Introduction
Esophageal cancer is a common malignant tumor with
an increasing incidence and mortality. Surgery combined with
radiotherapy and chemotherapy is indicated for esophageal cancer.
However, many patients are diagnosed in advanced stages of
esophageal cancer, resulting in poor treatment with poor prognosis
(1). Clinical practice suggests
(2) that recurrence and metastasis
are important factors contributing to the death of patients with
esophageal cancer. Epidermal growth factor receptor (EGF) is a
tyrosine kinase present on cell surface receptors, which affects
several growth factors.
Phosphorylated epidermal growth factor receptor
(P-EGFR) activates a variety of intracellular signaling pathways,
inducing cell proliferation and survival (3). Akt signaling pathway is an important
pathway regulating downstream signaling by EGFR (4). Phosphorylated Akt (p-Akt) activates Akt
signaling, and the downstream genes in the signaling pathways. It
controls the evolution, invasion, development and apoptosis of
cancer cells.
Esophageal squamous cell carcinoma (ESCC) is a
common pathological type of esophageal cancer. China has a high
proportion of ESCC, prompting studies investigating the genetic
mechanisms underlying the disease (5–8). However,
the pathogenesis of ESCC is not very clear, and the tumor markers
for the diagnosis of ESCC are few in number. Therefore, it is
imperative to undertake an in-depth study into the genetic and
molecular mechanisms underlying the pathogenesis of ESCC.
Development of ESCC markers with strong specificity and high
sensitivity, and molecular therapeutic targets has significant
clinical implications. In this study, we investigated 83 cases of
esophageal squamous carcinoma tissue compared with the
corresponding normal esophageal mucosa, from January 2009 to
October 2010 at the First Affiliated Hospital of Zhengzhou
University. The expression of P-EGFR and p-Akt was detected
immunohistochemically using the SP method. Its prognostic value was
analyzed to provide a valuable standard of reference for diagnosis
and prediction of survival.
Materials and methods
Materials
We studied 83 cases of esophageal squamous carcinoma
tissue and the corresponding normal esophageal mucosa, from January
2009 to October 2010 at the First Affiliated Hospital of Zhengzhou
University. Patients included 44 males and 39 females. The age
ranged from 34 to 68 years, with an average age of 56.2±33.5 years.
TNM staging revealed stage II in 23 cases, stage IIIa in 25 cases,
stage IIIb in 26 cases, and stage IV in 9 cases. Poor
differentiation was seen in 50 cases, moderate in 25 cases, and
high in 8 cases. Lymph node metastasis was observed in 43 cases,
and none in 40 cases. The tumor diameter was <3 cm in 26 cases,
3–5 cm in 32 cases, and >5 cm in 25 cases. The selected subjects
were not treated with neoadjuvant therapy. Seventy patients were
followed up over 60 months. This study was approved by the Ethics
Committee of the First Affiliated Hospital of Zhengzhou University.
Signed written informed consents were obtained from all
participants before the study.
Main reagents
Rabbit monoclonal P-EGFR antibody (dilution, 1:100;
cat. no. sc-120) and rabbit polyclonal p-Akt antibody (dilution:
1:100; cat. no. sc-8312) from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA), and xylene (Dongguan Jiabao Petroleum Chemical Co.,
Ltd., Dongguan, China).
Immunohistochemical assay
Sliced specimens were incubated at 58°C for ~3 h,
and subjected to xylene treatment for dewaxing. P-EGFR was detected
as follows using microwave repair at 650 W for 20 min and cooled to
room temperature, washed 3 times using Tween-phosphate-buffered
saline (PBS), first at 25°C for 16 h, and washed three times by
PBS, followed by DAB for coloration, and counterstained with
hematoxylin. After dehydration, it was observed under a light
microscope (BX-42; Olympus, Tokyo, Japan). PBS was used as a
substitute for the first antibody and as the negative control for
P-EGFR detection. The positive film was confirmed by Santa Cruz
Biotechnology, Inc. that provided the rabbit anti-human P-EGFR as a
positive control group.
The p-Akt was detected using boiling repair at 98°C
for 20 min, cooled to room temperature, and washed 3 times with
PBS. After incubation with the first antibody at 4°C for 16 h, it
was washed three times with PBS, and DAB for coloration, followed
by hematoxylin for counterstaining. After dehydration the specimen
was visualized under a light microscope. PBS was used as a
substitute for the first antibody with P-EGFR as the negative
control. The positive film was confirmed by Santa Cruz
Biotechnology, Inc. that provided rabbit anti-human p-Akt as a
positive control group.
Evaluation criterion
In the cytoplasm, cell membrane and nucleus, the
yellow particles were found to be positive cells. Each slice was
randomly selected under 5 high power fields, and the number of
positive cells in 100 cells was calculated.
Scoring criteria for P-EGFR expression level
(9): Absence of staining or <5%
tumor cell staining was scored as (−); 5–19% of tumor cell staining
was considered as (+); 20–50% was scored as (++), and >50% tumor
cell staining was determined as (+++).
Scoring criteria for p-Akt expression were (10): No staining or <30% staining was
determined as (−), >30% tumor cell staining was determined as
positive, yellow as (+), brownish yellow as (++), and brown as
(+++). The final results were as follows: (−) denoted negative
expression (PE-GFR−, p-Akt−); and (+), (++)
and (+++) represented positive expression (PE-GFR+,
p-Akt+).
Statistical analysis
The experimental data were analyzed by SPSS 21.0
software (IBM, Armonk, NY, USA). The two factors were analyzed by
non-parametric Spearman's rank correlation. Kaplan-Meier method was
used to analyze the survival based on single factor, and the
difference was tested by log-rank test. P<0.05 indicated
statistical significance. The survival curve was used for analysis
of the follow-up time and the survival condition of the
patients.
Results
Expression and distribution of P-EGFR
and P-Akt protein
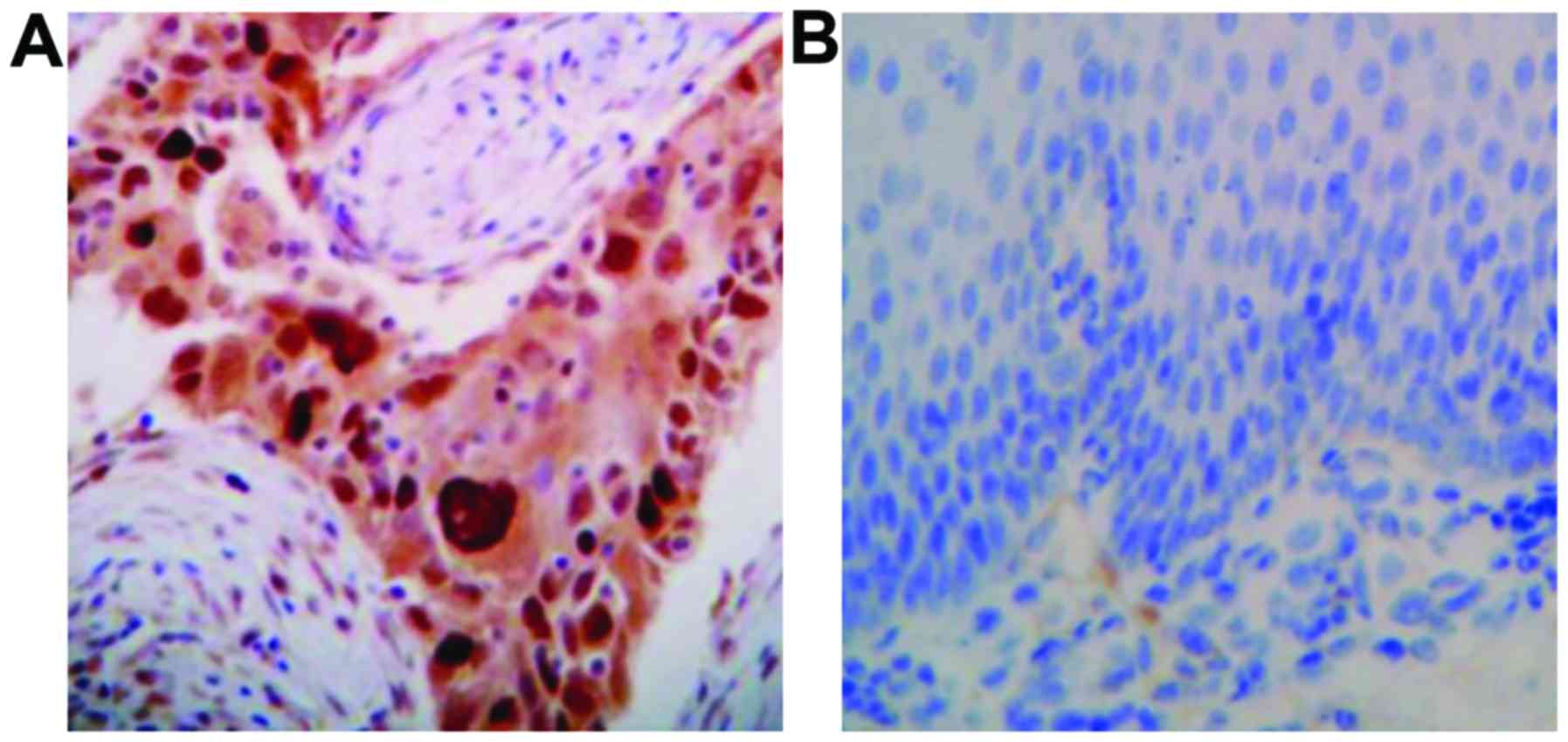
P-EGFR was mainly expressed in the cytoplasm or cell
membrane, with the positive expression was represented by brown
yellow particles (Fig. 1). The P-Akt
protein was expressed in the cytoplasm or nucleus, and the positive
expression was visualized as brownish yellow particles (Fig. 2).
The rate of positive expression of P-EGFR in ESCC
was 88% (73/83 cases), which was significantly higher than the rate
of 41% in the normal esophageal mucosa (34/83 cases) (P<0.05)
(Table I).
 | Table I.Expression of P-EGFR in esophageal
squamous cell carcinoma and adjacent normal esophageal tissues n
(%). |
Table I.
Expression of P-EGFR in esophageal
squamous cell carcinoma and adjacent normal esophageal tissues n
(%).
| Tissue | n | + | ++ | +++ | Total | − |
|---|
| ESCC tissue | 83 | 16 (19.3) | 20 (24.1) | 37 (44.6) | 73 (88) | 10 (12) |
| Adjacent normal
esophageal tissue | 83 | 24 (28.9) | 6 (7.2) | 4
(4.8) | 34 (41) | 49 (59) |
| χ2 |
|
|
|
|
| 16.753 |
| P-value |
|
|
|
|
| <0.05 |
The rate of P-Akt protein expression in ESCC was
90.4% (75/83 cases), which was significantly higher than in the
normal esophageal mucosa (27.7%; 23/83 cases) (P<0.05) (Table II).
 | Table II.Expression of p-Akt in ESCC and
adjacent normal esophageal tissues n (%). |
Table II.
Expression of p-Akt in ESCC and
adjacent normal esophageal tissues n (%).
| Tissue | n | + | ++ | +++ | Total | − |
|---|
| ESCC tissue | 83 | 18 (21.7) | 20 (24.1) | 37 (44.6) | 75 (90.4) | 8
(9.6) |
| Adjacent normal
esophageal tissue | 83 | 17 (20.5) | 4 (4.8) | 2 (2.4) | 23 (27.7) | 60 (72.3) |
| χ2 |
|
|
|
|
|
33.625 |
| P-value |
|
|
|
|
|
<0.05 |
Expression of P-EGFR and P-Akt protein
and clinical pathology of patients with ESCC
The expression of P-EGFR and P-Akt protein in
patients with ESCC was correlated with lymph node metastasis and
degree of differentiation (P<0.05), irrespective of sex, age,
tumor diameter or TNM stage (P>0.05; Table III).
 | Table III.Pathology of esophageal squamous cell
carcinoma: Expression of P-EGFR and P-Akt. |
Table III.
Pathology of esophageal squamous cell
carcinoma: Expression of P-EGFR and P-Akt.
| Clinical pathological
characteristics | n | P-EGFR-positive
cases | χ2
(P-value) | P-Akt-positive
cases | χ2
(P-value) |
|---|
| Sex |
| Male | 44 | 38 | 0.342 (>0.05) | 39 | 0.351 (>0.05) |
|
Female | 39 | 35 |
| 36 |
|
| Tumor diameter,
cm |
|
<3 | 26 | 23 | 1.163 (>0.05) | 23 | 2.505 (>0.05) |
| 3-5 | 32 | 28 |
| 29 |
|
|
>5 | 25 | 22 |
| 23 |
|
| TNM staging |
| II
stage | 23 | 21 | 5.326 (>0.05) | 21 | 1.634 (>0.05) |
| IIIa
stage | 25 | 22 |
| 23 |
|
| IIIb
stage | 26 | 23 |
| 24 |
|
| IV
stage | 9 | 7 |
|
|
|
| Degree of
differentiation |
| Low | 50 | 50 | 9.741 (<0.05) | 49 | 9.266 (>0.05) |
|
Middle | 25 | 19 |
| 22 |
|
| High | 8 | 4 |
| 4 |
|
| Lymph node
metastasis |
| Yes | 43 | 42 | 8.176 (<0.05) | 43 | 6.282 (<0.05) |
| No | 40 | 31 |
| 32 |
|
Expression of P-EGFR and P-Akt protein
in ESCC
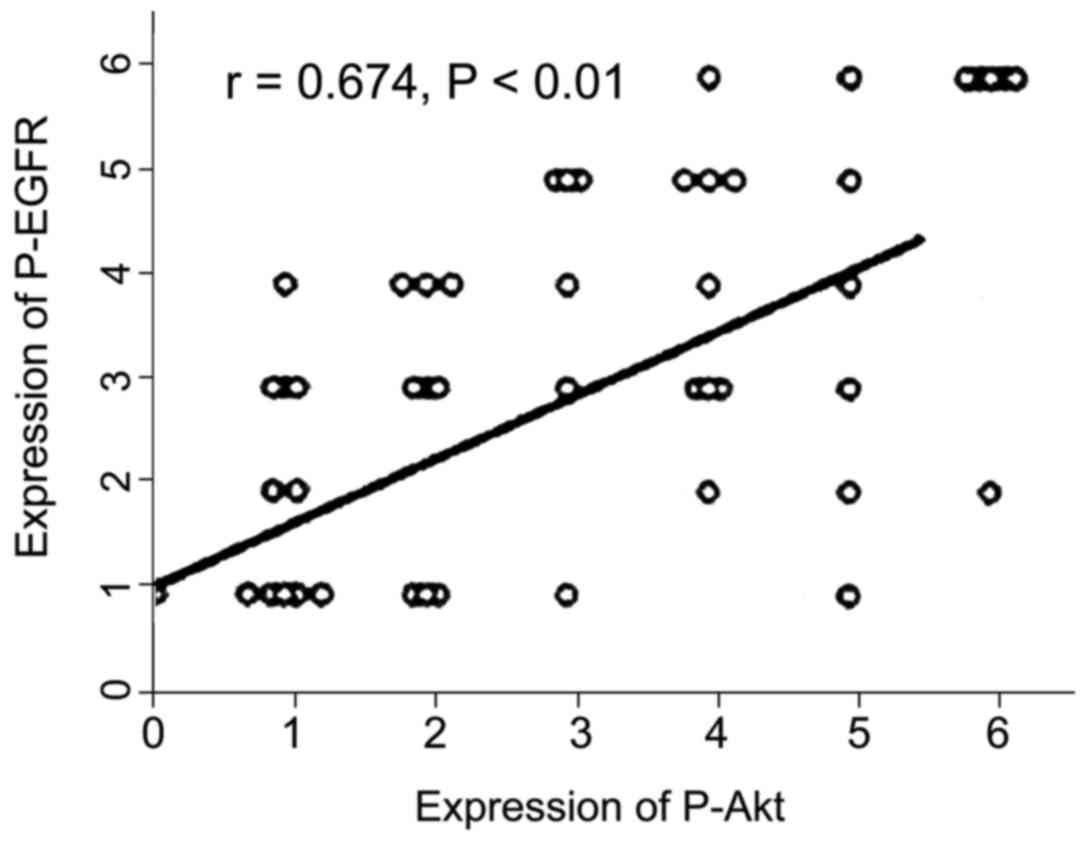
Pearson correlation analysis showed that the
expression of P-EGFR was positively correlated with that of P-Akt
protein in ESCC (r=0.674, P<0.01) (Fig. 3).
Relationship between P-EGFR and P-Akt
expression with survival time in patients with ESCC
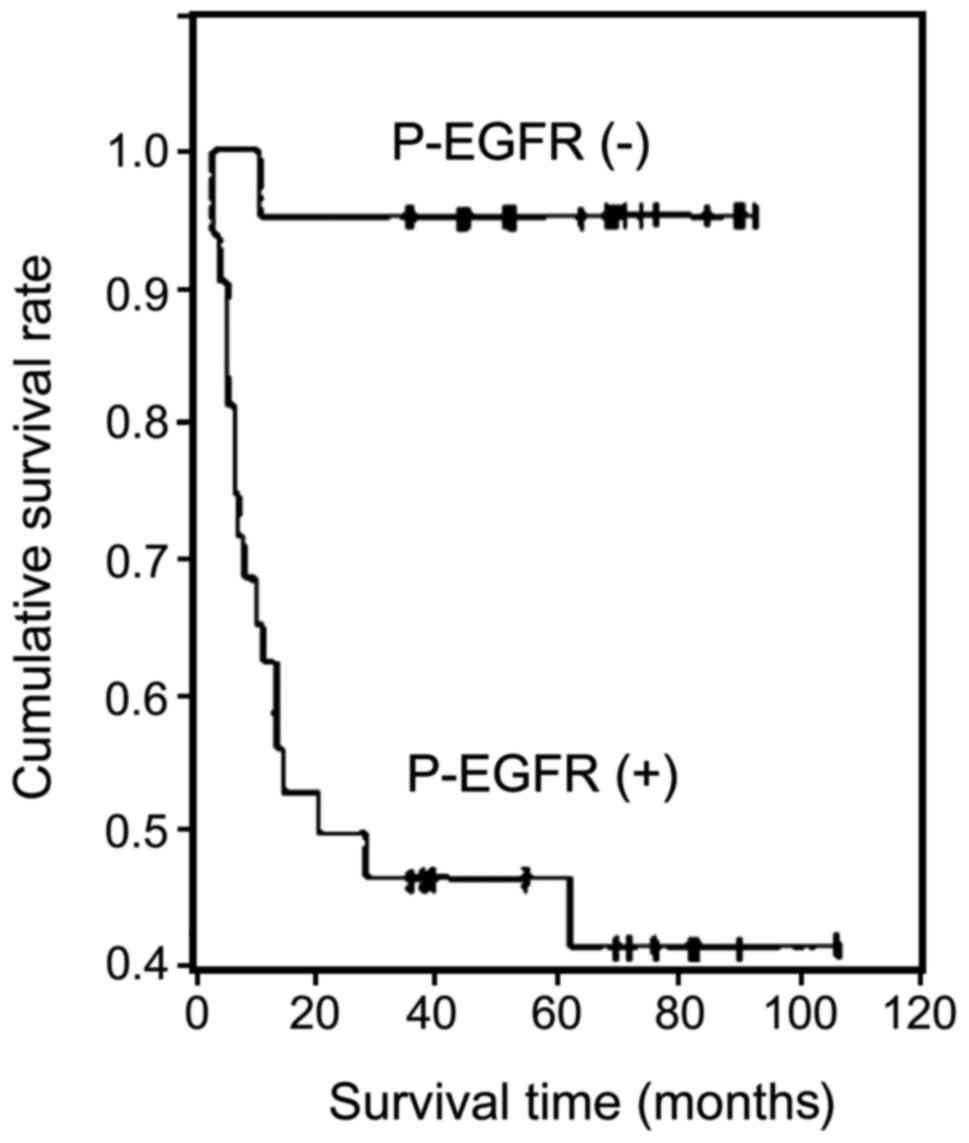
P-EGFR expression was negatively correlated with
survival time in patients with ESCC (r=−0.526, P<0.01).
Kaplan-Meier survival curves showed that the cumulative survival
rate of the P-EGFR-positive cases was significantly lower than that
of the P-EGFR-negative cases (P<0.01) (Fig. 4).
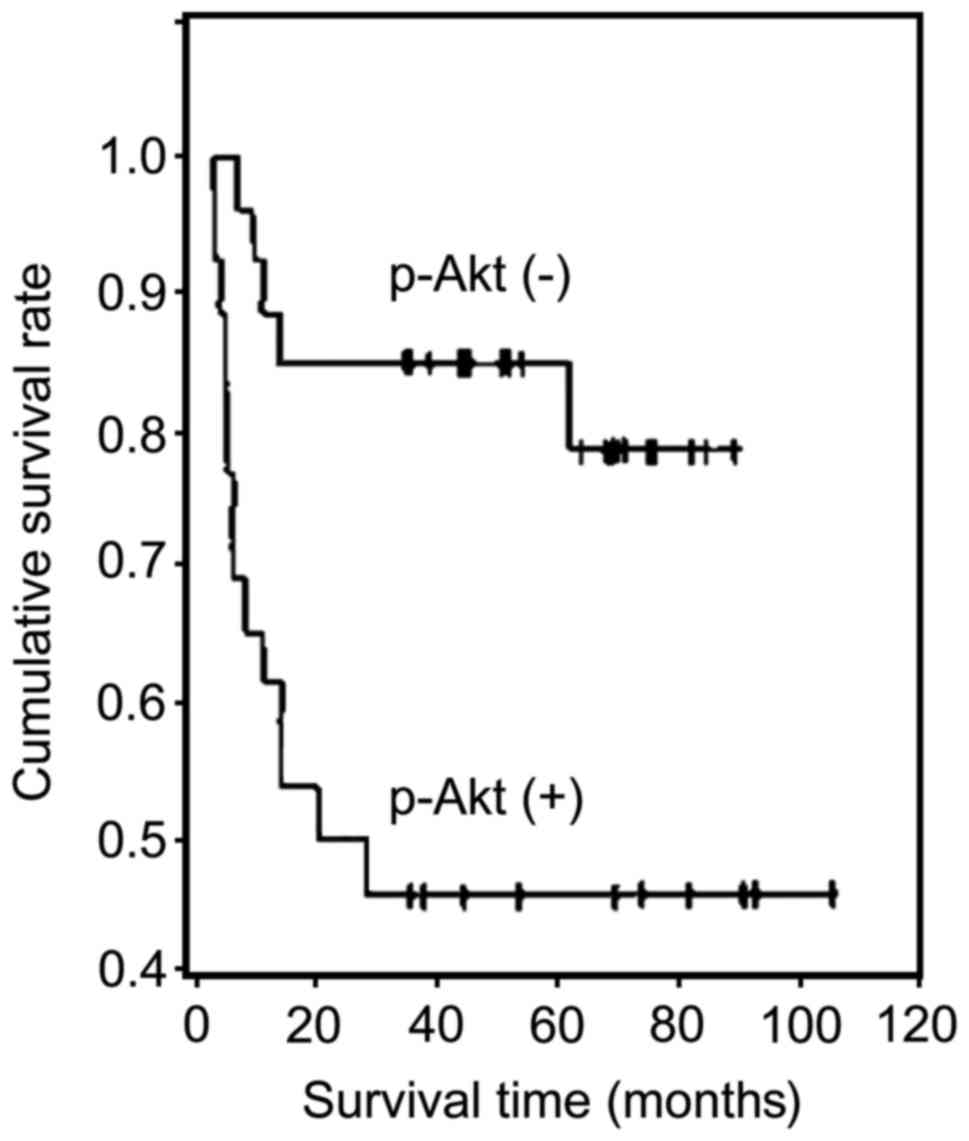
The expression of P-Akt was negatively correlated
with survival in patients with ESCC (r=−0.473, P<0.01).
Kaplan-Meier survival curves showed that the cumulative survival
rate of P-Akt-positive cases was significantly lower than that of
the P-Akt-negative cases (P<0.01) (Fig. 5).
Discussion
Esophageal cancer is a common malignant tumor of the
digestive tract, with a poor prognosis. It is generally divided
into squamous cell carcinoma and adenocarcinoma (11). However, there are regional differences
in the distribution of esophageal cancer: Adenocarcinoma is
prevalent in Europe and America, but squamous cell carcinoma occurs
predominantly in China. In recent years, several studies have
investigated esophageal cancer (12–14).
Clinical studies show (15) that
patients with ESCC are treated with chemotherapy. Results show that
the esophageal cancer is triggered by multiple factors in multiple
stages. The developmental course is divided into simple
hyperplasia, atypical hyperplasia, carcinoma in situ and
infiltrative cancer. A series of oncogenes and anti-oncogenes are
expressed. N-methyl-N nitrosourea alkyl induces ESCC in rats.
Experimental studies confirmed the occurrence of esophageal cancer
following long-term exposure to specific carcinogens (16). Therefore, researchers proposed several
models, such as nitrosamine carcinogenic model,
4-nitroquinoline-oxide model, ectopic transplantation, ESCC model,
and orthotopic transplantation of ESCC. Studies investigated the
etiology and development of ESCC. However, the specific regulatory
mechanism of ESCC and its pathogenesis remain obscure. The absence
of effective clinical treatment resulted in a high incidence of
ESCC, poor clinical prognosis, and high mortality rate.
Advances in molecular biology have shed new light on
the molecular markers of prognosis in ESCC, including the
expression of Fn14, VEGF, NGX6, COX-2, cyclin D1, E-cadherin, and
IMP3. The value of prognosis in ESCC is established. Recent studies
have indicated that (1,17) a high expression of EGFR is related to
prognosis of nasopharyngeal carcinoma. Therefore, we investigated
the molecular targeted therapies of cancer. Using EGFR as molecular
targets, drugs such as erlotinib and cetuximab have been developed.
P-EGFR belongs to the active form of EGFR. Studies have reported
that EGFR itself is not an important factor in cancer (such as
nasopharyngeal) cell proliferation. Elevated P-EGFR expression
plays a key role in the prevalence of cancer, and induces the
proliferation of cancer cells. However, researchers investigating
gastrointestinal carcinoid and pancreatic cancers detected
increased expression of P-EGFR and EGFR proteins. The study also
found that pancreatic cancer patients with low or no expression of
P-EGFR showed better prognosis than patients with high expression
of P-EGFR (18). Akt is highly
activated in tumors suggesting that the growth, differentiation and
proliferation of tumor cells, was abnormal. In vitro studies
suggest that the phosphorylation of Akt residues threonine 308 and
serine 473 was closely related to the activation of PI3K/Akt
signaling (19). However, the role
and clinical significance of P-Akt in the occurrence, development
and evolution of tumors in the human body is not very clear.
Cancer specimens derived from pathological archives
of immunohistochemical staining revealed gene products in patients
with tumor, and retrospective analysis of clinical data is an
important approach of clinical investigation. Tumor dissemination
in the body caused by cancer metastasis is refractory to surgical
treatment. Therefore, it is imperative to understand the factors
associated with tumor metastasis, and understand the mechanisms
underlying invasive cancer, to predict cancer metastasis and
clinical treatment.
In this study, we analyzed P-EGFR and P-Akt
expression in ESCC tissues and in the corresponding normal
esophageal mucosa immunohistochemically. We found a P-EGFR positive
expression rate of 88% in cancer tissues of ESCC, which was
significantly higher than the 41% found in normal esophageal mucosa
tissues (P<0.05). The positive rate of P-Akt protein expression
in the cancer tissue of patients with ESCC was 90.4%, which was
significantly higher than in the corresponding normal esophageal
mucosa tissues, at 27.7% (P<0.05). The positive rate of P-Akt
and P-EGFR protein expression in ESCC is correlated with lymph node
metastasis and differentiation (P<0.05) independent of sex, age,
tumor diameter and TNM stage (P>0.05). The level of P-Akt and
P-EGFR expression may be closely correlated with the occurrence and
evolution of ESCC.
Our analysis showed that the P-EGFR and P-Akt
protein expression in ESCC was positively correlated (r=0.674,
P<0.01). P-EGFR and P-Akt show a synergistic effect in
regulating the proliferation and survival of ESCC cells in
vivo.
We analyzed the follow-up data and survival time.
The results suggest that the expression of P-EGFR was negatively
correlated with the survival time of patients with ESCC (r=−0.526,
P<0.01). Kaplan-Meier survival curves showed that the cumulative
survival rate of the P-EGFR-positive cases was significantly lower
than that of the P-EGFR-negative cases (P<0.01). The expression
of P-Akt was negatively correlated with survival in patients with
ESCC (r=−0.473, P<0.01). Kaplan-Meier survival curves showed
that the cumulative survival rate of P-Akt-positive cases was
significantly lower than that of the P-Akt-negative cases
(P<0.01). P-Akt and P-EGFR promoted metastasis of ESCC and
shortened the survival of patients with ESCC.
In conclusion, the high expression of P-Akt and
P-EGFR is related to lymph node metastasis and differentiation of
ESCC. P-Akt and P-EGFR represent markers of ESCC. The combination
of P-EGFR and P-Akt levels is helpful in evaluating the severity of
ESCC and predicting their survival time.
References
|
1
|
Aleskandarany MA, Rakha EA, Ahmed MA, Powe
DG, Ellis IO and Green AR: Clinicopathologic and molecular
significance of phospho-Akt expression in early invasive breast
cancer. Breast Cancer Res Treat. 127:407–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hembrough T, Thyparambil S, Liao WL,
Darfler MM, Abdo J, Bengali KM, Taylor P, Tong J, Lara-Guerra H,
Waddell TK, et al: Selected reaction monitoring (SRM) analysis of
epidermal growth factor receptor (EGFR) in formalin fixed tumor
tissue. Clin Proteomics. 9:52012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdulwahab A, Sykes J, Kamel-Reid S, Chang
H and Brandwein JM: Therapy-related acute lymphoblastic leukemia is
more frequent than previously recognized and has a poor prognosis.
Cancer. 118:3962–3967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Licitra L, Störkel S, Kerr KM, Van Cutsem
E, Pirker R, Hirsch FR, Vermorken JB, von Heydebreck A, Esser R,
Celik I, et al: Predictive value of epidermal growth factor
receptor expression for first-line chemotherapy plus cetuximab in
patients with head and neck and colorectal cancer: analysis of data
from the EXTREME and CRYSTAL studies. Eur J Cancer. 49:1161–1168.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia M and Souchelnytstkyi S: Comments on
the cross-talk of TGFβ and EGF in cancer. Exp Oncol. 33:170–173.
2011.PubMed/NCBI
|
|
6
|
Mints M and Souchelnytskyi S: Impact of
combinations of EGF, TGFβ, 17β-oestradiol, and inhibitors of
corresponding pathways on proliferation of breast cancer cell
lines. Exp Oncol. 36:67–71. 2014.PubMed/NCBI
|
|
7
|
Cree IA: Designing personalised cancer
treatments. J Control Release. 172:405–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moghadamtousi SZ, Kadir HA, Paydar M,
Rouhollahi E and Karimian H: Annona muricata leaves induced
apoptosis in A549 cells through mitochondrial-mediated pathway and
involvement of NF-κB. BMC Complement Altern Med. 14:2992014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asare GA, Afriyie D, Ngala RA, Abutiate H,
Doku D, Mahmood SA and Rahman H: Antiproliferative activity of
aqueous leaf extract of Annona muricata L. on the prostate, BPH-1
cells, and some target genes. Integr Cancer Ther. 14:65–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mimeault M and Batra SK: Frequent gene
products and molecular pathways altered in prostate cancer- and
metastasis-initiating cells and their progenies and novel promising
multitargeted therapies. Mol Med. 17:949–964. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mimeault M, Johansson SL and Batra SK:
Pathobiological implications of the expression of EGFR, pAkt, NF-κB
and MIC-1 in prostate cancer stem cells and their progenies. PLoS
One. 7:e319192012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Llovet P, Sastre J, Ortega JS, Bando I,
Ferrer M, García-Alfonso P, Donnay O, Carrato A, Jiménez A, Aranda
E, et al: Prognostic value of BRAF PI3K, PTEN, EGFR copy number,
amphiregulin and epiregulin status in patients with KRAS codon 12
wild-type metastatic colorectal cancer receiving first-line
chemotherapy with anti-EGFR therapy. Mol Diagn Ther. 19:397–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pentheroudakis G, Kotoula V, De Roock W,
Kouvatseas G, Papakostas P, Makatsoris T, Papamichael D, Xanthakis
I, Sgouros J, Televantou D, et al: Biomarkers of benefit from
cetuximab-based therapy in metastatic colorectal cancer:
interaction of EGFR ligand expression with RAS/RAF, PIK3CA
genotypes. BMC Cancer. 13:492013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loupakis F, Cremolini C, Fioravanti A,
Orlandi P, Salvatore L, Masi G, Schirripa M, Di Desidero T,
Antoniotti C, Canu B, et al: EGFR ligands as pharmacodynamic
biomarkers in metastatic colorectal cancer patients treated with
cetuximab and irinotecan. Target Oncol. 9:205–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petrelli F, Borgonovo K and Barni S: The
predictive role of skin rash with cetuximab and panitumumab in
colorectal cancer patients: a systematic review and meta-analysis
of published trials. Target Oncol. 8:173–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li P, Yang R and Gao WQ: Contributions of
epithelial-mesenchymal transition and cancer stem cells to the
development of castration resistance of prostate cancer. Mol
Cancer. 13:552014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan Y, Zhang Y, Li Y, Hu H, Wang L, Li H,
Wang R, Ye T, Luo X, Zhang Y, et al: Prevalence, clinicopathologic
characteristics, and molecular associations of EGFR exon 20
insertion mutations in East Asian patients with lung
adenocarcinoma. Ann Surg Oncol. 21 Suppl 4:S490–S496. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng L, Ren W, Xie L, Li M, Liu J, Hu J,
Liu BR and Qian XP: Anti-EGFR MoAb treatment in colorectal cancer:
limitations, controversies, and contradictories. Cancer Chemother
Pharmacol. 74:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishikawa M, Miyake H and Masato F:
Enhanced sensitivity to sunitinib by inhibition of Akt-1 expression
in human castration-resistant prostate cancer PC3 cells both in
vitro and in vivo. Mol Cancer Ther. 13:1949–1960. 2014.
|