Introduction
In the United States, 46,420 patients were diagnosed
with and 39,590 patients succumbed to pancreatic cancer in 2014
(1). The overall 5-year relative
survival rate for patients with pancreatic cancer was 6% (1). At present, surgery is the only curative
therapeutic approach. However, only 10–15% of patients with
pancreatic cancer are considered suitable candidates for resection
at the time of diagnosis (2). The
palliative surgical procedures, including splanchnicectomy, biliary
bypass and gastric bypass are frequently performed, with a median
survival time of 6 months (3,4).
To improve the therapeutic effectiveness and reduce
side effects of treatments, the use of novel treatment techniques,
including intraoperative interstitial brachytherapy, have been
investigated. In 1934, the implantation of a radium needle was
utilized in seven patients with pancreatic cancer (5) with one of these patients surviving up to
2 years. Hilaris and Rousiss (6)
reported one of the earliest experiences of radioactive Iodine-125
(125I) seed implantation for the treatment of pancreatic
cancer in 98 patients. The median survival time was 7 months, and
one patient survived up to 5 years (6). Subsequent case studies using
125I as the implanted isotope have reported median
survival times between 7–14 months (7–9).
Patients with pancreatic cancer have been
demonstrated to benefit from 125I seed implantation
(10,11) However, a controlled study has not been
previously reported. To investigate the efficacy and safety of
125I seed implantation in the treatment of locally
advanced unresectable pancreatic head cancer, a prospective
nonrandomized study was performed.
Materials and methods
Patients
A consecutive series of 68 patients with locally
advanced unresectable pancreatic head carcinoma diagnosed following
surgical examination were enrolled in the present study between
January 2009 and December 2012 at The Third People's Hospital of
Chengdu (Chengdu, China). Among them, 35 patients underwent a
combination of surgical bypass (biliary and gastric bypass) and
permanent 125I seed implantation (group A), and 33
patients underwent biliary and gastric bypass (group B). The
selection of treatment method used was based on the decision of
each patient. Prior to making a decision, the patients were
appropriately informed about treatment methods and the possible
complications.
Inclusion criteria were a Karnofsky performance
status score (12) of ≥70, an
anticipated survival of ≥3 months, ability to undergo follow-up
assessment and no history of previous anticancer treatment.
Exclusion criterion was the existence of distant metastases. The
current study was approved by the Ethics Committee of The Third
People's Hospital of Chengdu and written informed consent was
obtained from all patients. Data collected prior to surgery
included demographics, physical examination results, blood test
results, abdominal computed tomography (CT), pain score and quality
of life (QOL) assessment. The largest diameter reported from the CT
report was defined as the tumor size.
Definitions
Locally advanced unresectable pancreatic head
carcinoma was defined as pathologically proven local invasion of
major visceral vessels and no evidence of metastases demonstrated
during explorative surgery (13). To
diagnose and predict the severity of pancreatitis, the 2012
revision of the Atlanta Classification of acute pancreatitis was
used (14). Pancreatic fistula was
defined as a drain output of any measurable volume of fluid on or
following postoperative day 3 with an amylase content >3 times
the serum amylase activity (15).
Biliary fistula was defined as persistence of biliary drainage for
>5 days (16). Delayed gastric
emptying (DGE) was defined as nasogastric drainage for >10 days
or a delay from regular diet until 14 days postoperatively
(17).
Technique of 125I
implantation
At the time of exploratory laparotomy, elevation of
the duodenum (Kocher procedure) was necessary in order to
accurately assess the posterior margin of the tumor in the
pancreatic head. The tumor size was determined subsequent to
measuring three mutually perpendicular dimensions of the tumor
(18). The implanted volume included
the tumor size plus 0.5 cm of peripheral tissue. The expected
number of implanted seeds was calculated according to the Cevc
equation (19).
Following the histologically confirmed diagnosis of
pancreatic carcinoma using fine needle aspiration biopsy, the
needles (18-gauge, hollow, stainless steel) were implanted into the
tumor and spaced at parallel intervals of 1.0 cm, extending ≥0.5 cm
beyond the margins of the mass. The depth of needle placement was
monitored by feeling the tip of the needle with the operating
finger of the radiation oncologist. If bile, blood, or pancreatic
juice issued from the needle when the stylet was withdrawn
therefrom, the needle was retracted a number of millimeters and the
stylet was left in place until the time of 125I seed
insertion (20). A Mick-applicator
(Mick Radio-Nuclear Instruments, Inc., Mount Vernon, NY, USA) was
then attached to each needle and the seeds (Shanghai Xinke
Pharmaceutical Co., Ltd., Shanghai, China) were implanted at 1.0 cm
intervals while withdrawing the needle. To minimize the dose to the
adjacent stomach and bowel and prevent pancreatic fistula, a
segment of omentum was placed over the implanted surface of the
pancreas. A median number of 27 seeds/patient (range, 20–39 seeds)
were implanted. During the surgery, surgeons wore lead aprons and
lead gloves. The exposure dose was measured using a personal
dosimeter worn on the chest.
Surgical procedure
All the patients underwent retrocolic
gastrojejunostomy and choledochojejunostomy regardless of
125I implantation. For the patient without the symptoms
and signs of duodenal obstruction and jaundice, prophylactic bypass
was performed. A total of 7 patients with severe malnutrition
underwent feeding jejunostomies. Patients in group A received
somatostatin analogues in order to prevent pancreatitis and
pancreatic fistula development. All patients were recommended to
undergo postoperative chemotherapy or radiotherapy. For different
reasons, only eight patients received chemotherapy consisted of
gemcitabine. The other patients refused to receive the
postoperative treatment.
Follow-up
Patients were observed monthly during the first year
following surgery and then at three month intervals. Evaluations
during the follow-up included physical examinations, blood tests,
chest X-ray, abdominal CT scan, QOL and pain score. During the
follow-up, collection of the patients' opinions was performed by a
doctor who was blinded to the study. The mean follow-up time was
11±6 months. Survival time was defined as the time span between
initial treatment and mortality or loss of contact. Compliance was
defined as the number of patients who completed the questionnaire
expressed as a proportion of the number of patients alive.
Response criteria
Response was evaluated according to the World Health
Organization criteria (21). A
complete response (CR) was defined as the disappearance of all
known lesions, without appearance of new lesions for ≥4 weeks. A
partial response (PR) was defined as ≥50% decrease in the maximum
transverse tumor measurements, with no appearance of new lesions on
two observations 4 weeks apart. No change (NC) was defined as
<50% decrease and <25% increase in the size of measurable
lesions. Progressive disease (PD) was defined as ≥25% increase in
the size of one or more measurable lesions or the appearance of new
lesions. Time to progression was determined as the interval between
the date of first treatment and the date at which PD was first
observed.
Quality of life
For prospective measurement of QOL, the standard
Chinese version of the European Organization for Research and
Treatment of Cancer Quality of Life Core Questionnaire (QLQ-C30;
version 3.0) was used (22). The
QLQ-C30 consists of 30 items pertaining to 5 functional scales,
symptoms and global quality of life (22). Its feasibility has previously been
validated for patients in China (23).
Baseline measurements were performed prior to
surgery. Subsequent questionnaires were completed at 1, 3, 6, 9 and
12 months following surgery. In accordance with Heek et al
(24), the global health status,
physical and emotional functioning and all gastrointestinal (GI)
symptom scales of the QLQ-C30 provide the appropriate information.
Therefore, an overall digestive symptom scale including nausea and
vomiting, appetite loss, constipation and diarrhea was created.
Pain score
Pain intensity was quantified using a specially
designed pain score (25), including
two subjective items, the patient's self-estimation of intensity of
pain using a visual analog scale and the frequency of pain attacks,
and two objective items, analgesic medication taken and the time
periods of inability to work. The sum of the median values divided
by 4 provided the final pain score.
Statistical analysis
Data are expressed as the mean ± standard deviation
or number and percentage. All statistical analyses were performed
with SPSS (version 16.0; SPSS Inc., Chicago, IL, USA).
χ2 or Fisher's exact tests were applied for categorical
data and the Mann-Whitney U test was used for numerical data. The
Kaplan-Meier estimator method was used to analyze survival and
levels of significance were determined with the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Study population
In group A, one patient was uncontactable during the
follow-up. In group B, one patient was excluded from the analysis
as the final pathology of the intraoperative specimen revealed a
benign tumor. From the remaining 66 patients, 34 patients (52%)
underwent a combination of surgical bypass and permanent
125I seed implantation, and 32 patients (48%) underwent
biliary and gastric bypass. The characteristics of the patients
included in the study were similar across the two groups (Table I).
 | Table I.Clinicopathological characteristics of
patients with locally advanced unresectable pancreatic head
carcinoma. |
Table I.
Clinicopathological characteristics of
patients with locally advanced unresectable pancreatic head
carcinoma.
|
| Value |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | Group A, n=34 | Group B, n=32 | P-value |
|---|
| Age,
yearsa | 56±9 | 57±10 |
0.878 |
| Genderb |
|
|
|
|
Male | 21 (62) | 18 (56) |
0.649 |
|
Female | 13 (38) | 14 (44) |
|
| Preoperative
symptomsb |
|
|
|
|
Abdominal pain | 25 (74) | 27 (84) |
0.281 |
|
Jaundice | 32 (94) | 31 (97) | >0.999 |
| Weight
loss | 31 (91) | 28 (88) |
0.705 |
| KPSa | 81±7 | 78±6 |
0.107 |
|
Bilirubin, mg/dla | 15±5 | 14±6 |
0.792 |
|
CA19-9a | 567±286 | 600±307 |
0.768 |
| TNM
Stageb |
|
|
|
|
T3N0-1M0 | 11 (32) | 15 (47) |
0.228 |
|
T4N0-1M0 | 23 (68) | 17 (53) |
|
| Tumor size,
mma | 43±6 | 42±6 |
0.566 |
Postoperative complications and
hospitalization stay
Mortality, morbidity and length of hospital stay are
described in Table II. No mortality
occurred during the perioperative period in the two groups. In
group A, one patient had mild acute pancreatitis that was resolved
with the use of somatostatin analogues. The incidence of DGE was
not significantly influenced by 125I seed implant. All
patients with postoperative DGE were successfully treated
conservatively. There were two pancreatic fistulas in group A, and
one biliary and one GI fistula in group B. All the fistulas were
treated without surgery. In each group, one patient required
re-exploration for significant anastomotic bleeding. The duration
of stay in hospital was 15±4 days in group A and 13±3 days in group
B (P=0.104). During the follow-up, two patients in group A were
diagnosed with gastric ulcer. In addition, one patient in group A
had two seeds and another patient had three seeds that migrated to
the liver.
 | Table II.Postoperative complications and
length of hospital stay of patients with locally advanced
unresectable pancreatic head carcinoma. |
Table II.
Postoperative complications and
length of hospital stay of patients with locally advanced
unresectable pancreatic head carcinoma.
|
| Value |
|
|---|
|
|
|
|
|---|
| Postoperative
characteristics | Group A, n=34 | Group B, n=32 | P-value |
|---|
|
Pancreatitisa | 1 (3) | 0 (0) | 1.0 |
| GI
bleedinga | 1 (3) | 1 (3) | 1.0 |
| Pancreatic
fistulaa | 2 (6) | 0 (0) |
0.493 |
| GI
fistulaa | 0 (0) | 1 (3) |
0.493 |
| Biliary
fistulaa | 0 (0) | 1 (3) |
0.493 |
| DGEa | 5 (15) | 2 (6) |
0.427 |
| Gastric
ulcera | 2 (6) | 0 (0) |
0.493 |
| Length of hospital
stay, daysb | 15±4 | 13±3 |
0.104 |
Response
The tumor responses are presented in Table III. In group A, there were no cases
of CR. A total of 19 patients presented with PR and NC was observed
in 7 patients. PD was observed in 8 patients, and all presented
with extra-pancreatic metastases. In addition, 6 patients presented
with local progression with increase in the size of the primary
tumor mass. In group B, the overall response rate was 0%. The tumor
was rated stable in 5 patients, and the other patients developed
extrapancreatic metastases and simultaneous increase in the size of
the pancreatic mass. The mean time until disease progression was
significantly increased in group A compared to group B (8±1 vs. 5±2
months; P<0.001).
 | Table III.Response to treatment of patients
with locally advanced unresectable pancreatic head carcinoma. |
Table III.
Response to treatment of patients
with locally advanced unresectable pancreatic head carcinoma.
|
| Value |
|
|---|
|
|
|
|
|---|
| Response | Group A (n=34) | Group B (n=32) | P-value |
|---|
|
Completea | 0 (0) | 0 (0) | 0.806 |
|
Partiala | 19 (56) | 0 (0) | 0.000 |
| No
changea | 7 (21) | 5 (16) | 0.427 |
|
Progressiona | 8 (24) | 27 (84) | 0.013 |
| Time to
progression, monthsb | 8±1 | 5±2 | <0.0001 |
Survival
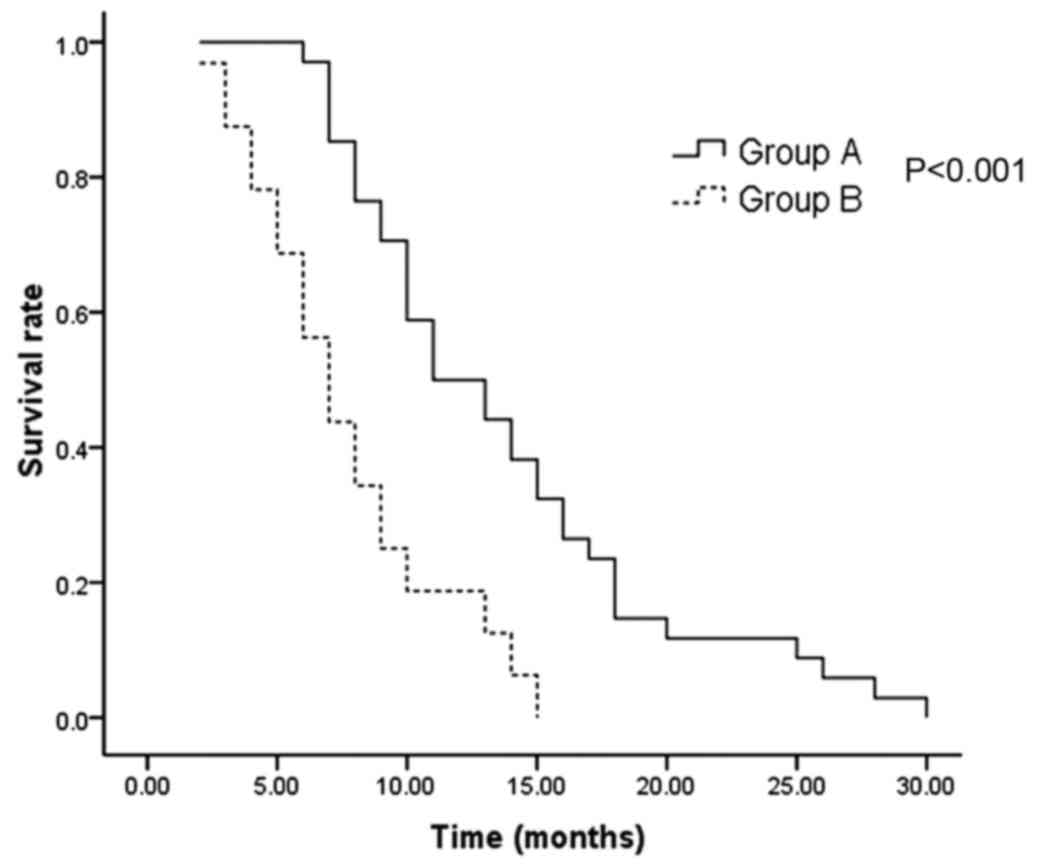
The median survival time was significantly longer in
group A compared to the patients in group B (11 vs. 7 months;
P<0.001). (Fig. 1). The 1 and
2-year survival rates were 50 and 12%, respectively in group A, as
opposed to 19 and 0%, respectively in group B.
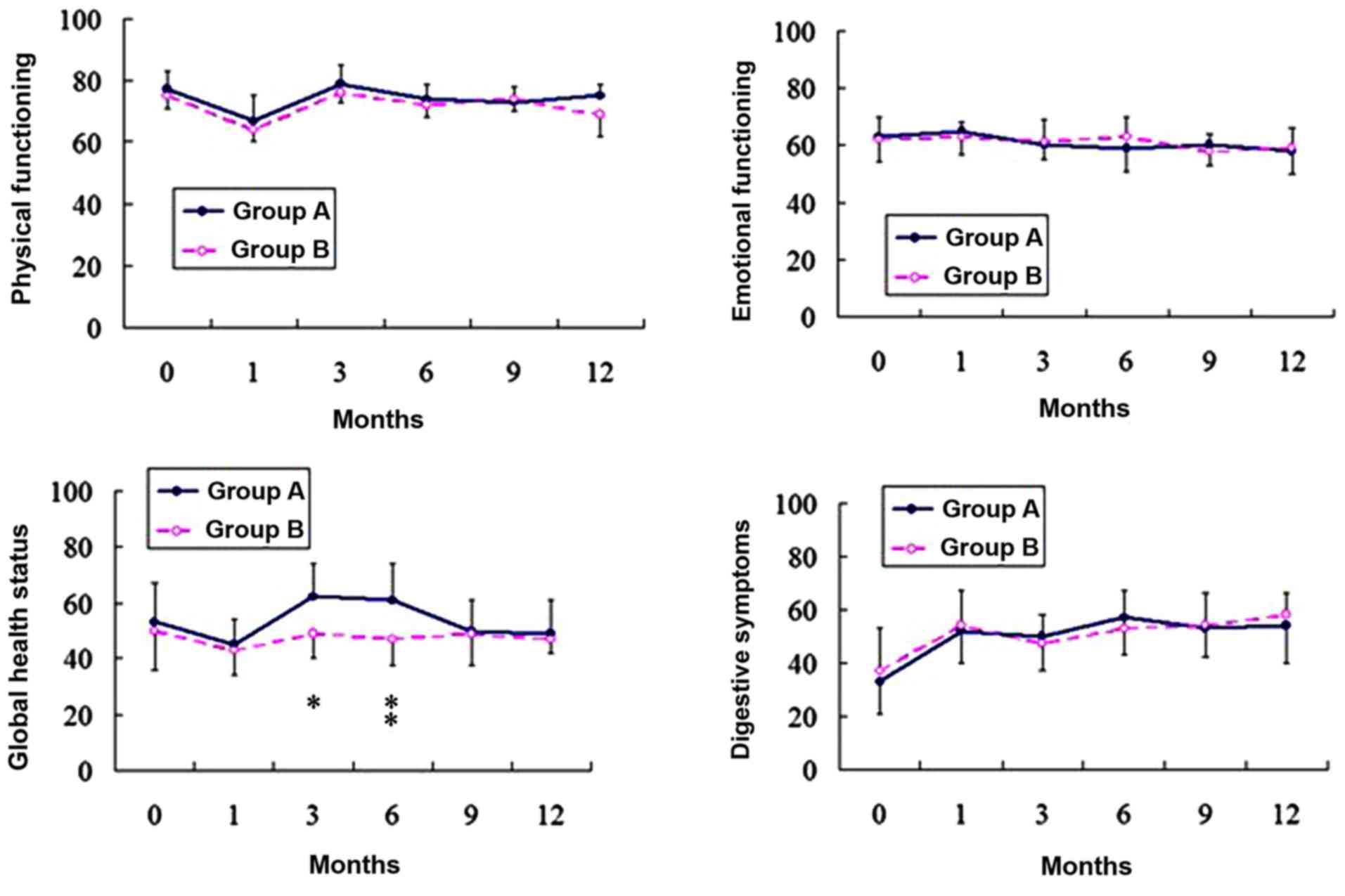
Quality of life
Compliance with questionnaire completion was
comparable in the two groups (Table
IV). Data on the QOL scales from the questionnaires were
plotted graphically. Representative graphs are illustrated in
Fig. 2 for each aspect of QOL. Prior
to surgery, the patients in the two groups were comparable with
respect to all scales. At 1 month following surgery, the two groups
revealed a significant decrease in physical functioning compared to
the preoperative status, but this had returned to preoperative
values by 3 months' post-surgery. There were no significant changes
in emotional functioning following surgery in either of the two
groups. Global health status decreased following surgery in group
B, however this decrease was not statistically significant. Global
health status decreased in group A at 1 month following surgery.
This values of this status improved between 3 and 6 months
following surgery, and 6 months later it had returned to the
preoperative status. The digestive symptoms were significantly more
pronounced following the two surgical procedures.
 | Table IV.Compliance to questionnaires during
the follow-up of patients with locally advanced unresectable
pancreatic head carcinoma. |
Table IV.
Compliance to questionnaires during
the follow-up of patients with locally advanced unresectable
pancreatic head carcinoma.
|
| No. of patients
(%) |
|
|---|
|
|
|
|
|---|
| Months following
surgery | Group A | Group B | P-value |
|---|
| Baseline | 34/34 (100) | 32/32
(100) | 0.806 |
| 1 | 30/34 (88) | 27/32 (84) | 0.122 |
| 3 | 32/34 (94) | 24/28 (86) | 0.233 |
| 6 | 25/33 (76) | 13/18 (72) | 0.955 |
| 9 | 19/24 (79) | 5/8
(63) | 0.116 |
| 12 | 12/17 (71) | 3/6
(50) | 0.621 |
Pain score
Fig. 3 illustrates a
graphic description of the pain scores. No significant differences
were observed in pain intensity prior to surgery between the two
groups. The pain scores were stable over the course of the study
for patients in group B. For patients in group A, a significant
reduction was observed in pain scores that persisted for 9 months
following surgery. At one month following surgery, the pain score
indicated a 51% reduction from the baseline in group A (49±20 vs.
24±10, respectively; P<0.001). A total of 3 patients with severe
pain who were completely relieved from pain had no pain recurrence
prior to mortality.
Discussion
In the present study, 3 and 6% patients suffered
from pancreatitis and pancreatic fistulas following surgery in
group A, respectively. To minimize postoperative complications,
certain measures are used, including intraoperative ultrasound
guidance, suturing of pinholes, a segment of omentum placed over
the implanted surface of the pancreas and somatostatin analogue
treatment following surgery. There was no statistically significant
difference in morbidity rates between the two groups. It was
demonstrated that the implantation of 125I seeds did not
increase the duration of hospital stay. In addition, the results of
the present study suggested that 125I seed implantation
for unresectable pancreatic head cancer is feasible and safe.
Ma et al (26)
revealed that 125I seed implantation effectively
inhibited pancreatic tumor growth and reduced tumor volume.
125I irradiation-induced apoptosis and DNA
hypomethylation are two important mechanisms underlying the
therapeutic effect of low-energy 125I seed implantation.
In the present study, it was identified that 125I seed
implantation provided more improved tumor responses, however the CR
rate was identified to be 0% in group A. Zou et al (27) demonstrated that intraoperative
radiofrequency ablation combined with 125I seed
implantation is an effective procedure for the treatment of
unresectable pancreatic cancer. The rate of CR and PR was 21.8 and
56.3%, respectively. Jin et al (28) performed a study on 22 patients with
advanced pancreatic cancer who underwent endoscopic
ultrasound-guided interstitial implantation of 125I
seeds combined with routine gemcitabine-based fluorouracil
chemotherapy. Rates of complete and partial remission in the 22
patients were reported as 0 and 13.6%, respectively. The lower rate
of overall response was attributed to 18 patients with tumor stage
III–IV, according to the International Union Against Cancer (UICC)
classifications for pancreatic cancer set up in 2002 (29).
In the present study, it was demonstrated that
125I seed implantation was beneficial for the extension
of survival. Wang et al (11)
reported that the median survival time for 125I seed
implantation alone was 7 months. Du et al (30) reported the long-term effect of
gemcitabine-combined endoscopic ultrasonography-guided
125I seed implantation in pancreatic cancer. It was
demonstrated that the median survival time was 4 months in the seed
implantation-only group. The median survival times of the two
studies (11,30) described are shorter compared to the
results of the present study. These differences may be due to a
higher proportion of patients with non-metastatic locally advanced
tumors in the present study.
The aim of treatment for patients with unresectable
pancreatic cancer is to improve the quality of their remaining life
(31). In the present study, the
physical function and global health status were demonstrated to
decrease following surgery in the two groups. The scores recovered
to preoperative levels of QOL within 3 months following surgery.
The only exception was global health status, which remained stable
in group B. On the symptom scale, digestive symptoms worsened in
the two groups. This result may be attributed to: The
reconstruction of the digestive tract changes the normal anatomy of
upper gut; and/or the radiation of 125I seeds having a
negative effect on adjacent organs.
In the present study, a pain score was calculated
using a visual analog scale of pain, frequency of pain attacks and
pain-associated sick leave. In addition, analgesic medication was
applied to quantify pain intensity more distinctly. It was
demonstrated that 125I seeds implantation resulted in
more precise pain relief.
With the development of therapeutic methods, biliary
and digestive stenoses can be endoscopically treated in patients
with unresectable pancreatic cancer. However, Ueda et al
(32) compared palliative surgical
biliary bypass to endoscopic biliary stenting for unresectable
pancreatic cancer, whereby a lower morbidity, lower mortality and
more effective long-term palliation was demonstrated in the
surgical biliary bypass group. Prophylactic surgical biliary bypass
with gastrointestinal bypass may be a good treatment option for
non-jaundiced patients undergoing chemotherapy for unresectable
pancreatic cancer. Randomized controlled trials have shown
prophylactic gastrojejunostomy to significantly decrease the
incidence rate of late gastric outlet obstruction without altering
the postoperative mortality or morbidity rates, or prolonging
hospital stay compared to biliary bypass alone (24,33). Mann
et al (34) demonstrated that
surgical combined biliary and gastric bypass offers effective
long-term palliation of biliary and gastric outlet obstruction in
patients with unresectable malignant disease. Low mortality and
morbidity rates suggest that this should be used as a first line
therapy in patients who are considered unresectable at
laparotomy.
In conclusion, the results of the present study
suggest that brachytherapy using 125I seed implantation
is feasible, safe and effective for the treatment of patients with
unresectable pancreatic cancer. Brachytherapy using 125I
seed implantation provides satisfactory QOL and produces adequate
pain relief.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
von Wichert G, Seufferlein T and Adler G:
Palliative treatment of pancreatic cancer. J Dig Dis. 9:1–7. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Pimpec Barthes F, Chapuis O, Riquet M,
Cuttat JF, Peillon C, Mouroux J and Jancovici R: Thoracoscopic
splanchnicectomy for control of intractable pain in pancreatic
cancer. Ann Thorac Surg. 65:810–813. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isla AM, Worthington T, Kakkar AK and
Williamson RC: A continuing role for surgical bypass in the
palliative treatment of pancreatic carcinoma. Dig Surg. 17:143–146.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Handley WS: Pancreatic cancer and the
treatment by implanted radium. Ann Surg. 100:215–222. 1934.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hilaris B and Rousiss K: Cancer of the
pancreasHilaris BS: Handbook of interstitial brachytherapy. Acton,
Mass: Publishing Sciences Group (PSG); pp. 251–262. 1975
|
|
7
|
Syed AM, Puthawala AA and Neblett DL:
Interstitial iodine-125 implant in the management of unresectable
pancreatic carcinoma. Cancer. 52:808–813. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Jiang Y, Li J, Tian S, Ran W and
Xiu D: Intraoperative ultrasound-guided iodine-125 seed
implantation for unresectable pancreatic carcinoma. J Exp Clin
Cancer Res. 28:882009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whittington R, Solin L, Mohiuddin M,
Cantor RI, Rosato FE, Biermann WA, Weiss SM and Pajak TF:
Multimodality therapy of localized unresectable pancreatic
adenocarcinoma. Cancer. 54:1991–1998. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhongmin W, Yu L, Fenju L, Kemin C and
Gang H: Clinical efficacy of CT-guided iodine-125 seed implantation
therapy in patients with advanced pancreatic cancer. Eur Radiol.
20:1786–1791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Wang J, Jiang Y, Li J, Tian S, Ran
W, Xiu D and Gao Y: The investigation of 125I seed implantation as
a salvage modality for unresectable pancreatic carcinoma. J Exp
Clin Cancer Res. 32:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yates JW, Chalmer B and Mckegney FP:
Evaluation of patients with advanced cancer using the karnofsky
performance status. Cancer. 45:2220–2224. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verslype C, Van Cutsem E, Dicato M,
Cascinu S, Cunningham D, Diaz-Rubio E, Glimelius B, Haller D,
Haustermans K, Heinemann V, et al: The management of pancreatic
cancer. Current expert opinion and recommendations derived from the
8th World congress on gastrointestinal cancer, Barcelona, 2006. Ann
Oncol. 18 Suppl 7:vii1–vii10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group: Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bassi C, Dervenis C, Butturini G,
Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W
and Buchler M; International Study Group on Pancreatic Fistula
Definition, : Postoperative pancreatic fistula: An international
study group (ISGPF) definition. Surgery. 138:8–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murakami Y, Uemura K, Hayashidani Y, Sudo
T, Hashimoto Y, Nakagawa N, Ohge H and Sueda T: No mortality after
150 consecutive pancreatoduodenctomies with duct-to-mucosa
pancreaticogastrostomy. J Surg Oncol. 97:205–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park YC, Kim SW, Jang JY, Ahn YJ and Park
YH: Factors influencing delayed gastric emptying after
pylorus-preserving pancreatoduodenectomy. J Am Coll Surg.
196:859–865. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bodner WR and Hilaris BS: Brachytherapy
and pancreatic cancer. Semin Surg Oncol. 13:204–207. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Monk BJ, Tewari KS, Puthawala AA, Syed Am,
Haugen JA and Burger RA: Treatment of recurrent gynecologic
malignancies with iodine-125 permanent interstitial irradiation.
Int J Radiat Oncol Biol Phys. 52:806–815. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dobelbower RR Jr, Merrick HW III, Ahuja RK
and Skeel RT: 125I interstitial implant, precision high-dose
external beam therapy, and 5-FU for unresectable adenocarcinoma of
pancreas and extrahepatic biliary tree. Cancer. 58:2185–2195. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aaronson NK, Ahmedzai S, Bergman B,
Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman
SB, de Haes JC, et al: The European Organization for Research and
Treatment of Cancer QLQ-C30: A quality-of-life instrument for use
in international clinical trials in oncology. J Natl Cancer Inst.
85:365–376. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao H and Kanda K: Testing psychometric
properties of the standard Chinese version of the European
Organization for Research and Treatment of Cancer Quality of Life
Core Questionnaire 30 (EORTC QLQ-C30). J Epidemiol. 14:193–203.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Heek NT, De Castro SM, van Eijck CH,
van Geenen RC, Hesselink EJ, Breslau PJ, Tran TC, Kazemier G,
Visser MR, Busch OR, et al: The need for a prophylactic
gastrojejunostomy for unresectable periampullary cancer: A
prospective randomizedmulticenter trial with special focus on
assessment of quality of life. Ann Surg. 238:894–905. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bloechle C, Izbicki JR, Knoefel WT,
Kuechler T and Broelsch CE: Quality of life in chronic
pancreatitis: Results after duodenum-preserving resection of the
head of the pancreas. Pancreas. 11:77–85. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma JX, Jin ZD, Si PR, Liu Y, Lu Z, Wu HY,
Pan X, Wang LW, Gong YF, Gao J and Zhao-shen L: Continuous and
low-energy 125I seed irradiation changes DNA methyltransferases
expression patterns and inhibits pancreatic cancer tumor growth. J
Exp Clin Cancer Res. 30:352011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou YP, Li WM, Zheng F, Li FC, Huang H, Du
JD and Liu HR: Intraoperative radiofrequency ablation combined with
125 iodine seed implantation for unresectable pancreatic cancer.
World J Gastroenterol. 16:5104–5110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin Z, Du Y, Li Z, Jiang Y, Chen J and Liu
Y: Endoscopic ultrasonography-guided interstitial implantation of
iodine 125-seeds combined with chemotherapy in the treatment of
unresectable pancreatic carcinoma: a prospective pilot study.
Endoscopy. 40:314–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Freelove R and Walling AD: Pancreatic
cancer: Diagnosis and management. Am Fam Physician. 73:485–492.
2006.PubMed/NCBI
|
|
30
|
Du Y, Jin Z, Meng H, Zou D, Chen J, Liu Y,
Zhan X, Wang D, Liao Z and Li Z: Long-term effect of
gemcitabine-combined endoscopic ultrasonography-guided
brachytherapy in pancreatic cancer. J Interv Gastroenterol.
3:18–24. 2013. View
Article : Google Scholar
|
|
31
|
Kostro J and Sledziński Z: Quality of life
after surgical treatment of pancreatic cancer. Acta Chir Belg.
108:679–684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ueda J, Kayashima T, Mori Y, Ohtsuka T,
Takahata S, Nakamura M and Tanaka M: Hepaticocholecystojejunostomy
as effective palliative biliary bypass for unresectable pancreatic
cancer. Hepatogastroenterology. 61:197–202. 2014.PubMed/NCBI
|
|
33
|
Lillemoe KD, Cameron JL, Hardacre JM, Sohn
TA, Sauter PK, Coleman J, Pitt HA and Yeo CJ: Is prophylactic
gastrojejunostomy indicated for unresectable periampullary
carcinoma? A prospective randomized trial. Ann Surg. 230:322–330.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mann CD, Thomasset SC, Johnson NA, Garcea
G, Neal CP, Dennison AR and Berry DP: Combined biliary and gastric
bypass procedures as effective palliation for unresectable
malignant disease. ANZ J Surg. 79:471–475. 2009. View Article : Google Scholar : PubMed/NCBI
|