2596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh H, Nugent Z, Mahmud SM, Demers AA
and Bernstein CN: Predictors of colorectal cancer after negative
colonoscopy: A population-based study. Am J Gastroenterol.
105:663–673; quiz 674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pickhardt PJ, Kim DH, Pooler BD, Hinshaw
JL, Barlow D, Jensen D, Reichelderfer M and Cash BD: Assessment of
volumetric growth rates of small colorectal polyps with CT
colonography: A longitudinal study of natural history. Lancet
Oncol. 14:711–720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oka S, Tanaka S, Nakadoi K, Asayama N and
Chayama K: Endoscopic features and management of diminutive
colorectal submucosal invasive carcinoma. Dig Endosc. 26 Suppl
2:S78–S83. 2014. View Article : Google Scholar
|
|
27
|
Matsuda T, Fujii T, Sano Y, Kudo S, Oda Y,
Igarashi M, Iishi H, Murakami Y, Ishikawa H, Shimoda T, et al:
Five-year incidence of advanced neoplasia after initial colonoscopy
in Japan: A multicenter retrospective cohort study. Jpn J Clin
Oncol. 39:435–442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ignjatovic A, East JE, Suzuki N, Vance M,
Guenther T and Saunders BP: Optical diagnosis of small colorectal
polyps at routine colonoscopy (Detect InSpect ChAracterise Resect
and Discard; DISCARD trial): A prospective cohort study. Lancet
Oncol. 10:1171–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rex DK, Kahi C, O'Brien M, Levin TR, Pohl
H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J and
Lieberman DA: The American society for gastrointestinal endoscopy
PIVI (preservation and incorporation of valuable endoscopic
innovations) on real-time endoscopic assessment of the histology of
diminutive colorectal polyps. Gastrointest Endosc. 73:419–422.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gupta N, Bansal A, Rao D, Early DS,
Jonnalagadda S, Edmundowicz SA, Sharma P and Rastogi A: Accuracy of
in vivo optical diagnosis of colon polyp histology by narrow-band
imaging in predicting colonoscopy surveillance intervals.
Gastrointest Endosc. 75:494–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sibon I and Orgogozo JM: Antiplatelet drug
discontinuation is a risk factor for ischemic stroke. Neurology.
62:1187–1189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blacker DJ, Wijdicks EF and McClelland RL:
Stroke risk in anticoagulated patients with atrial fibrillation
undergoing endoscopy. Neurology. 61:964–968. 2003. View Article : Google Scholar : PubMed/NCBI
|