Introduction
The incidence of thyroid carcinoma has increased
rapidly over the past 3 decades (1).
However, the reason for this increase in incidence remains to be
elucidated (2). Certain studies have
reported that this increase may be due to the high detection rate
resulting from the increasing prevalence of thyroid ultrasonography
and subsequent fine-needle aspiration cytology (3–5). A
previous study suggested that the number of teenagers (<20
years) with the disease, in which screening for thyroid carcinoma
is not performed, is increasing (6).
Papillary thyroid carcinoma (PTC) is the primary subtype of thyroid
cancer that has contributed to this increased incidence globally.
The etiological factors for the development of papillary thyroid
carcinoma include exogenous factors, including radiation exposure,
high iodine intake, Hashimoto's thyroiditis and obesity, and
endogenous factors, such as certain gene mutations that have been
demonstrated to serve a key role in progression of thyroid
tumorigenesis (7–9). The incidence of PTC in females is more
than twice than in males, which may be due to the fact that
estrogen may promote the proliferation of thyroid carcinoma cells
(10). Although there have been a
number of hypotheses regarding thyroid tumorigenesis, further study
is required.
Lysosomal protein transmembrane 4-β (LAPTM4B) is
typically highly expressed in hepatocellular carcinoma in
comparison with normal liver cells (11). LAPTM4B is located on chromosome 8q22.1
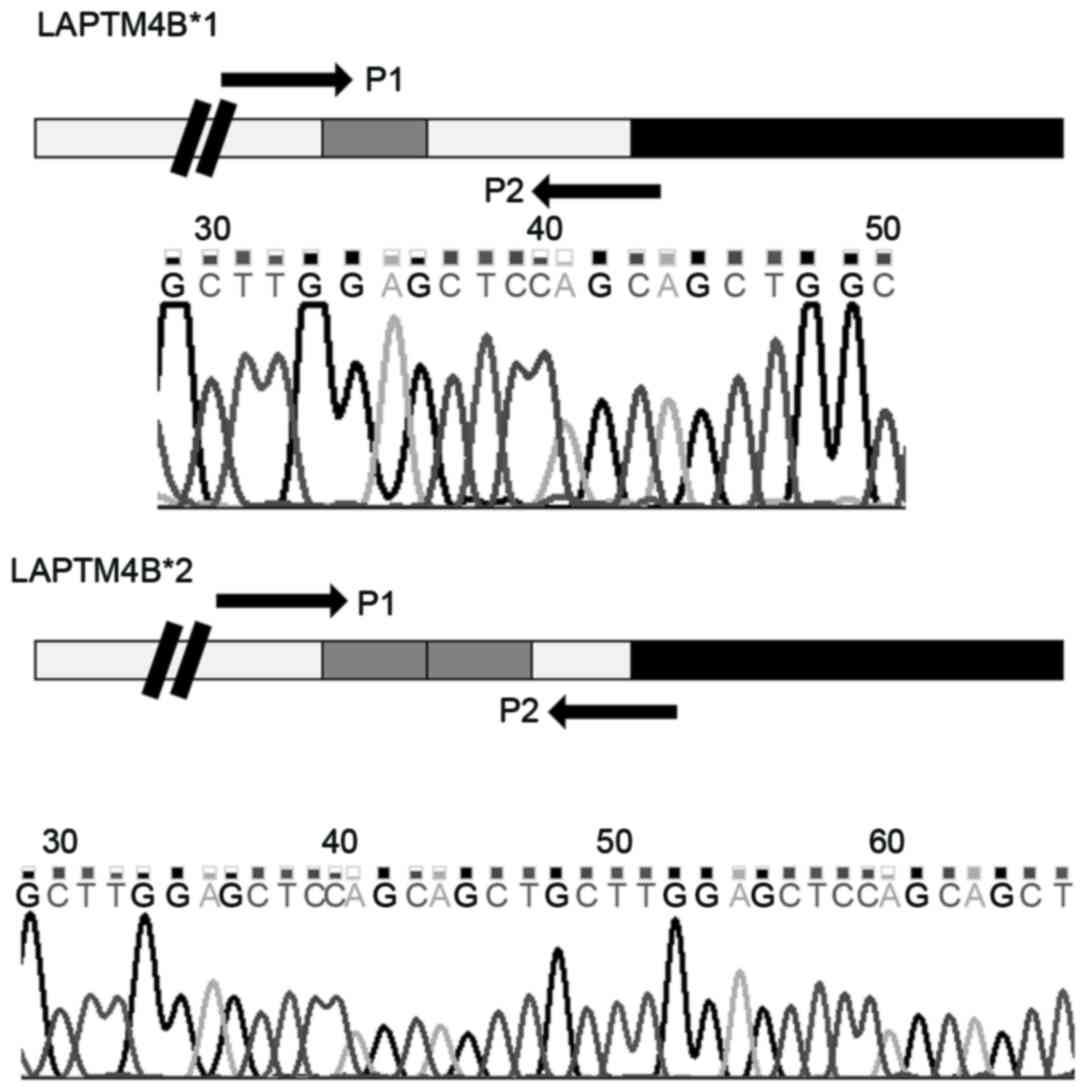
and includes 7 exons and 6 introns (12). In total two alleles of LAPTM4B have
been identified, termed LAPTM4B*1 allele and LAPTM4B*2 allele
(12). The difference between these
alleles is that LAPTM4B*2 has an extra 19 base pairs (bp) sequence
arranged in tandem at the 5′untranslated region (UTR) in exon 1
(12). The two alleles generate three
potential genotypes: LAPTM4B*1/1; LAPTM4B*1/2; LAPTM4B*2/2
(12).
Previous studies have established that LAPTM4B gene
polymorphism is associated with tumor susceptibility; notably,
LAPTM4B*2 is associated with a high risk of breast (13), stomach (14), lung (15), colon (16), endometrial (17), cervical (18), gall bladder (19), ovarian cancer and melanoma (20,21).
Nevertheless, no significant difference in the frequency of
LAPTM4B*2 is observed in patients with nasopharyngeal carcinoma and
esophageal carcinoma, compared with corresponding controls
(16,22). These results suggest that LAPTM4B*2
may be specifically associated with susceptibility to certain
tumors. Papillary thyroid carcinoma is a well-differentiated
carcinoma that has not yet been associated with LAPTM4B gene
polymorphism. Therefore, in the present study, a case-control assay
was performed to assess LAPTM4B gene polymorphism association with
susceptibility to PTC in the female Chinese population. A
dual-luciferase reporter assay was also performed in two types of
thyroid papillary cells to assess the transcriptional activity of
LAPTM4B*1 compared with LAPTM4B*2.
Materials and methods
Patients and tissue samples
Blood samples were collected in EDTA-containing
tubes from 183 patients with PTC who underwent surgical resection
at Beijing Cancer Hospital Affiliated with Peking University
(Beijing, China) from April 2015 to September 2015 and frozen at
−20°C. Each patient provided informed consent for participation in
the present study. Tumor-Node-Metastasis (TNM) staging was
performed by Yue Meng for each patient following standard TNM
guidelines (23). Clinicopathological
characteristics that were evaluated included sex, age, tumor size,
tumor location, body mass index (BMI), tumor invasive condition and
central lymph node status. The clinicopathological features of
female patients also included menopause and the number of
pregnancies. The data of healthy control patients was obtained from
previously published studies by Cheng et al (16) and Liu et al (14) and the consent for these data was
obtained at the time of initial data retrieval.
DNA extraction and polymerase chain
reaction (PCR)
Frozen blood samples were thawed in a water bath at
room temperature and genomic DNA was extracted from 600 µl of each
blood sample using a DNA extraction kit (Tiangen Biotech Co.,
Beijing, China; cat. no., DP304-03), according to the
manufacturer's protocol. For each sample, the genotype of LAPTM4B
was identified by PCR, using the following primers: forward,
5′-GCCGACTAGGGGACTGGCGGA-3′; and reverse,
5′-CGAGAGCTCCGAGCTTCTGCC-3′. The PCR mixture (25 µl) contained 2X
PCR Taq mix (12.5 µl; KT-201; Tiangen Biotech Co., Beijing, China),
1 µl template DNA at final concentration 100 ng/µl, 0.5 µl β-actin
forward primer at a final concentration 5 µM, 0.5 µl β-actin
reverse primer at a final concentration 5 µM, 1 µl LAPTM4B forward
primer at a final concentration 10 µM, 1 µl LAPTM4B reverse primer
at a final concentration 10 µM and 9.5 µl ddH2O.0.5 U Taq
polymerase (Tiangen Biotech Co.) and 1 µl template DNA at a final
concentration 100 ng/µl. Human β-actin was used as an internal
positive control using the following primers: Forward,
5′-TCACCAACTGGGACGACAT-3′; and reverse, 5′-AGGTAGTCAGTCAGGTCCCG-3′.
The PCR conditions were 95°C denaturation for 5 min, 30 cycles of
30 sec each at 94°C, 30 sec at 64.1°C and 30 sec at 72°C, followed
by extension at 72°C for 5 min. The PCR products were analyzed by
electrophoresis using a 2.5% agarose gel and visualized with
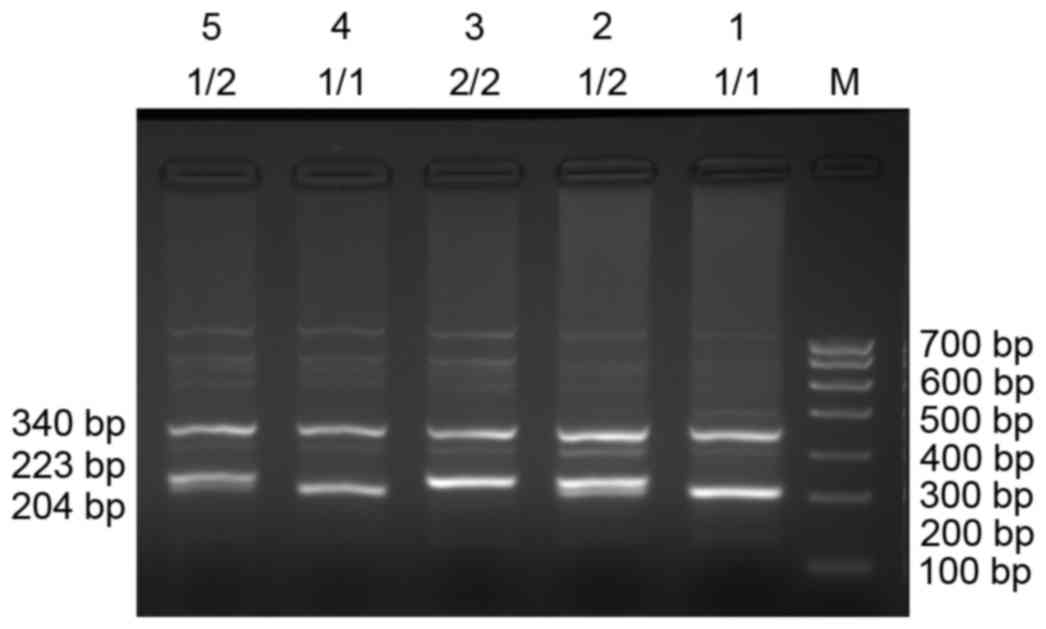
ethidium bromide. The LAPTM4B*1/1 genotype produced a 204 bp band,
the LAPTM4B*2/2 genotype produced a 223 bp band. The LAPTM4B*1/2
genotype produced 204 and 223-bp bands. The products of PCR were
sent to SBS Genetech Co., Ltd (Beijing, China) for sequencing.
Cells and culture conditions
Human PTC TPC-1 and B-CPAP cell lines, were
maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and penicillin streptomycin
solution (cat. no., SV30010; HyClone; GE Healthcare Life Sciences,
Little Chalfont, UK) at 1X working concentration. The cells were
cultured at 37°C in a 5% CO2 atmosphere. The cells were
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China).
Transient transfection and dual
luciferase reporter assay
The PTC cells were seeded into 24-well plates
(1×105 cells/well), and incubated for 12 h at 37°C in
complete medium. The TPC1 and B-CPAP cells were transfected with
0.5 µg of PGL-3 reporter constructs with a number of LAPTM4B
promoter inserts and pGL-3 promoter vector; all truncated plasmids
were conserved by the laboratory [Key Laboratory of Carcinogenesis
and Translational Research (Ministry of Education), Department of
Clinical Laboratory, Peking University Cancer Hospital &
Institute, Beijing, China] (24).
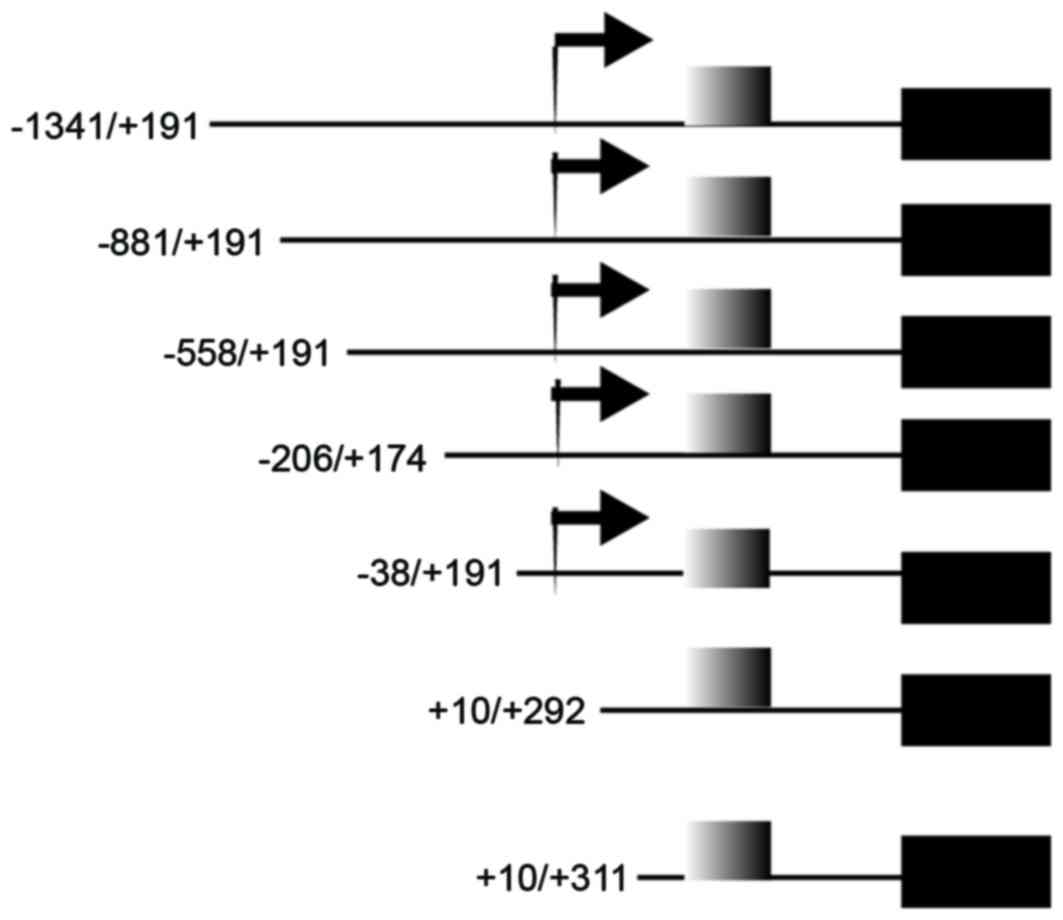
Different regions of LAPTM4B promoter were inserted to PGL-3
reporter constructs: −1341/+191, −881/+191, −558/+191, −206/+174,
−38/+191, +1−/+292 and +10/+311. A 0.02-µg phRL-CMV plasmid (E6271;
Promega Corporation, Madison, WI, USA) was transfected in each well
at the same time to normalize for the transfection efficiency.
Transfection was performed using Lipofectamine 3000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. At 48 h following transfection, cell
lysates were collected for luciferase activity detection using the
DualGlo Luciferase Assay system (Promega Corporation), according to
the manufacturer's protocol. All reactions were performed in
triplicate and results were normalized to the Renilla
luciferase activity. The luciferase value of all the plasmids was
compared with that of the pGL-3 promoter (positive control), which
was set as 100%.
Statistics analysis
All data were analyzed using SPSS v22.0 software
(IBM Corp., Armonk, NY, USA). Genotypic frequencies were tested for
Hardy-Weinberg equilibrium using the χ2 test. Fisher's
exact test was used to calculate the genotypic frequency and other
parametric distributions between cancer cases and controls.
Associations between genotypes and the risk of thyroid papillary
cancer were estimated using odds ratios (ORs) and 95% confidence
intervals (CIs), which were computed by using unconditional
logistic regression models. All ORs were adjusted for age and sex,
as appropriate. A one-way analysis of variance was applied for the
comparison of luciferase report assay data, followed by a least
significant difference (LSD) multiple-range post-hoc test to detect
significant differences between different groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
LAPTM4B genotypes
The three different genotypes were identified using
PCR: LAPTM4B*1/1, homozygotes; LAPTM4B*2/2, homozygotes; and
LAPTM4B*1/2 heterozygotes (Fig. 1).
The LAPTM4B*1/1 genotype was identified by the presence of a 204-bp
band, the LAPTM4B*2/2 genotype was identified by the presence of a
223-bp band. The presence of 204-bp and 223-bp bands together
identified the heterozygotic LAPTM4B*1/2 genotype. The human
β-actin PCR product was a 340-bp band that appeared in all internal
controls. PCR products were sequenced and the results are presented
in Fig. 2.
LAPTM4B genotypes and PTC
susceptibility
The frequency of LAPTM4B genotypes of 183 patients
(P=0.37) and 697 health controls (P=0.352) were in accordance with
Hardy-Weinberg equilibrium distribution. There were statistically
significant differences in age and sex between patients with PTC
and healthy controls in the present study (Table I). Following OR adjustments by sex and
age, the risk of PTC in patients with the LAPTM4B*2/2 genotype was
identified to be 2.765-fold higher compared with that in samples
with the LAPTM4B*1/1 genotype. The risk of PTC in patients with a
copy of LAPTM4B*2 is 1.516-fold higher compared with that in
patients with only LAPTM4B*1 (Table
II).
 | Table I.General characterization of case and
control group. |
Table I.
General characterization of case and
control group.
| Characteristics | Controls, n (%)
n=697 | Thyroid carcinoma, n
(%) n=183 | P-valuea |
|---|
| Sex |
|
| P<0.001 |
|
Female | 249 (35.7) | 132 (72.1) |
|
| Male | 448 (64.2) | 51
(27.8) |
|
| Age, years |
|
| P<0.001 |
|
≤40 | 110 (15.8) | 87
(47.5) |
|
|
41–50 | 223 (32.0) | 49
(26.8) |
|
|
51–60 | 142 (20.4) | 31
(16.9) |
|
|
61–70 | 106 (15.2) | 15 (8.2) |
|
|
>70 | 116 (16.6) | 1
(0.6) |
|
 | Table II.Genotype and allelic frequencies of
LAPTM4B gene polymorphism in PTC patients and controls. |
Table II.
Genotype and allelic frequencies of
LAPTM4B gene polymorphism in PTC patients and controls.
| LAPTM4B status | Healthy
controlsa, n (%)
n=697 | PTC, n (%)
n=183 | Odds
ratiob (95% CI) | P-value |
|---|
| Allele |
|
|
|
|
| *1 | 1,058 (75.9) | 253 (69.1) |
|
|
| *2 | 336
(24.1) | 113 (30.9) | 1.516
(1.140–2.017) | 0.004 |
| Genotype |
|
|
|
|
|
*1/1 | 397
(57.0) | 90
(49.2) |
|
|
|
*1/2 | 264
(37.8) | 73
(39.9) | 1.352
(0.919–1.987) | 0.125 |
|
*2/2 | 36
(5.2) | 20
(10.9) | 2.765
(1.384–5.524) | 0.004 |
Genotypes of LAPTM4B and clinical
parameters
By analyzing the clinical parameters of patients
with PTC and healthy controls, the female sex was identified to be
significantly associated with LAPTM4B 2/2 genotype, whereas age,
central lymph node metastasis condition, invasive condition of the
tumor, tumor size, BMI, tumor location and TMN stage were not
significantly associated with a specific LAPTM4B genotype (Table III).
 | Table III.Association between LAPTM4B genotypes
and clinical characteristics of papillary thyroid carcinoma. |
Table III.
Association between LAPTM4B genotypes
and clinical characteristics of papillary thyroid carcinoma.
|
| LAPTM4B
genotype |
|
|---|
|
|
|
|
|---|
|
Characteristics | 1/1 | 1/2 | 2/2 | P-value |
|---|
| Age, years |
|
|
| 0.705 |
|
>40 | 50 | 37 | 12 |
|
|
≤40 | 40 | 36 | 8 |
|
| Sex |
|
|
| 0.046 |
|
Female | 64 | 49 | 19 |
|
|
Male | 26 | 24 | 1 |
|
| CLNM |
|
|
| 0.219 |
|
Positive | 56 | 53 | 11 |
|
|
Negative | 34 | 20 | 9 |
|
| Invasive tumor |
|
|
| 0.531 |
|
Yes | 49 | 39 | 8 |
|
| No | 41 | 34 | 12 |
|
| Tumor size, cm |
|
|
| 0.836 |
| ≥1 | 40 | 26 | 8 |
|
|
<1 | 46 | 42 | 11 |
|
|
Unknown | 4 | 5 | 1 |
|
| TNM stage |
|
|
| 0.290 |
|
I–II | 58 | 41 | 10 |
|
|
III–IV | 19 | 16 | 8 |
|
|
Unknown | 13 | 16 | 2 |
|
| Tumor location |
|
|
| 0.777 |
| One
side | 17 | 8 | 4 |
|
| Two
sides | 1 | 3 | 0 |
|
|
Isthmus | 3 | 2 | 1 |
|
| Two
sides and isthmus | 7 | 7 | 1 |
|
| One
side and isthmus | 62 | 53 | 14 |
|
| BMI |
|
|
| 0.531 |
|
>25 | 49 | 39 | 8 |
|
|
≤25 | 41 | 34 | 12 |
|
LAPTM4B genotypes and PTC
susceptibility compared between females and males
As sex was associated with a certain LAPTM4B
genotype, the LAPTM4B genotypes of females and males were analyzed
separately. In the female patients that were analyzed, a
LAPTM4B*2/2 genotype increased the risk of developing PTC
5.494-fold compared with the LAPTM4B*1/1 genotype. A female
carrying a copy of LAPTM4B*2 had a 1.968-fold increased risk of PTC
compared with those carrying only copies of LAPTM4B*1 alone
(Table IV). However, no
statistically significant difference in LAPTM4B genotype frequency
was exhibited between the PTC patients and the healthy controls in
males (Table V).
 | Table IV.Frequency of LAPTM4B in female PTC
patients and healthy controls. |
Table IV.
Frequency of LAPTM4B in female PTC
patients and healthy controls.
| LAPTM4B status | Healthy controls, n
(%) n=249 | PTC, n (%)
n=132 | Odds ratio (95%
CI) | P-value |
|---|
| Allele |
|
|
|
|
| *1 | 395 (79.3) | 177 (67.0) |
|
|
| *2 | 103 (20.7) | 87
(33.0) | 1.968
(1.363–2.841) |
<0.001a |
| Genotype |
|
|
|
|
|
*1/1 | 156 (62.7) | 64
(48.5) |
|
|
|
*1/2 | 83
(33.3) | 49
(37.1) | 1.443
(0.880–2.365) |
0.146b |
|
*2/2 | 10 (4.0) | 19
(14.4) | 5.494
(2.198–13.730) |
<0.001b |
 | Table V.The frequency of LAPTM4B in male
papillary thyroid carcinoma patients and male healthy controls. |
Table V.
The frequency of LAPTM4B in male
papillary thyroid carcinoma patients and male healthy controls.
| LAPTM4B status | Healthy controls, n
(%) n=448 | PTC, n (%)
n=51 | OR (95% CI) | P-value |
|---|
| Allele |
|
|
|
|
| *1 | 663 (74.0) | 76 (74.5) |
|
|
| *2 | 233 (26.0) | 26 (25.5) | 0.996
(0.615–1.612) | 0.986a |
| Genotype |
|
|
|
|
|
*1/1 | 241 (53.8) | 26 (50.9) |
|
|
|
*1/2 | 181 (40.4) | 24 (47.1) | 1.187
(0.649–2.169) | 0.577b |
|
*2/2 | 26 (5.8) | 1 (2.0) | 0.445
(0.057–3.481) | 0.440b |
Association between LAPTvM4B genotype
and clinical parameters in female patients
The clinical parameters and genotypes of LAPTM4B
were further analyzed in the female participants. There was no
significant association between age, central lymph node metastasis,
invasive condition of the tumor, tumor size, TNM stage, tumor
location, BMI, number of pregnancies or menopause condition and
LAPTM4B genotype (Table VI).
 | Table VI.Association between LAPTM4B genotypes
and clinical characteristics of papillary thyroid carcinoma in the
female group. |
Table VI.
Association between LAPTM4B genotypes
and clinical characteristics of papillary thyroid carcinoma in the
female group.
|
| LAPTM4B
genotype |
|
|---|
|
|
|
|
|---|
|
Characteristics | *1/1 (n=64) | *1/2 (n=49) | *2/2 (n=19) | P-value |
|---|
| Age, years |
|
|
| 0.745 |
|
>40 | 34 | 27 | 12 |
|
|
≤40 | 30 | 22 | 7 |
|
|
CLNMa |
|
|
| 0.084 |
|
Positive | 21 | 10 | 9 |
|
|
Negative | 43 | 39 | 10 |
|
| Invasive tumor |
|
|
| 0.640 |
|
Yes | 28 | 24 | 7 |
|
| No | 36 | 25 | 12 |
|
| Tumor size, cm |
|
|
| 0.816 |
| ≥1 | 28 | 16 | 7 |
|
|
<1 | 33 | 31 | 11 |
|
|
Unknown | 3 | 2 | 1 |
|
| TNM stage |
|
|
| 0.228 |
|
I–II | 41 | 27 | 9 |
|
|
III–IV | 16 | 11 | 8 |
|
|
Unknown | 7 | 11 | 2 |
|
| Tumor location |
|
|
| 0.856 |
| One
side | 10 | 6 | 4 |
|
| Two
sides | 0 | 2 | 0 |
|
|
Isthmus | 2 | 2 | 1 |
|
| Two
sides and isthmus | 4 | 3 | 1 |
|
| One
side and isthmus | 48 | 36 | 13 |
|
| BMI |
|
|
| 0.64 |
|
>25 | 28 | 24 | 7 |
|
|
≤25 | 36 | 25 | 12 |
|
| Pregnancies |
|
|
| 0.852 |
| 0 | 12 | 10 | 4 |
|
| 1 | 37 | 32 | 14 |
|
| ≥2 | 10 | 6 | 1 |
|
|
Unknown | 5 | 1 | 0 |
|
| Menopause |
|
|
| 0.138 |
|
Yes | 16 | 12 | 9 |
|
| No | 48 | 37 | 10 |
|
Dual luciferase reporter assay
The difference between LAPTM4B*1 and LAPTM4B*2 is
the presence of a 19-bp sequence located in the 5′UTR of exon 1.
Thus, the current study hypothesized that the extra 19-bp sequence
may affect the transcriptional activity of LAPTM4B*2, resulting in
an altered biological function that causes the patients that carry
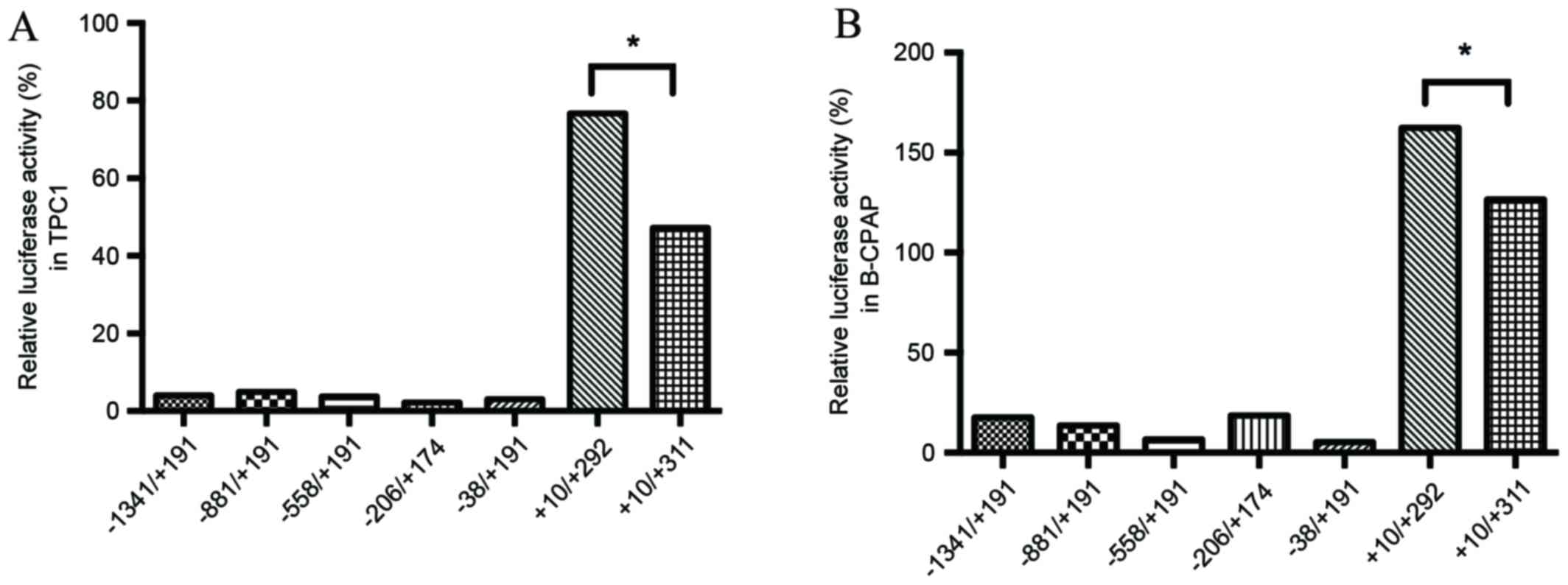
LAPTM4B*2 to be susceptible to PTC. In total seven types of
luciferase reporter plasmid were transfected into TPC1 and B-CPAP
cells (Fig. 3). The +10/+292 plasmid,
which contains one 19-bp sequence, had the highest transcription
activity, and the +10/+311 plasmid, including an extra 19-bp
sequence, had the second highest (Fig.
4). It is possible that there may be transcription factors that
bind to the extra 19-bp sequence, increasing transcriptional
activity; however, the susceptibility of individuals with the
LAPTM4B*2 to PTC is not caused by transcriptional regulation of the
polymorphism region in these two cell lines.
Discussion
PTC is the most frequently occurring
well-differentiated form of thyroid carcinoma (25). In recent years, PTC has become the
most common type of cancer in female patients (26). Gene mutation, rearrangement and
certain genetic polymorphisms are hypothesized to be the
etiological factors associated with the development of PTC
(27–29). In addition, female hormones may also
promote susceptibility to PTC in females (30). However, the etiology of PTC remains to
be elucidated. The present study has demonstrated that LAPTM4B gene
polymorphism is a susceptibility factor to papillary thyroid
carcinoma in Chinese females. A female patient carrying a copy of
LAPTM4B*2 is more susceptible to PTC compared with those carrying
only LAPTM4B*1.
In the present study, a dual luciferase reporter
assay was performed, demonstrating that the transcriptional
activity of LAPTM4B*1 was higher compared with that of LAPTM4B*2 in
PTC cells. Previous clinical studies have revealed that the
overexpression of LAPTM4B gene facilitates tumorigenesis by
promoting cell proliferation (31–33),
inhibiting apoptosis (34) and
initiating autophagy (35) in
numerous types of carcinoma. Therefore, the current study
hypothesized that LAPTM4B*2 may have an increased transcriptional
activity compared with that LAPTM4B*1, which may explain why people
with LAPTM4B allele *2 demonstrate increased susceptibility to PTC.
However, the transcription activity of LAPTM4B*2 was reduced
compared with that of LAPTM4B*1 in PTC cells. Therefore, the
underlying mechanism as to why patients with LAPTM4B*2 are more
susceptible to PTC is not associated with the transcriptional
regulation of the polymorphism region in TPC1 and B-CPAP cell
lines.
Screening the sequence of the LAPTM4B*2 and
LAPTM4B*1, the extra 19 bp sequence may directly induce the
skipping of the termination codon, which would promote the
protein-code frame lengthened of 53 amino acids at the N terminus
and produce a variation of the functional protein compared with
that of LAPTM4B*1 (Fig. 5). The N
terminus of the LAPTM4B protein serves a key role in cell signal
transduction, ligand-receptor binding (12) and certain other fields such as cell
invasion, migration and cell proliferation (36). Therefore, the protein encoded by
LAPTM4B*2 may promote the progression of tumor development.
The present study compared the LAPTM4B genotypes
between PTC patients and a healthy control group. The LAPTM4B*2/*2
genotype distribution frequency and LAPTM4B*2 distribution
frequency between the two groups were significantly different.
However, the analysis of clinical parameters revealed that the
LAPTM4B genotype frequency was associated with the female sex, so
LAPTM4B genotype frequency was analyzed separately for males and
females. A significant difference in LAPTM4B genotype frequency was
observed between female patients with PTC and female healthy
controls. This result verified that females have increased
susceptibility to PTC; however, the negative results observed in
the male group requires verification in a larger sample.
Previous studies have revealed that individuals with
the LAPTM4B*1/*2 genotype are also susceptible to numerous types of
tumor (11,12,19).
Nevertheless, the present study into PTC indicates that only those
with the LAPTM4B*2/*2 genotype are more susceptible to PTC. Unlike
less-differentiated tumors, which are more aggressive and impart a
poorer prognosis, well-differentiated PTC may require homozygotic
LAPTM4B*2/2 to drive tumorigenesis.
In conclusion, the present study demonstrated that
the presence of the LAPTM4B*2 allele is associated with increased
susceptibility of Chinese females to PTC, and that this
susceptibility is not caused by transcriptional regulation mediated
by the 19 bp addition in LAPTM4B*2.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (No. 81572910).
Glossary
Abbreviations
Abbreviations:
|
LAPTM4B
|
lysosomal protein transmembrane
4-β
|
|
PTC
|
papillary thyroid carcinoma
|
|
ORs
|
odd ratios
|
|
CI
|
confidence interval
|
|
BMI
|
body mass index
|
References
|
1
|
Ito Y, Nikiforov YE, Schlumberger M and
Vigneri R: Increasing incidence of thyroid cancer: controversies
explored. Nat Rev Endocrinol. 9:178–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aschebrook-Kilfoy B, Grogan RH, Ward MH,
Kaplan E and Devesa SS: Follicular thyroid cancer incidence
patterns in the United States, 1980–2009. Thyroid. 23:1015–1021.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davies L and Welch H: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaccarella S, Dal Maso L, Laversanne M,
Bray F, Plummer M and Franceschi S: The impact of diagnostic
changes on the rise in thyroid cancer incidence: A population-based
study in selected high-resource countries. Thyroid. 25:1127–1136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vergamini LB, Frazier AL, Abrantes FL,
Ribeiro KB and Rodriguez-Galindo C: Increase in the incidence of
differentiated thyroid carcinoma in children, adolescents and young
adults: A population-based study. J Pediatr. 164:1481–1485. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu L, Port M, Landi S, Gemignani F,
Cipollini M, Elisei R, Goudeva L, Müller JA, Nerlich K, Pellegrini
G, et al: Obesity and the risk of papillary thyroid cancer: A
pooled analysis of three case-control studies. Thyroid. 24:966–974.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou M, Baitei EY, Al-Rijjal RA, Parhar RS,
Al-Mohanna FA, Kimura S, Pritchard C, Binessa HA, Alzahrani AS,
Al-Khalaf HH, et al: TSH overcomes Braf(V600E)-induced senescence
to promote tumor progression via downregulation of p53 expression
in papillary thyroid cancer. Oncogene. 35:1909–1918. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen GG, Vlantis AC, Zeng Q and van
Hasselt CA: Regulation of cell growth by estrogen signaling and
potential targets in thyroid cancer. Curr Cancer Drug Targets.
8:367–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang H, Lin M, Xiong F, Yang Y, Nie X,
McNutt MA and Zhou R: Combined lysosomal protein transmembrane 4
beta-35 and argininosuccinate synthetase expression predicts
clinical outcome in hepatocellular carcinoma patients. Surg Today.
41:810–817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao GZ, Zhou RL, Zhang QY, Zhang Y, Liu
JJ, Rui JA, Wei X and Ye DX: Molecular cloning and characterization
of LAPTM4B, a novel gene upregulated in hepatocellular carcinoma.
Oncogene. 22:5060–5069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan M, Liu Y, Zhou R and Zhang Q:
Association of LAPTM4B gene polymorphism with breast cancer
susceptibility. Cancer epidemiol. 36:364–368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Zhang QY, Qian N and Zhou RL:
Relationship between LAPTM4B gene polymorphism and susceptibility
of gastric cancer. Ann Oncol. 18:311–316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng LJ, Zhang QY, Liu B and Zhou RL:
Relationship between LAPTM4B gene polymorphism and susceptibility
of lung cancer. Beijing Da Xue Xue Bao. 37:302–305. 2005.(In
Chinese). PubMed/NCBI
|
|
16
|
Cheng XJ, Xu W, Zhang QY and Zhou RL:
Relationship between LAPTM4B gene polymorphism and susceptibility
of colorectal and esophageal cancers. Ann Oncol. 19:527–532. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng F, Li H, Zhou R, Luo C, Hu Y and Lou
G: LAPTM4B gene polymorphism and endometrial carcinoma risk and
prognosis. Biomarkers. 18:136–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng F, Song H, Luo C, Yin M, Xu Y, Liu H,
Zhou R and Lou G: Correlation of LAPTM4B polymorphisms with
cervical carcinoma. Cancer. 117:2652–2658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang H, Zhai G, Ji X, Xiong F, Su J and
McNutt MA: Correlation of LAPTM4B polymorphisms with gallbladder
carcinoma susceptibility in Chinese patients. Med Oncol.
29:2809–2813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Y, Liu Y, Zhou R, Meng F, Gao Y, Yang
S, Li X, Yang M and Lou G: LAPTM4B polymorphisms is associated with
ovarian cancer susceptibility and its prognosis. Jpn J Clin Oncol.
42:413–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Zhou R, Xu J and Zhang Q:
Relationship between LAPTM4B gene polymorphism and susceptibility
of malignant melanoma in Chinese patients. Transl Oncol. 7:638–643.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang B, Xu J, Zhou R and Zhang Q:
Association of LAPTM4B gene polymorphism with nasopharyngeal
carcinoma susceptibility in a Chinese population. Med Oncol.
30:4702013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cuccurullo V and Mansi L: AJCC Cancer
Staging Handbook: From the AJCC Cancer Staging Manual (7th
edition). European Journal of Nuclear Medicine and Molecular
Imaging. 38:408. 2011. View Article : Google Scholar
|
|
24
|
Zhang M, Xu JJ, Zhou RL and Zhang QY: cAMP
responsive element binding protein-1 is a transcription factor of
lysosomal-associated protein transmembrane-4 Beta in human breast
cancer cells. PLoS one. 8:e575202013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aschebrook-Kilfoy B, Ward MH, Sabra MM and
Devesa SS: Thyroid cancer incidence patterns in the United States
by histologic type, 1992–2006. Thyroid. 21:125–134. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao J, Huang X, Yang M, Li M and Zheng J:
Association between the FTOrs8050136 polymorphism and cancer risk:
A meta-analysis. Fam cancer. 15:145–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee YC, Chung JH, Kim SK, Rhee SY, Chon S,
Oh SJ, Hong IK and Eun YG: Association between interleukin
17/interleukin 17 receptor gene polymorphisms and papillary thyroid
cancer in Korean population. Cytokine. 71:283–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soares P, Trovisco V, Rocha AS, Lima J,
Castro P, Preto A, Máximo V, Botelho T, Seruca R and
Sobrinho-Simões M: BRAF mutations and RET/PTC rearrangements are
alternative events in the etiopathogenesis of PTC. Oncogene.
22:4578–4580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horn-Ross PL, Canchola AJ, Ma H, Reynolds
P and Bernstein L: Hormonal factors and the risk of papillary
thyroid cancer in the California Teachers Study cohort. Cancer
Epidemiol Biomarkers Prev. 20:1751–1759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Milkereit R, Persaud A, Vanoaica L, Guetg
A, Verrey F and Rotin D: LAPTM4b recruits the LAT1-4F2hc Leu
transporter to lysosomes and promotes mTORC1 activation. Nat
commun. 6:72502015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng F, Chen X, Song H and Lou G: LAPTM4B
down regulation inhibits the proliferation, invasion and
angiogenesis of HeLa cells in vitro. Cell Physiol Biochem.
37:890–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou L, He XD, Yu JC, Zhou RL, Yang H, Qu
Q and Rui JA: Overexpression of LAPTM4B promotes growth of
gallbladder carcinoma cells in vitro. Am J Surg. 199:515–521. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou L, He XD, Yu JC, Zhou RL, Shan Y and
Rui JA: Overexpression of LAPTM4B-35 attenuates epirubucin-induced
apoptosis of gallbladder carcinoma GBC-SD cells. Surgery.
150:25–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan X, Thapa N, Sun Y and Anderson RA: A
kinase-independent role for EGF receptor in autophagy initiation.
Cell. 160:145–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu X, Xiong F, Wei X, Yang H and Zhou R:
LAPTM4B-35, a novel tetratransmembrane protein and its PPRP motif
play critical roles in proliferation and metastatic potential of
hepatocellular carcinoma cells. Cancer sci. 100:2335–2340. 2009.
View Article : Google Scholar : PubMed/NCBI
|