Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the most common gastrointestinal malignancies in China, and its
associated tumor invasion and metastasis are important contributing
factors towards patient mortality and presents a serious risk to
health (1,2). A previous study suggested that ESCC
primarily exhibits metastasis via lymphatic vessels in the early
stages (1,2). Therefore, investigating the factors that
may affect lymph angiogenesis in ESCC, as well as the molecular
mechanisms underlying lymphatic metastasis, may have vital clinical
significance towards the diagnosis and treatment of ESCC (3). Vascular endothelial growth factor C
(VEGF-C) is a member of the vascular endothelial growth factor
family, and previous studies have demonstrated that VEGF-C
participates in the lymph angiogenesis of a variety of malignant
tumors, resulting in the lymph node metastasis of tumors and thus
affecting prognosis (4–6). Tumor metastasis-related gene 1 (MTA1)
occupies a specific position in the entire tumor metastasis-related
gene family. A previous study demonstrated that MTA1 protein is a
subunit of the nucleosome remodeling and histone deacetylase NuRD
(7). It regulates the state of
histone deacetylase (HDAC) and nucleosomes by interacting with HDAC
so as to regulate its biological functions. In addition, previous
studies have implicated MTA1 in multiple types of cancer, including
gastric, colon, breast, non-small cell lung, prostate, ovarian and
esophageal cancer, and, head and neck squamous cell carcinoma
(8–10). Previous studies have primarily focused
on the role of MTA1 in tumor angiogenesis, suggesting that it
serves crucial functions in tumor angiogenesis in addition to
promoting the invasion and metastasis of tumors, while its roles in
lymph angiogenesis remain unclear (7–10).
Therefore, the present study used immunohistochemistry to detect
MTA1, VEGF-C and carcinoembryonic antigen M2A monoclonal antibody
(D2-40) in resected tissue samples from patients with ESCC, aiming
to investigate the roles of MTA1 and VEGF-C in lymph angiogenesis
and lymph node metastasis in ESCC, and the associations between
them.
Materials and methods
Sample sources
The ESCC tissues were surgically resected and
collected from 107 patients with ESCC from the Department of
Cardiothoracic Surgery, Suining Central Hospital (Suining, China)
from March 2013 to January 2014, and 56 samples of normal
esophageal tissues were also collected during the same period at
the same location. Clinicopathological data for all patients is
provided in Table I. All tissue
samples were paraffin-embedded for storage prior to further
experiments. All patients involved did not receive preoperative
radiotherapy, chemotherapy, traditional Chinese medicine treatment
or any other type of treatment, and had no family history of the
disease. The diagnosis for all patients with ESCC was confirmed by
postoperative pathology, and tumor-node-metastasis (TNM) staging
and tumor grading were performed based on the ESCC staging criteria
of American Joint Committee on Cancer/Union for International
Cancer Control 7th edition, 2009 (2).
The present study was performed in accordance with the declaration
of Helsinki and was approved by the Ethics Committee of Suining
Central Hospital. Written informed consent was obtained from all
patients, prior to enrollment in the present study.
 | Table I.Clinicopathological data of the 107
patients with ESCC. |
Table I.
Clinicopathological data of the 107
patients with ESCC.
| Clinicopathological
data | Distribution of
patients |
|---|
| Age (mean ± standard
deviation) | 61.15±7.12 |
| Sex
(male:female) | 86:21 |
| Lesion length
(≥5/<5 cm) | 35/72 |
| Tumor type (visual
assessment) (ulcerative/fungating/medullary/rosive) | 76/13/10/8 |
| Differentiation
degree (high/medium/low) | 24/65/18 |
| TNM staging
(I/II/III) | 10/50/47 |
| T stage
(T1/T2/T3/T4) | 12/25/64/6 |
| N stage
(N0/N1/N3/N) | 44/39/19/5 |
Experimental methods and
immunohistochemical evaluations
The paraffin-embedded tissues were cut into 4 µm
sections and stored at 4°C, followed by dewaxing, hydration and
closure in 3% hydrogen peroxide in darkness for 20 min at 20°C. The
tissue sections were then washed with distilled water for 5 min
three times. Subsequently, antigen retrieval was performed as
follows: A plastic section tank, filled with 0.01 mol/l citric acid
buffer (pH 6.0), was placed into a microwave for 10-min pre-heating
(high-middle power), then tissue sections were added and microwave
antigen retrieval was performed for 20 min (high power), 10 min
(middle-high power) prior to cooling to room temperature. Tissue
sections were again washed with PBS for 5 min three times.
Subsequently, the mouse anti-human tumor MTA1 (A11; no. sc-17773;
1:50 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
rabbit anti-human VEGF-C (H-190; no. sc-9047; 1:50 dilution; Santa
Cruz Biotechnology, Inc.) and mouse anti-human D2-40
immunohistochemical monoclonal antibodies (no. MAB-0567; 1:100
dilution; Fuzhou Maxin Biotech Co., Ltd., Fuzhou, China) were added
dropwise, then placed into a wetbox for 1 h at 37°C, prior to
overnight storage at 4°C. The sections were then washed with PBS
for 5 min three times. Secondary antibodies (Envision™ kit and
chromogenic agents; Dako REAL™ EnVision™ Detection System,
Peroxidase/DAB+, Rabbit/Mouse; cat. no. K5007) were then added
dropwise, placed into a wetbox for 1 h culture at 37°C, prior to
washing with PBS for 5 min three times. The residual PBS on the
tissue sections was discarded and DAB solution was added and
samples were incubated for 10 sec at room temperature. The tissue
sections were placed into distilled water and hematoxylin
re-staining was performed for 3 min at room temperature. Following
hydrochloric acid-ethanol differentiation, the tissue sections were
washed with running water for 10 min and dehydrated sequentially
using 85, 95 and 100% ethanol for 5 min at each gradient, and
finally mounted with green mounting glue.
PBS was used to replace the primary antibodies for
the control tissues and breast tissues with clear expression were
used as the positive control (11).
Two experienced pathologists who were blinded to the identity of
each sample conducted the determination of immunohistochemical
results independently. The tissue sections were scored according to
the proportion of positive cells: 0, ≤5%; 1, 6–25%; 2, 26–50%; 3,
51–75%; 4, 76–100%. The staining intensity was scored as follows:
0, no signal; 1, pale yellow; 2, buffy; 3, brownish. Comprehensive
evaluation: The results were then scored based on the product of
the positive cell proportion and the staining intensity: 0, 0–1
score; 1, 2–4 score; 2, 6–8 score; 3, 9–12 score; high expression,
≥2 points; low expression, <2 points (12,13). LVD
in ESCC tissue sections that were single-stained for D2-40 was
analyzed as previously described. Tumoral LVD located at the
periphery of tissue, within 2 mm of the tumor and adjacent to the
invasive front was assessed. Five areas where the most lymphatic
vessels could be observed were chosen using light microscopy at ×40
magnification. LVD was assessed by counting all the stained vessels
in each area at ×200 magnification. The assessed mean number of
lymphatic vessels was determined and expressed as LVD (14).
Statistical methods
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) was
used to analyze the experimental data. The χ2 test was used to
analyze the differences between high and low expression levels of
MTA1 and VEFG-C, and Kruskal-Wallis one-way analysis of variance
was used to analyze the measurement data among >3 groups. The
correlations between MTA1 and VEGF-C expression levels were
analyzed using Spearman's ρ correlation analysis. Comparison of the
means between the groups was performed using a Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of positive
immunohistochemical expression
Immunohistochemical analysis results suggested that
the positive signal of MTA1 protein was located in the nuclei of
ESCC cells (Fig. 1) and the positive
signal of VEGF-C protein was located in the cytoplasm of ESCC cells
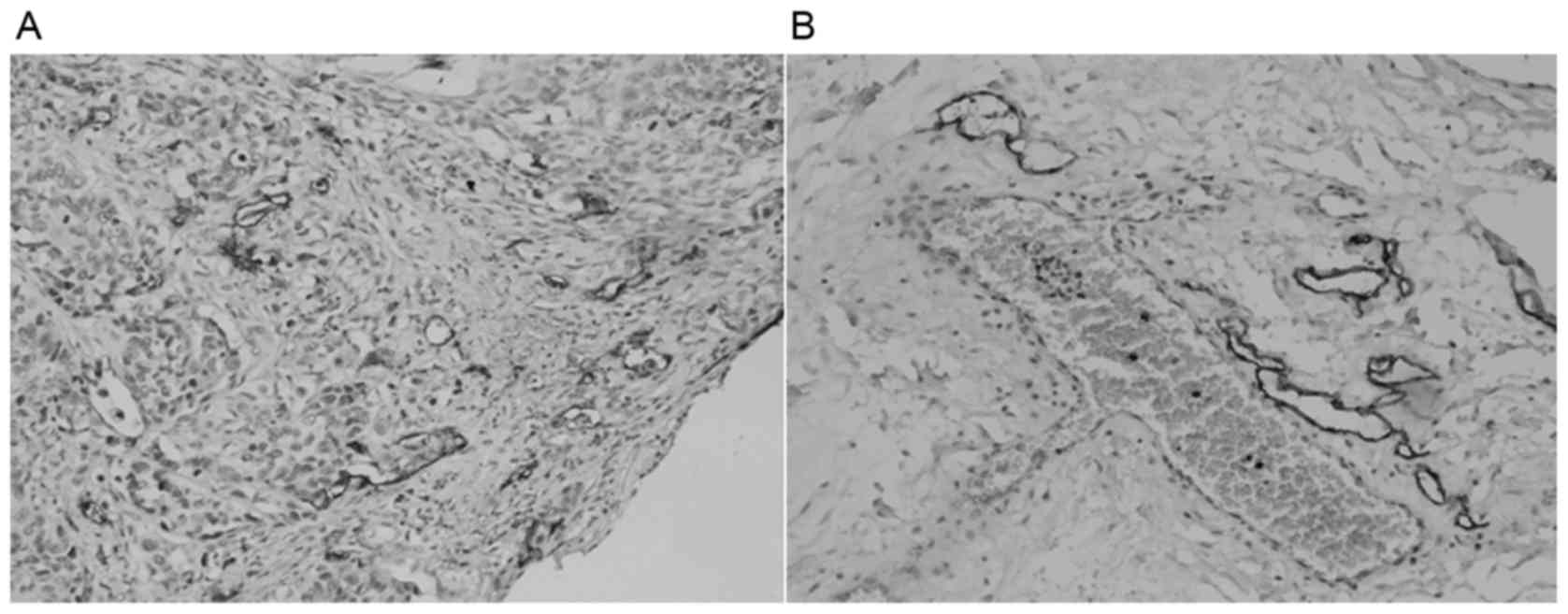
(Fig. 2). D2-40 staining was mainly
located in the tumor stroma microenvironment surrounding the ESCC
tumor tissues (Fig. 3).
Expression levels of MTA1 and VEGF-C
and its correlations with the clinicopathological parameters in
ESCC tissues
In ESCC, the protein expression level of MTA1 was
50.4% and that of VEGF-C was 58.8%, which are significantly high
compared with those in the normal esophageal tissues (P<0.05).
Among the 107 ESCC tissue samples, the expression levels of MTA1
and VEGF-C proteins in the group with lymph node metastasis were
58.7 and 68.3%, respectively, and those in the group without lymph
node metastasis were 38.6 and 45.3%, respectively, differences that
were statistically significant (P<0.05). In the T3/4 group, the
expression level of MTA1 and VEGF-C proteins was 58.6 and 65.7%,
respectively, and in the T1/2 group it was 35.1 and 45.9%,
respectively, differences that were also statistically significant
(P<0.05). There was no correlation between high expression
levels of MTA1 and VEGF-C proteins and the patient age, sex,
differentiation degree or tumor size (P>0.05; Table II).
 | Table II.Associations between the expression
levels of MTA1 and VEGF-C and the clinicopathological parameters of
patients with ESCC. |
Table II.
Associations between the expression
levels of MTA1 and VEGF-C and the clinicopathological parameters of
patients with ESCC.
|
|
| MTA1 |
|
| VEGF-C |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Parameters | Cases | Low expression | High expression | χ2 | P-value | Low expression | High expression | χ2 | P-value |
|---|
| Age |
| ≥60
years | 64 | 31 | 33 | 0.076 | 0.782 | 30 | 34 | 2.177 | 0.140 |
| <60
years | 43 | 22 | 21 |
|
| 14 | 29 |
|
|
| Sex |
| Male | 86 | 41 | 45 | 0.605 | 0.487 | 34 | 52 | 0.456 | 0.500 |
|
Female | 21 | 12 | 9 |
|
| 10 | 11 |
|
|
| Tumor size |
| ≥5
cm | 35 | 21 | 14 | 2.280 | 0.131 | 12 | 23 | 1.004 | 0.316 |
| <5
cm | 72 | 32 | 40 |
|
| 32 | 40 |
|
|
| Differentiation
degree |
|
Middle-high
differentiation | 89 | 43 | 46 | 0.314 | 0.575 | 36 | 53 | 0.099 | 0.753 |
| Low
differentiation | 18 | 10 | 8 |
|
| 8 | 10 |
|
|
| T stage |
| T1/2
stage | 37 | 24 | 13 | 5.319 | 0.021a | 20 | 17 | 3.907 | 0.048a |
| T3/4
stage | 70 | 29 | 41 |
|
| 24 | 46 |
|
|
| Lymph node
metastasis |
|
Yes | 63 | 26 | 37 | 4.184 | 0.041a | 20 | 43 | 5.562 | 0.018a |
|
N/A | 44 | 27 | 17 |
|
| 24 | 20 |
|
|
Associations between the expression
levels of MTA1 and VEGF-C and patient clinical staging and lymph
vessel density in ESCC
The high expression levels of MTA1 and VEGF-C
proteins in various TNM stage ESCC tissue samples were compared
using Kruskal-Wallis one-way analysis of variance, and the
differences were statistically significant (P<0.05; Table III). In the group with high
expression levels of MTA1, the micro-lymphatic density (LVD) was
15.784±3.874/high-power field (HPF), which was significantly
different compared with in the group with low MTA1 expression
levels (11.550±3.341/HPF; P<0.05). In the group with a high
expression level of VEGF-C, LVD was 15.333±3.803/HPF, which was
significantly different compared with a low expression level of
VEGF-C in the group with low LVD (11.333±3.556/HPF; P<0.05).
 | Table III.Associations between the expression
levels of MTA1 and VEGF-C with clinical staging of ESCC. |
Table III.
Associations between the expression
levels of MTA1 and VEGF-C with clinical staging of ESCC.
|
| MTA1 |
| VEGF-C |
|
|---|
|
|
|
|
|
|
|---|
| Stage | Low expression | High
expression | Sum | χ2 | Low expression | High
expression | Sum | χ2 |
|---|
| I | 7 | 3 | 10 | 6.407 | 7 | 3 | 10 | 6.058 |
| II | 29 | 21 | 50 | P=0.041 | 22 | 28 | 50 | P=0.048 |
| III | 17 | 30 | 47 |
| 14 | 33 | 47 |
|
| Sum | 53 | 54 | 107 |
| 44 | 63 | 107 |
|
Association between the protein
expression levels of MTA1 and VEGF-C in ESCC
The protein expression levels of MTA1 and VEGF-C in
ESCC were determined using Spearman's rank correlation test. The
results revealed that they were positively correlated (r=0.512;
P<0.000; Table IV).
 | Table IV.Associations between the protein
expression levels of MTA1 and VEGF-C in ESCC. |
Table IV.
Associations between the protein
expression levels of MTA1 and VEGF-C in ESCC.
|
| VEGF-C |
|
|---|
|
|
|
|
|---|
| MTA1 | 0 point | 1 point | 2 points | 3 points | Sum |
|---|
| 0 point | 10 | 5 | 4 | 2 | 21 |
| 1 point | 3 | 13 | 13 | 3 | 32 |
| 2 points | 1 | 7 | 15 | 4 | 27 |
| 3 points | 0 | 5 | 6 | 16 | 27 |
| Sum | 14 | 30 | 38 | 25 | 107 |
Discussion
Cases of esophageal cancer in China are typically
ESCC, which is the fifth most common type of cancer in China and
has an associated mortality rate ranked as the fourth highest among
malignant types of cancer (3). ESCC
may be spread in vivo via the direct invasion of surrounding
tissues, including lymphatic metastasis, hematogenous metastasis
and implantation metastasis (2).
Invasion and metastasis remain important factors that affect the
post-operative 5-year survival rate of patients with ESCC (1,2). The
esophagus lacks the serosal layer and is rich in lymphatic
drainage; therefore, ESCC may be transferred mainly via lymphatic
metastasis in the early stages. Furthermore, investigating the
factors that affect lymph angiogenesis in ESCC, as well as the
molecular mechanisms underlying lymphatic metastasis, may have
important clinical value for the diagnosis and treatment ESCC
(15,16).
It has previously been demonstrated that D2-40 was a
lymphatic endothelial cell marker with high specificity, thus the
present study used D2-40 to mark the lymphatic endothelial cells in
ESCC and normal esophageal tissues, and to determine the LVD
(17,18). The present study revealed that LVD was
high in the tumor microenvironment surrounding ESCC, and were
irregular in shape and exhibited the extended state. Whereas LVD
was low in the central region of ESCC, mostly with strip-like
shapes and exhibiting the blocking state, with only a few
exhibiting the extended state, similar to a previous study by
Padera et al (19). These
structural characteristics may allow micro-lymphatic vessels to
become the direct channel for the invasion and metastasis of
malignant tumors. In the present study, the LVD in the ESCC group
(13.688±4.183) was significantly different compared with that in
the normal esophageal group (9.165±2.284; P<0.05). LVD in the
surrounding area (stroma) of ESCC was significantly higher compared
with those in the central region of ESCC and normal esophageal
tissues; the comparison of LVD between the 44 cases without lymph
node metastasis (12.474±4.647) and 63 cases with lymph node
metastasis (14.676±3.473), revealed that LVD in the group with
lymph node metastasis was significantly higher compared with in
tissues without lymph node metastasis, and the difference was
statistically significant (P<0.05). Therefore, LVD in ESCC was
increased compared with control tissue, and LVD was associated with
lymph node metastasis.
MTA1 is a novel tumor metastasis-associated gene,
highly expressed in normal human testis, with no or low expression
in other non-cancerous tissues; it may be upregulated to various
degrees in numerous types of cancer and is closely associated with
tumor metastasis and invasion (11,20).
However, these previous studies were limited to the investigation
of associations between MTA1 and prognosis, or between MTA1 and
tumor angiogenesis, and did not investigate the associations
between MTA1 and tumor lymph angiogenesis. The present study
demonstrated that the expression level of MTA1 protein in ESCC was
significantly higher compared with in normal esophageal tissues
(P<0.05). MTA1 protein was primarily expressed in the nuclei of
ESCC cells, and with increasing ESCC stage, the protein expression
levels of MTA1 gradually increased. Among the 63 patients with ESCC
lymph node metastasis, 37 exhibited high expression levels of MTA1
protein. Among the 44 patients with ESCC without lymph node
metastasis, 17 exhibited high expression levels of MTA1 protein.
For each group, the difference was statistically significant
(P<0.05). In ESCC, LVD in the group with high expression of MTA1
was significantly high compared with in the group with low MTA1
expression (P<0.05), which indicated that the MTA1 gene may
serve a promoting role in the development, lymph angiogenesis and
lymph node metastasis of ESCC.
Previous studies have revealed that VEGF-C is
overexpressed in numerous types of human malignancies, and
participates in the lymph angiogenesis of certain malignant types
of cancer, resulting in the progression and lymph node metastasis
of primary tumors and ultimately affecting patient prognosis
(21–23). The results of the present study
suggested that VEGF-C protein was predominantly expressed in the
cytoplasm of ESCC cells, and with increasing tumor stage the
protein expression levels of VEGF-C were also increased, suggesting
that the VEGF-C gene may serve a specific promoting role in the
development of ESCC. The protein expression level of VEGF-C in ESCC
was significantly high, compared with in the normal esophageal
tissues (P<0.05). Peng et al (24) retrieved the VEGF-associated literature
in PubMed and Embase databases and performed a meta-analysis of 19
studies that met the final conditions (including 1,453 patient
cases), and concluded that the overexpression of VEGF-C was
positively associated with the poor prognosis of ESCC. In
particular, Asian patients with ESCC and positive VEGF-C expression
exhibited a poorer prognosis when compared with those patients with
negative VEGF-C expression level (23). VEGF-C was an effective indicator of
the prognosis of ESCC; however, prospective studies are required
for further confirmation (24). In
the present study, among the 63 patients with ESSC and lymph node
metastasis, 43 exhibited a high protein expression level of VEGF-C,
and among the 44 ESSC patients without lymph node metastasis, 20
had high protein expression levels of VEGF-C, a difference that was
statistically significant (P<0.05). In ESCC tissues, LVD in the
group with a high VEGF-C expression was significantly higher
compared with in the group with low VEGF-C expression (P<0.05).
Therefore, the present study hypothesized that detecting the
expression level of VEGF-C in biopsy specimens may aid the
prediction of lymph node metastasis in ESCC.
Moon et al (25) revealed that MTA1 increased the
expression and stability of hypoxia-inducible factor 1-α (HIF-1α),
and that HIF-1α also promoted tumor angiogenesis by regulating
VEGF; a high expression level of MTA1 was also indicated to
increase the metastatic potential of tumor cells (26). To the best of our knowledge, no
previous studies have investigated the association between MTA1 and
VEGF-C expression in ESCC, in addition to their association with
tumor lymph angiogenesis. Using the χ2 test, the present
study revealed significant differences between the expression
levels of MTA1 and VEGF-C present in the ESCC cases with lymph node
metastasis (37/63, 43/63) and those in the ESCC cases without lymph
node metastasis (17/44, 20/44; P=0.041, P=0.018, respectively). In
addition, LVD in the ESCC cases with high expression levels of MTA1
and VEGF-C was significantly higher compared with in the ESCC cases
with low expression levels of MTA1 and VEGF-C (P<0.05). This
suggested that the expression levels of MTA1 and VEGF-C may be
closely associated with lymph angiogenesis in ESCC, and that with
increasing expression levels the likelihood of lymph node
metastasis in ESCC may be correspondingly increased. The Spearman's
ρ test revealed that the protein expression levels of MTA1 and
VEGF-C were positively associated in ESCC (r=0.512; P<0.000),
indicating that MTA1 and VEGF-C may serve synergic roles in the
lymph angiogenesis of ESCC, which could co-promote the growth and
lymphatic metastasis of ESCC. Therefore, according a study
performed by Moon et al (25),
the present study hypothesized that MTA1 may adhere to HIF-1α,
thereby regulating the expression of VEGF-C and promoting lymph
angiogenesis in ESCC; however, the specific underlying regulatory
mechanisms require further experimental study.
References
|
1
|
Lin CS, Cheng CT, Liu CY, Lee MY, Hsiao
MC, Shih CH and Liu CC: Radical lymph node dissection in primary
esophagectomy for esophageal squamous cell carcinoma. Ann Thorac
Surg. 100:278–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Handbook. 7th.
Springer; New York, NY: 2010
|
|
3
|
Xu XL, Zheng WH, Zhu SM, Zhao A and Mao
WM: The prognostic impact of lymph node involvement in large scale
operable node-positive esophageal squamous cell carcinoma patients:
A 10-year experience. PLoS One. 10:e01330762015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng Y, Wang W, Hu J, Ma J, Zhang Y and
Zhang J: Expression of VEGF-C and VEGF-D as significant markers for
assessment of lymphangiogenesis and lymph node metastasis in
non-small cell lung cancer. Anat Rec (Hoboken). 293:802–812. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wakisaka N, Hirota K, Kondo S,
Sawada-Kitamura S, Endo K, Murono S and Yoshizaki T: Induction of
lymphangiogenesis through vascular endothelial growth
factor-C/vascular endothelial growth factor receptor 3 axis and its
correlation with lymph node metastasis in nasopharyngeal carcinoma.
Oral Oncol. 48:703–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pérez D, Rohde A, Callejón G, Pérez-Ruiz
E, Rodrigo I, Rivas-Ruiz F, Ramos B, Medina F, Villatoro R, Redondo
M, et al: Correlation between serum levels of vascular endothelial
growth factor-C and sentinel lymph node status in early breast
cancer. Tumour Biol. 36:9285–9293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li DQ, Suresh SB, Sujit SS, Eswaran J and
Kumar R: Metastasis-associated protein 1/nucleosome remodeling and
histone deacetylase complex in cancer. Cancer Res. 72:387–394.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li SH, Tian H, Yue WM, Li L, Li WJ, Chen
ZT, Hu WS, Zhu YC and Qi L: Overexpression of metastasis-associated
protein 1 is significantly correlated with tumor angiogenesis and
poor survival in patients with early-stage non-small cell lung
cancer. Ann Surg Oncol. 18:2048–2056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kai L, Wang J, Ivanovic M, Chung YT,
Laskin WB, Schulze-Hoepfner F, Mirochnik Y, Satcher RL Jr and
Levenson AS: Targeting prostate cancer angiogenesis through
metastasis-associated protein 1 (MTA1). Prostate. 71:268–280. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prisco MG, Zannoni GF, De Stefano I,
Vellone VG, Tortorella L, Fagotti A, Mereu L, Scambia G and Gallo
D: Prognostic role of metastasis tumor antigen 1 in patients with
ovarian cancer: A clinical study. Hum Pathol. 43:282–288. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagaraj SR, Shilpa P, Rachaiah K and
Salimath BP: Crosstalk between VEGF and MTA1 signaling pathway to
aggressiveness of breast carcinoma. Mol Carcinog. 54:333–350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hofer MD, Kuefer R, Varambally S, Li H, Ma
J, Shapiro GI, Gschwend JE, Hautmann RE, Sanda MG, Giehl K, et al:
The role of metastasis-associated protein 1 in prostate cancer
progression. Cancer Res. 64:825–829. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyake K, Yoshizumi T, Imura S, Sugimoto
K, Batmunkh E, Kanemura H, Morine Y and Shimada M: Expression of
hypoxiainducible actor-1alpha, histone deacetylase 1, and
metastasis-associated protein 1 in pancreatic carcinoma:
Correlation with poor prognosis with possible regulation. Pancreas.
36:e1–e9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aishima S, Nishihara Y, Iguchi T, Taguchi
K, Taketomi A, Maehara Y and Tsuneyoshi M: Lymphatic spread is
related to VEGF-C expression and D2-40-positive myofibroblasts in
intrahepatic cholangiocarcinoma. Mod Pathol. 21:256–264. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sawyers CL: The cancer biomarker problem.
Nature. 452:548–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zamanian-Azodi M, Rezaei-Tavirani M,
Hasanzadeh H, Rahmati Rad S and Dalilan S: Introducing biomarker
panel in esophageal, gastric, and colon cancers; A proteomic
approach. Gastroenterol Hepatol Bed Bench. 8:6–18. 2015.PubMed/NCBI
|
|
17
|
Saad RS, Lindner JL, Liu Y and Silverman
JF: Lymphatic vessel density as prognostic marker in esophageal
adenocarcinoma. Am J Clin Pathol. 131:92–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Straume O, Jackson DG and Akslen LA:
Independent prognostic impact lymphatic vessel density and presence
of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer
Res. 9:250–256. 2003.PubMed/NCBI
|
|
19
|
Padera TP, Kadambi A, di Tomaso E,
Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark
EJ, et al: Lymphatic metastasis in the absence of functional
intratumor lymphatics. Science. 296:1883–1886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng X, Du L, Wang C, Yang Y, Li J, Liu H,
Zhang J, Wang L, Zhang X, Li W, et al: Close association of
metastasis-associated protein 1 overexpression with increased
angiogenesis and poor survival in patients with histologically
node-negative gastric cancer. World J Surg. 37:792–798. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie LX, Zhai TT, Yang LP, Yang E, Zhang
XH, Chen JY and Zhang H: Lymphangiogenesis and prognostic
significance of vascular endothelial growth factor C in
gastro-oesophageal junction adenocarcinoma. Int J Exp Pathol.
94:39–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ochi N, Matsuo Y, Sawai H, Yasuda A,
Takahashi H, Sato M, Funahashi H, Okada Y and Manabe T: Vascular
endothelial growth factor-C secreted by pancreatic cancer cell line
promotes lymphatic endothelial cell migration in an in vitro model
of tumor lymphangiogenesis. Pancreas. 34:444–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang CA, Harrell JC, Iwanaga R, Jedlicka P
and Ford HL: Vascular endothelial growth factor C promotes breast
cancer progression via a novel antioxidant mechanism that involves
regulation of superoxide dismutase 3. Breast Cancer Res.
16:4622014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng J, Shao N, Peng H and Chen LQ:
Prognostic significance of vascular endothelial growth factor
expession in esophageal carcinoma: A meta-analysis. J BUON.
18:398–406. 2013.PubMed/NCBI
|
|
25
|
Moon HE, Cheon H, Chun KH, Lee SK, Kim YS,
Jung BK, Park JA, Kim SH, Jeong JW and Lee MS:
Metastasis-associated protein 1 enhances angiogenesis by
stabilization of HIF-1α. Oncol Rep. 16:929–935. 2006.PubMed/NCBI
|
|
26
|
Du B, Yang ZY, Zhong XY, Fang M, Yan YR,
Qi GL, Pan YL and Zhou XL: Metastasis-associated protein 1 induces
VEGF-C and facilitates lymphangiogenesis in colorectal cancer.
World J Gastroenterol. 17:1219–1226. 2011. View Article : Google Scholar : PubMed/NCBI
|