Introduction
Tumor progression is a complex phenomenon that
involves the interaction between tumor cells and the surrounding
tissues, promoting a plastic phenotype referred to as the
epithelial-mesenchymal transition (EMT) (1). This process involves alterations in
cell-cell and cell-matrix interactions, and consequently the
acquisition of a motile phenotype (1,2). The
molecules involved in cell-cell interactions assume a significant
role in physiological events and pathological conditions such as
inflammatory and neoplastic processes (2). Among such molecules, calcium-dependent
transmembrane glycoproteins (epithelial-cadherin) expressed in
epithelial tissues, termed E-cadherins, are highlighted (3).
E-cadherin is associated with a group of
intracellular proteins termed catenins, which bind to the actin
filaments of the cytoskeleton (3).
Cadherins and catenins form a single functional complex so that the
deletion or mutation of one of these proteins may result in a loss
of function or the disruption of cell-cell adhesion (4). Studies have revealed that the
E-cadherin/β-catenin complex (EβC) is a key regulatory factor
required for the maintenance of normal intercellular adhesion in
carcinomas, acting as an invasion suppressor molecule (1,2,5). Consequently, a loss of EβC function
allows or increases the risk of neoplastic invasion of the
underlying normal tissue (5). Changes
to E-cadherin expression patterns may, therefore, be a vital step
in the development and progression of malignant tumors (5,6).
During the early stages of glandular tumors in
situ, malignant luminal cells are surrounded by an intact layer
of myoepithelial cells (7). However,
this condition is short-lived, as myoepithelial cells rapidly
disappear (8,9). A number of hydrolytic enzymes, released
either by the tumor cells or by cells surrounding the tumor,
combined with certain growth factors may favor disruption of the
basement membrane, contributing to EMT (10). Matrix metalloproteinases (MMPs) act by
degrading almost all components of the basement membrane and the
extracellular matrix (ECM) (11). In
malignant disease, metastasis-associated MMP overexpression has
been reported to be associated with cytokines such as epidermal
growth factor (EGF), which in turn may serve a role in modulating a
developing metastatic potential in neoplastic cells (12).
EGF has been extensively investigated in invasive
tumor phenotypes, and several in vitro studies have
demonstrated the proliferative effect of EGF on a variety of cell
types (4,13,14).
Additionally, EGF performs an essential role in promoting a
migratory phenotype, even in benign cells (4,14). When
EGF binds to its receptor, E-cadherin is internalized from the cell
membrane, reducing cell-cell contact, which in turn weakens the
epithelial layer and induces EMT (15,16). EGF
has been revealed to upregulate a number of MMPs in squamous cell
carcinoma cells, as well as in myoepithelial cells (15).
Considering the importance of myoepithelial cells to
the invasive behavior of cancer cells in salivary gland neoplasms
(17,18), the present study aimed to evaluate the
role of EGF on E-cadherin/β-catenin gene expression and MMP-2
secretion in an in vitro model of tumorigenesis, which
mimics a situation where in situ neoplastic cells of oral
carcinoma are surrounded by benign myoepithelial cells (19).
Materials and methods
Cell culture
The present study was approved by the Research
Ethics Committee of the São Leopoldo Mandic Dental Institute and
Research Center (Campinas, Brazil; approval no. 1.468.890/2016).
Benign myoepithelial cells were obtained from explants of excised
salivary gland pleomorphic adenoma (PA), as previously described
(18).
Cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
supplemented with 1% antimycotic-antibiotic solution (10,000 U/ml
penicillin, 10 mg/ml streptomycin and 25 mg/ml amphotericin B in
0.9% sodium chloride; Sigma-Aldrich; Merck KGaA) containing 10%
donor calf serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Cells were then seeded onto 60-mm diameter plastic
culture dishes (110 cells/mm2) and incubated under
standard cell culture conditions (37°C, 100% humidity, 95% air and
5% CO2). Once 100% confluence was reached, the cells
were detached using 0.05% trypsin and subcultured at a density of
110 cells/mm2 in 20 µg/ml fibronectin substratum
(Sigma-Aldrich; Merck KGaA). The cells were then seeded either onto
polystyrene plates or 13-mm coverslips for subsequent experiments.
The benign myoepithelial cells from PA seeded either on the
polystyrene plates or coverslips were cultured in DMEM for 24 h
(37°C, 100% humidity, 95% air and 5% CO2) prior to
supplementation with malignant conditioned medium, which contains
floating vital malignant cells that have lost their ability to
adhere to polystyrene, and according to the method described by
Martinez et al (18,19), is likely to contain malignant stem
cells.
For cell induction using malignant conditioned
medium in vitro, CAL27 squamous cell carcinoma cells were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cultured as aforementioned. Cell culture medium (DMEM) was
changed 48 h prior to use. Benign myoepithelial cells cultured in
DMEM for 24 h were then incubated for four days with the unfiltered
malignant conditioned medium (37°C, 100% humidity, 95% air and 5%
CO2), which contained non-adhered malignant epithelial
cells. EGF was subsequently added at 5 or 10 ng/ml (BD Biosciences,
San Jose, CA, USA). As a control group, myoepithelial cells were
cultured in conventional non-conditioned DMEM.
Indirect immunofluorescence
Cells grown on coverslips were fixed in absolute
methanol for 6 min at −20°C and rinsed with PBS, followed by
blocking with 1% bovine albumin (catalog no. A2058; Sigma-Aldrich;
Merck KGaA) in PBS for 30 min at room temperature. Polyclonal
rabbit E-cadherin (catalog no. H-108; dilution, 1:50; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and monoclonal mouse
β-catenin (catalog no. 610154; dilution, 1:50; BD Biosciences)
primary antibodies were incubated for 1 h at room temperature.
Control staining was performed using PBS in place of the primary
antibody. The secondary antibody used was either biotinylated goat
anti-rabbit (catalog no. 4050-08; dilution) or goat anti-mouse IgG
(catalog no. 1031-08; dilution, 1:150) (both from Vector
Laboratories, Inc., Burlingame, CA, USA), which were incubated for
30 min at room temperature. Fluorescein-streptavidin conjugate
(catalog no. SA-5001; dilution, 1:150; Vector Laboratories, Inc.)
was used for the second step. The preparations were rinsed and
mounted using DAPI-associated Vectashield® (catalog no. H-1200;
Vector Laboratories, Inc.) and the slides were maintained at 4°C
for up to 24 h to prevent loss of fluorescence. The images were
assessed immediately after staining using a Zeiss Axioskop 2
conventional fluorescence microscope (original magnification, ×400;
Carl Zeiss MicroImaging GmbH, Jena, Germany) equipped with 63X Plan
Apochromatic 1.4NA and 100X Plan Apochromatic 1.4NA objectives
(Carl Zeiss MicroImaging GmbH).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells cultured under
differing conditions using TRIzol® reagent (Molecular Research
Center, Cincinnati, OH, USA). The RNA samples were treated with
DNase (1:1, DNase I, RNase-free kit; catalog no. EN0521; Fermentas;
Thermo Fisher Scientific, Inc.). Reverse transcription was
performed using the Superscript III First-Strand cDNA Synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The primer sets were as follows: β-catenin
forward, 5′-GCAGTTCGCCTTCACTATGGA-3′ and reverse,
5′-GACAAAGGGCAAGATTTCGAATC-3′; E-cadherin forward,
5′-TCATGAGTGTCCCCCGGTAT-3′ and reverse,
5′-TCAAACACGAGCAGAGAATCATAAG-3′; GAPDH (used as the internal
reference gene) forward, 5′-TGGCAAAGTGGAGATTGTTGCC-3′ and reverse,
5′-AAGATGGTGATGGGCTTCCCG-3′. RT-qPCR was performed using a 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with SYBR-Green/ROX (Maxima; Fermentas; Thermo Fisher
Scientific, Inc.), as the detection dye. Cycling conditions were as
follows: 10 min at 95°C followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. Quantification of the data was performed using
the 7500 software (7500 Fast Red Time PCR systems v2.0.6; Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the relative
expression levels were calculated according to the comparative Cq
method, as 2−ΔΔCq (20).
Each qPCR experiment was repeated three times.
ELISA
MMP-2 quantification was performed using the human
MMP-2 ELISA kit (catalog no. DY902; R&D Systems, Inc.,
Minneapolis, MN, USA). Coating containing the capture antibody and
blocking were provided in the kit and performed according to the
manufacturer's protocol. The reactions were performed on a 96-well
plate (catalog no. 80040LE 0903; Apogent Technologies, Inc.,
Milwaukee, MI, USA), according to the manufacturer's
recommendations. Supernatant obtained from the cell cultures were
harvested and centrifuged at 5,000 × g at 4°C for 15 min, 100 µl.
The wells were then rinsed three times with washing buffer (0.05%
Tween-20 in PBS, pH 7.2) prior to adding the standard (provided in
the kit) and supernatant in duplicate wells. Following incubation
for 2 h at room temperature, the plates were washed again and
incubated with 100 µl of the detection antibody at room temperature
for 2 h. The plates were then rinsed three times (0.05% Tween-20 in
PBS, pH 7.2), treated with 100 µl of streptavidin-horseradish
peroxidase for 20 min at room temperature and rinsed three times
with washing buffer (0.05% Tween-20 in PBS, pH 7.2). Substrate
solution (100 µl; provided in the kit) was added to each well and
incubated for 15 min in the dark at room temperature. The reaction
was stopped by adding 50 µl stop solution (provided in the kit),
and the color was measured at 450 nm, using an automated microplate
spectrophotometer (Epoch), associated with the software GenS
(v1.10.8) (both from BioTek Instruments, Inc., Winooski, VT, USA).
Total MMP was quantified in ng/ml. Results were calculated using
the standard curves created in each assay. The ELISA assays were
performed in a blind manner and in triplicate.
Statistical analysis
Data are expressed as the mean ± standard deviation.
In order to compare the results between distinct conditions,
one-way analysis of variance was used with Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. All statistical calculations were performed with
GraphPad Prism v 6.0 software (GraphPad Software, Inc., La Jolla,
CA, USA).
Results
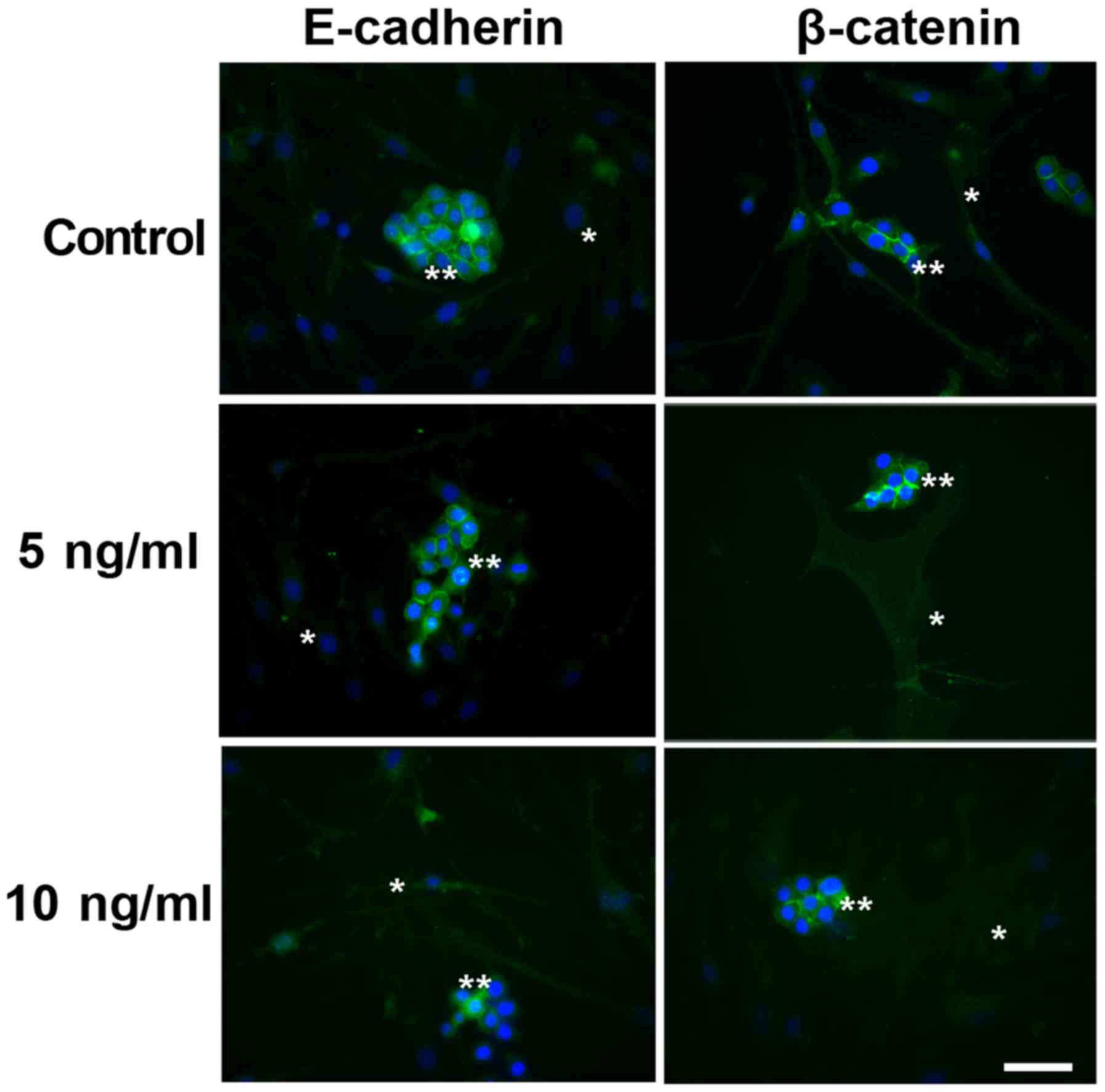
Indirect immunofluorescence of
E-cadherin and ß-catenin
Indirect immunofluorescence to E-cadherin and
β-catenin in the co-cultured cells under different conditions is
illustrated in Fig. 1. E-cadherin and
β-catenin were observed to be immunoexpressed in the cytoplasm of
the malignant cells under all studied conditions. No immunostaining
was observed in the myoepithelial cells, even following EGF
supplementation.
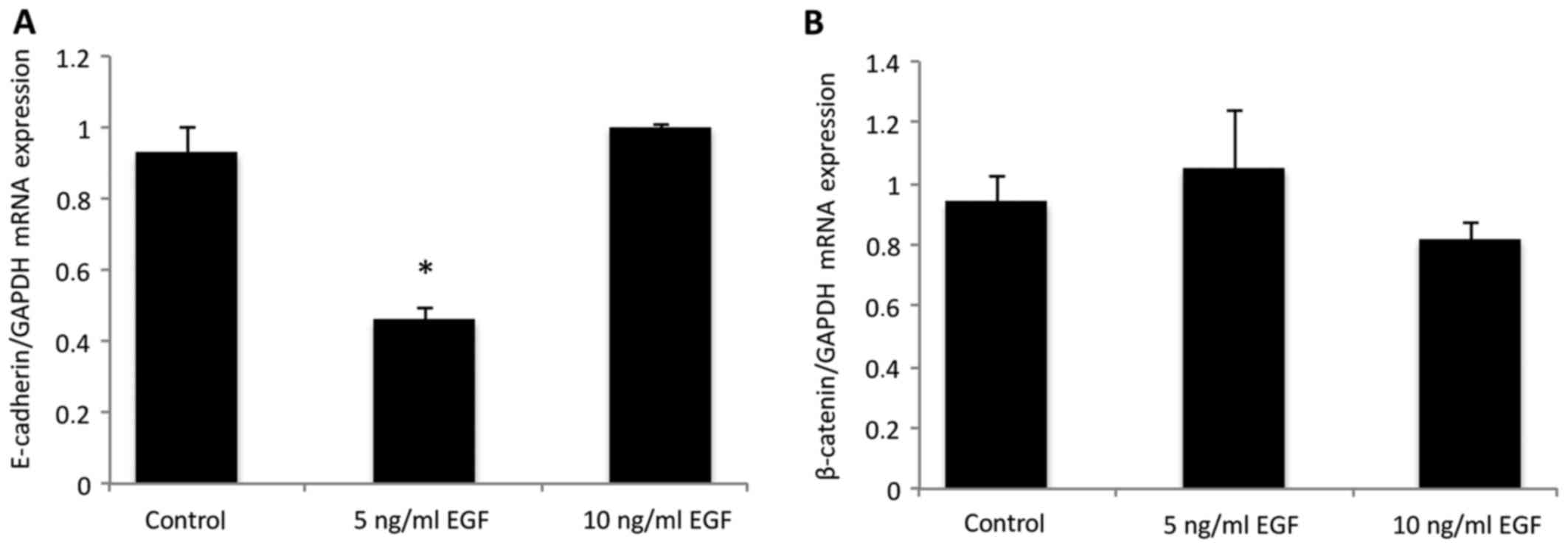
mRNA E-cadherin and ß-catenin
expression
In order to quantify the results observed using
immunofluorescence, the expression of E-cadherin and β-catenin was
assessed using qPCR (Fig. 2)
following EGF supplementation. E-cadherin expression was
significantly downregulated in the cells treated with 5 ng/ml EGF,
when compared with the control group (P<0.05; Fig. 2A), which was statistically similar to
the group treated with 10 ng/ml EGF (P>0.05). No difference was
observed in β-catenin mRNA expression levels following EGF
supplementation (Fig. 2B), as
compared with the control (P>0.05).
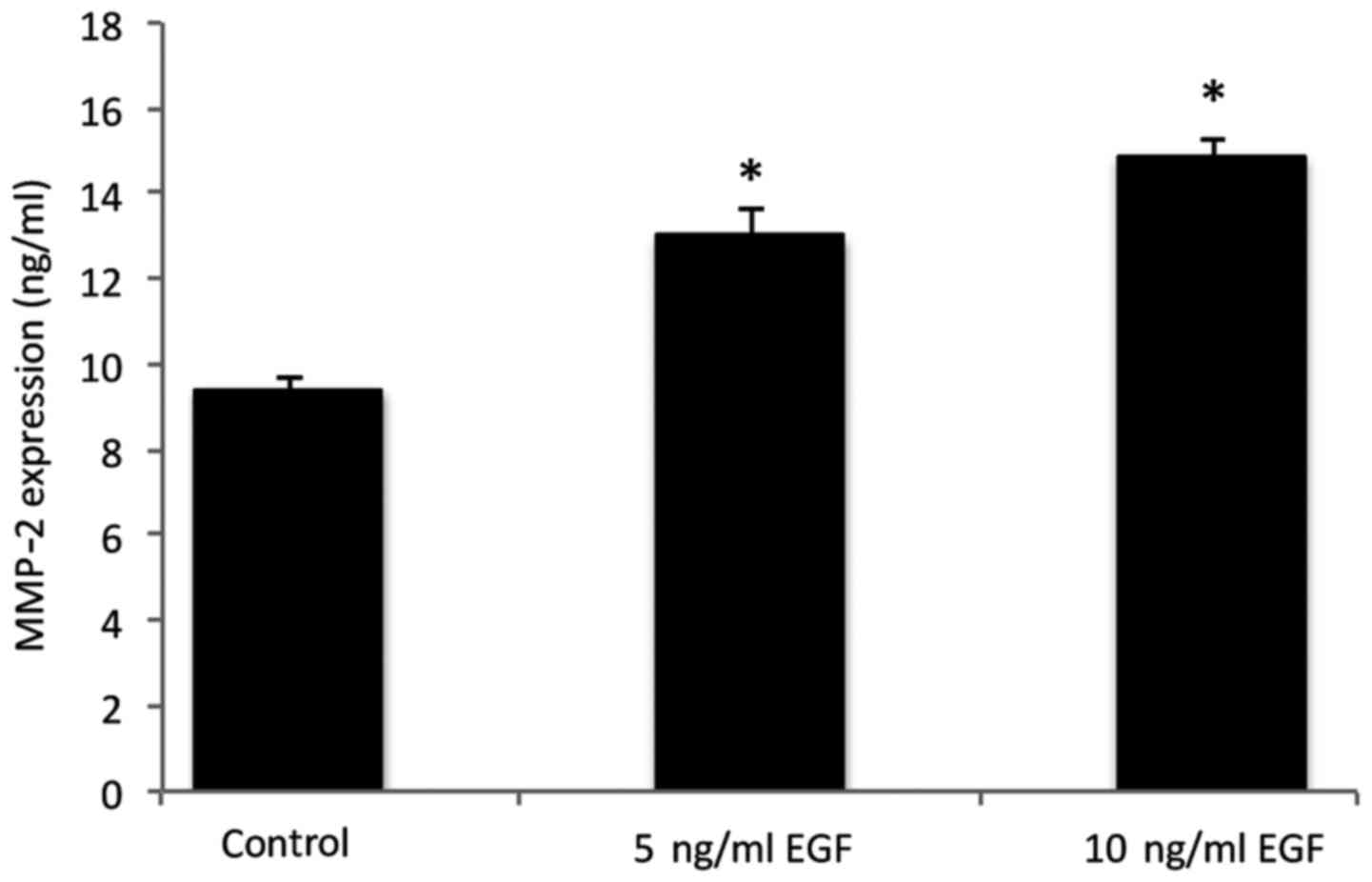
MMP-2 protein secretion
MMP-2 levels in the co-cultured cells under
different conditions are presented in Fig. 3. The results indicated an increase in
MMP-2 secretion in the cells following supplementation with 5 and
10 ng/ml EGF, when compared with the control group (P<0.05).
Discussion
Metastasis is a process that involves several
sequential and interrelated steps, including detachment, migration,
invasion and adhesion, and in this context, the tumor
microenvironment has been widely studied and is considered an
important factor in the so named ‘invasion-metastasis cascade’
(21,22). Growth factors are secreted by normal
and cancerous cells within the tumor microenvironment, and are
involved in tumor initiation and progression (4). EGF has been revealed to be associated
with cell proliferation and migration in contexts such as salivary
gland tumors (17) and EMT (15).
EMT is a result of paracrine signaling by
stromal-derived factors, and is also a potential source of cells
within the stromal compartment; cross-talk between neoplastic and
stromal cells may affect the production and secretion of a number
of molecules and matrix proteases, including the
E-cadherin/β-catenin complex, which is responsible for maintaining
adhesive properties, and MMPs, which have previously been
implicated in degrading components of the basement membrane
(10).
In the present in vitro study, MMP-2
secretion and E-cadherin/β-catenin expression were analyzed
following four days of cell culture, wherein myoepithelial cells,
mimicking an in situ condition, surrounded carcinoma cells.
As co-culture time progresses, benign cells begin to disappear
until only malignant cells are left (8), and such interactions between neoplastic
cells and the stroma can no longer be detected. This behavior is
associated with basement membrane disruption through the action of
MMPs, which culminates in EMT-associated invasion (15). Such findings corroborate an invasive
behavior by the malignant cells, which occurs in the presence of
EGF downregulating E-cadherin expression and increasing MMP-2
secretion.
In epithelial cells, EGF has been associated with
increasing MMP secretion, contributing to a metastatic phenotype by
modifying certain ECM components and promoting a motile behavior,
which is typical of EMT (12). In
addition, MMP-2 is known to promote modifications to cell
morphology and phenotype in vitro (13), which can directly affect cell adhesion
(11). Furthermore, MMP-2, which is
one of the major enzymes involved in degrading collagen types I and
IV as well as the ECM, is overexpressed in highly metastatic
tumors, demonstrating the importance of this molecule in cancer
invasion and metastasis (23).
E-cadherin expression is impaired in a number of
tumor types, and its loss from the cell surface due to genetic or
epigenetic events leads to disruption of cell contacts, tumor cell
detachment, shape change and local invasion, all of which are
events known to initiate EMT (24).
In this setting, β-catenin is considered as one of the most
important factors involved in decreasing cell-cell interactions in
malignant epithelial cells (25). In
an environment of degradation and destabilization of cell-cell
adhesion and decreased E-cadherin expression, β-catenin is released
into the cytoplasm causing it to accumulate and eventually lead to
nuclear translocation (25). Cadherin
switching is a hallmark of EMT, where E-cadherin can be replaced by
an abnormal expression of N-cadherin without changes in E-cadherin
levels (26). This event may be
affected by cytokines and growth factors (27). It has previously been reported that
EGF is also involved in regulating cell adhesion in several solid
malignancies (28), and that EGF
stimulation causes E-cadherin relocation from the membrane to the
perinuclear area during EMT in head and neck squamous cell
carcinoma (29).
Dynamic evaluation of certain factors, such as EGF,
with regard to E-cadherin/β-catenin expression may yield data
concerning key biological processes in tumorigenesis in
vitro. However, the use of E-cadherin/β-catenin as prognostic
markers in salivary gland tumors containing myoepithelial cells,
for instance, may not necessarily generate definitive predictive
values (30). Furuse et al
(30) demonstrated that such
molecules may be immunoexpressed in normal salivary glands as well
as in malignant neoplastic tissues, be it invasive or non-invasive.
In such cross-sectional studies, biological timing is difficult to
predict histologically. Additionally, although a quantitative
difference may exist between the physiological and neoplastic
expression of E-cadherin/β-catenin, as demonstrated in the present
in vitro study, such differences are likely to go undetected
using qualitative methods.
In conclusion, the present study demonstrated that
EGF performs an important role in the EMT process by altering the
expression of the E-cadherin/β-catenin complex and increasing MMP-2
secretion. This could, in turn, favor the dissolution of the
basement membrane, aiding the maintenance of malignant cell
clusters in detriment of stromal cells and encouraging an invasive
phenotype, based on this in vitro model of
tumorigenesis.
Acknowledgements
The authors wish to thank Ms Pollyanna Tombini
Montaldi (Department of Oral Pathology, Immunology and Molecular
Biology, São Leopoldo Mandic Institute and Research Center) and Mrs
Vanessa Araújo (Department of Oral Pathology, Immunology and
Molecular Biology, São Leopoldo Mandic Institute and Research
Center) for their excellent technical expertise and assistance and
the Brazilian Council for Scientific and Technological Development
(grant no. 471153/2013-3).
References
|
1
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khew-Goodall Y and Wadham C: A perspective
on regulation of cell-cell adhesion and epithelial-mesenchymal
transition: Known and novel. Cells Tissues Organs. 179:81–86. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stappert J and Kemler R: The cadherin
superfamily. Adv Mol Cell Biol. 28:27–63. 1999. View Article : Google Scholar
|
|
4
|
Lu Z, Ghosh S, Wang Z and Hunter T:
Downregulation of caveolin-1 function by EGF leads to the loss of
E-cadherin, increased transcriptional activity of beta-catenin, and
enhanced tumor cell invasion. Cancer Cell. 4:499–515. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wijnhoven BP, Dinjens WN and Pignatelli M:
E-cadherin-catenin cell-cell adhesion complex and human cancer. Br
J Surg. 87:992–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pećina-Slaus N: Tumor supressor gene
E-cadherin and its role in normal and malignant cells. Cancer Cell
Int. 3:172003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Altemani A, Martins MT, Freitas L, Soares
F, Araújo NS and Araújo VC: Carcinoma ex pleomorphic adenoma
(CXPA): Immunoprofile of the cells involved in carcinomatous
progression. Histopathology. 46:635–641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martinez EF, de Araújo NS and de Araújo
VC: How do benign myoepithelial cells from in situ areas of
carcinoma ex-pleomorphic adenoma favor tumor progression? J Cell
Commun Signal. 9:279–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silva CA, Martinez EF, Demasi AP, Altemani
A, da Silveira Bossonaro JP, Araújo NS and de Araújo VC: Cellular
senescence and autophagy of myoepithelial cells are involved in the
progression of in situ areas of carcinoma ex-pleomorphic adenoma to
invasive carcinoma. An in vitro model. J Cell Commun Signal.
9:255–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5(pii): E172016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilkins-Port CE and Higgins PJ: Regulation
of extracellular matrix remodeling following transforming growth
factor-beta1/epidermal growth factor-stimulated
epithelial-mesenchymal transition in human premalignant
keratinocytes. Cells Tissues Organs. 185:116–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Antoniades HN and Owen AJ: Growth factors
and regulation of cell growth. Annu Rev Med. 33:445–463. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Navarini NF, Araújo VC, Brown AL,
Passador-Santos F, Souza IF, Napimoga MH, Araújo NS and Martinez
EF: The EGF signaling pathway influences cell migration and the
secretion of metalloproteinases by myoepithelial cells in
pleomorphic adenoma. Tumour Biol. 36:205–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed S and Nawshad A: Complexity in
interpretation of embryonic epithelial-mesenchymal transition in
response to transforming growth factor-beta signaling. Cells
Tissues Organs. 185:131–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barsky SH and Karlin NJ: Mechanisms of
disease: Breast tumor pathogenesis and the role of the
myoepithelial cell. Nat Clin Pract Oncol. 3:138–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinez EF, Demasi AP, Napimoga MH,
Arana-Chavez VE, Altemani A, de Araújo NS and de Araújo VC: In
vitro influence of the extracellular matrix in myoepithelial cells
stimulated by malignant conditioned medium. Oral Oncol. 48:102–109.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martinez EF, Montaldi PT, de Araújo NS,
Altemani A and de Araújo VC: A proposal of an in vitro model which
mimics in situ areas of carcinoma. J Cell Commun Signal. 6:107–109.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McSherry EA, Donatello S, Hopkins AM and
McDonnell S: Molecular basis of invasion in breast cancer. Cell Mol
Life Sci. 64:3201–3218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho WJ, Chow AK, Schulz R and Daniel EE:
Matrix metalloproteinase-2, caveolins, focal adhesion kinase and
c-Kit in cells of the mouse myocardium. J Cell Mol Med.
11:1069–1086. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brooks SA, Lomax-Browne HJ, Carter TM,
Kinch CE and Hall DM: Molecular interactions in cancer cell
metastasis. Acta Histochem. 112:3–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Umbreit C, Flanjak J, Weiss C, Erben P,
Aderhold C, Faber A, Stern-Straeter J, Hoermann K and Schultz JD:
Incomplete epithelial-mesenchymal transition in p16-positive
squamous cell carcinoma cells correlates with β-catenin expression.
Anticancer Res. 34:7061–7069. 2014.PubMed/NCBI
|
|
26
|
Bryan RT: Cell adhesion and urothelial
bladder cancer: The role of cadherin switching and related
phenomena. Philos Trans R Soc Lond B Biol Sci. 370:201400422015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121:727–735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith A, Teknos TN and Pan Q: Epithelial
to mesenchymal transition in head and neck squamous cell carcinoma.
Oral Oncol. 49:287–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holz C, Niehr F, Boyko M, Hristozova T,
Distel L, Budach V and Tinhofer I:
Epithelial-mesenchymal-transition induced by EGFR activation
interferes with cell migration and response to irradiation and
cetuximab in head and neck cancer cells. Radiother Oncol.
101:158–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Furuse C, Cury PR, Altemani A, dos Santos
Pinto D Jr, de Araújo NS and de Araújo VC: Beta-catenin and
E-cadherin expression in salivary gland tumors. Int J Surg Pathol.
14:212–217. 2006. View Article : Google Scholar : PubMed/NCBI
|