Introduction
Gliomas are the most frequent and aggressive
malignant tumors, with an average survival time of 12 months
(1–3).
A major cause of the failure of conventional treatments is the
highly invasive and diffusively infiltrative nature of these tumors
(4,5).
Despite advances in surgery and adjuvant therapy, the survival time
of patients with malignant glioma has changed little over the past
decades (6,7). With the development of molecular
biology, gene therapy is becoming the focus of tumor therapy.
Therefore, identifying molecular mechanisms and novel tumor
therapeutic targets is critical and necessary for this incurable
cancer.
β-transducin repeat-containing protein (β-TrCP), as
the substrate recognition subunit for the E3 ubiquitin ligases,
utilizes seven WD40 repeats to interact with substrates
phosphorylated within the DSG (X)2+nS destruction motifs
and is involved in the degradation of numerous proteins in cell
signaling and cell cycle regulation (8–10). β-TrCP
is involved in major regulatory mechanisms, including cell cycle
progression, metabolism, development and immunity (11–14).
Notably, two β-TrCP proteins are expressed in humans. β-TrCP1 is
encoded by BTRC, and β-TrCP2 encoded by FBXW11, also known as HOS
or β-TRCP2 (15,16). The ubiquitin proteasome pathway serves
a pivotal role in controlling the degradation of the majority of
regulatory proteins in mammalian cells (17,18) and
regulates a number of cellular processes by facilitating the timely
destruction of key regulatory proteins by the 26S proteasome
complex (19). In this pathway,
protein ubiquitination involves the concerted action of the E1
ubiquitin-activating enzyme, an E2 ubiquitin-conjugating enzyme and
an E3 ubiquitin-protein ligase, of which delivers multiple
ubiquitin molecules to the target protein (20–22).
Diverse β-TrCP substrates involved in different
normal and malignant pathways have been identified, includingBMI-1
(23), IκB (24), β-catenin (25,26),
vascular endothelial growth factor receptor 2 (VEGFR2) (27), metastasis suppressor protein 1 (MTSS1)
(28), Emi1 (29), SNAI1 (30), and M-phase inducer phosphatase 1
(Cdc25A) (9). Zhong et al
(28) reported that β-TrCP
targetsMTSS1 for ubiquitination-mediated destruction, to promote
breast and prostate cancer cell proliferation and migration.
However, Shaik et al (27)
demonstrated that β-TrCP suppresses angiogenesis and thyroid cancer
cell migration by promoting ubiquitination and destruction of
VEGFR2. In addition, β-TrCP may inhibit growth and invasiveness of
lung cancer cells (31). Therefore,
the roles of β-TrCP are different in different types of tumors.
Previous studies by the authors have demonstrated
that β-TrCP protein expression levels were significantly lower in
glioma compared with non-tumorous human brain tissues and that low
β-TrCP expression indicated poor prognosis in patients with glioma
(32). In the present study, it was
additionally observed that β-TrCP affected migration, invasion and
proliferation of human glioma cells.
Materials and methods
Antibodies and reagents
The rabbit polyclonal anti-β-TrCP antibody was
purchased from Abcam (ab71753; 1:500; Cambridge, UK). The rabbit
polyclonal anti-Flag antibody was purchased from EarthOx Life
Sciences (EO22230; 1:2,000; Millbrae, CA, USA). The rabbit
monoclonal anti-β-actin antibody was purchased from EMD Millipore
(04–1116; 1:2,000; Billerica, MA, USA).
Cell culture and plasmid
transfection
Human glioma U251 and U87 cell lines were purchased
from Shanghai Cell Bank of the Type Culture Collection Committee of
the Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in DMEM/F-12 media (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Evergreen Biological Engineering Co., Hangzhou, China) in a
humidified incubator with 5% CO2 at 37°C. For transfection, U251 or
U87 cells were transfected with β-TrCP plasmid (1 µg; plasmid
10865; Addgene, Inc., Cambridge, MA, USA) using Lipofectamine
2000® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's specifications. U251 or U87 cells
transfected with an empty plamid were used as controls.
Western blot analysis
Following 24 h transfection with the β-TrCP plasmid,
total protein from the transfected U251/U87 cells was extracted
using lysis buffer (RIPA, 1 ml; Aprotinin, 1 µl, 2 µg/ml;
Leupeptin, (1–10) µl, 10–100 µM; Pepstatin A, 1 µl, 1 µM;
PMSF, 5 µl 0.5 mM; Benzamidine, 1 µl, 4 mM; DTT, 1 µl, 1 mM)
consisting of protease inhibitors. The protein lysates (80 µg) were
subjected to 10% SDS-PAGE, then transferred to polyvinylidene
fluoride (PVDF) membrane (Merck KGaA, Darmstadt, Germany), and
probed with primary antibodies (β-TrCP, 1:500; β-actin, 1:2,000;
anti-Flag, 1:2,000) for target bands at 4°C overnight for blocking
and secondary antibodies (7074; 1:4,000; Cell Signaling Technology,
Inc., Danvers, MA, USA) at room temperature for 2 h. Bound
antibodies were detected by the Pierce ECL Plus Western Blotting
substrate (Thermo Fisher Scientific, Inc.) and exposed to X-ray
films. The PVDF membranes were washed (3 times for 15 min each)
using washing buffer (TBST) following incubation with antibodies.
Band densities were quantified using Image J software (1.42q,
National Institutes of Health, Bethesda, MD, USA). The relative
amount of proteins was determined by normalizing the densitometry
value of interest to that of the internal loading control. Western
blotting was performed for three times.
Wound healing assay
A total of 24 h following transfection with β-TrCP
plasmid (1.5×105 cells per hole), a rectangular lesion was created
using a plastic pipette tip and the monolayer was rinsed twice for
1 min with PBS and incubated in serum-free media [Dulbecco's
modified Eagle's medium (DMEM); Gibco; Thermo Fisher Scientific,
Inc.] at 37°C for 24 h. Subsequently, 5 randomly selected fields at
the lesion border were acquired under an inverted microscope
(magnification, ×100; Olympus Corporation, Tokyo, Japan). U251 or
U87 cells transfected with empty plasmids were used as
controls.
Transwell invasion assay
Cell invasion assays were performed using a
Transwell system that incorporated a polycarbonate filter membrane
with a diameter of 6.5 mm and pore size of 8 µm (Corning
Incorporated, Corning, NY, USA), according to the manufacturer's
protocol. To assess invasion, filters were precoated with 10 µg
Matrigel (BD Biosciences, Franklin Lakes, NJ). A pretreated cell
suspension (1×105) in serum-free culture media (Gibco; Thermo
Fisher Scientific, Inc.) was added into the inserts, and each
insert was placed in the lower chamber filled with culture media
containing 10% FBS as a chemoattractant. Following 24 h incubation
at 37°C, the non-invasive cells were removed from the upper
chamber. The filters were fixed with methanol for 15 min and
stained with a 0.1% crystal violet solution at 37°C for 10 min. A
total of 5 fields of adherent cells in each well were randomly
photographed under an inverted microscope (magnification, ×100;
IX71; Olympus, Japan) and counted. The same experimental design was
used for the migration experiments, except that filters were not
precoated with Matrigel. U251 or U87 cells transfected with empty
plamids were used as controls.
5-ethynyl-2′-deoxyuridine (EdU)
assay
The effects on proliferative ability of U251 and U87
cells was measured by EdU incorporation assay using the EdU assay
kit (Guangzhou RiboBio Co., Ltd., Guangzhou, China) subsequent to
β-TrCP plasmids transfection according to the manufacture's
protocol. Briefly, U251 or U87cells at 4×103 cells/well were
cultured in triplicate in 96-well plates and transfected with
β-TrCP plasmids for 24 h. The cells were subsequently exposed to 50
µM EdU for an additional 2 h at 37°C. The cells were fixed with 4%
formaldehyde for 30 min at room temperature and treated with 2
mg/ml glycine to neutralize the formaldehyde, then treated with
0.5% Triton X-100 for 15 min at room temperature for
permeabilization. Following 3 washes with PBS for 15 min, 100 µl 1x
Apollo reaction cocktail (Guangzhou RiboBio Co., Ltd.) was added
into each well for 30 min. Subsequently, the DNA contents of the
cells in each well were stained with 100 µl 1X Hoechst 33342
(Beyotime Institute of Biotechnology, Haimen, China) for 30 min and
visualized under a fluorescence microscope (magnification, ×100;
IX71; Olympus, Japan). U251 or U87 cells transfected with empty
plamids were used as controls.
Statistical analysis
The SPSS package (version 16.0; SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analyses. To distinguish
the difference between the treatment and control groups, the
statistical significance was determined using Student's t-test.
Data was presented as the mean ± standard error. P<0.05 was
considered to indicate a statistically significant difference.
Results
Validation of β-TrCP plasmid
transfection
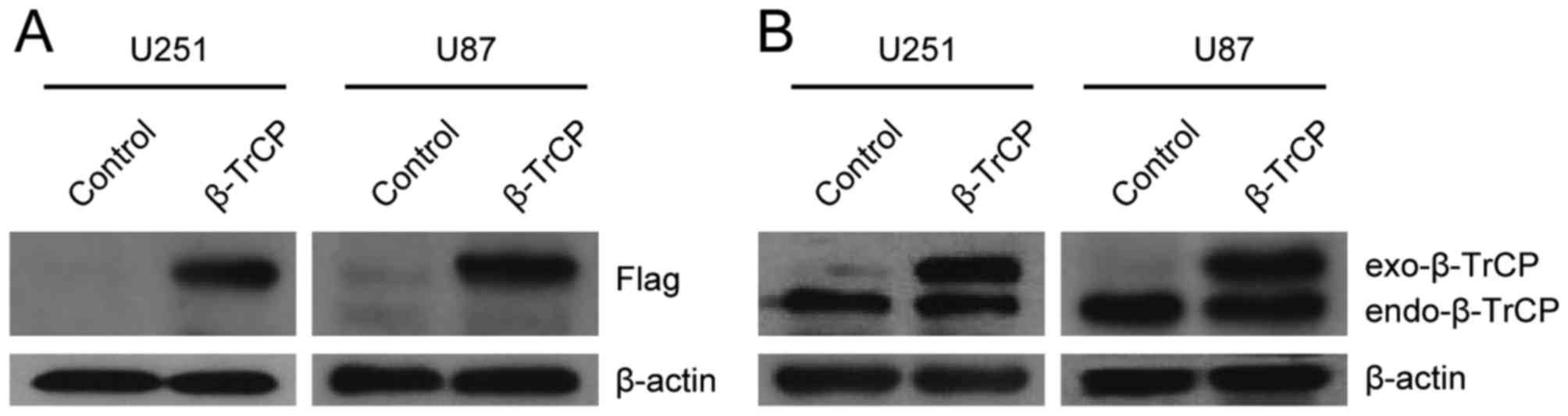
The effect of β-TrCP plasmid transfection was
validated in U251 and U87 cells (Fig. 1A
and B). The exogenous β-TrCP and endogenous β-TrCP were
well-expressed when immunoblotted with anti-Flag or with
anti-β-TrCP antibodies.
Effect of β-TrCP on glioma cell
migration
Whether overexpression of β-TrCP affects the
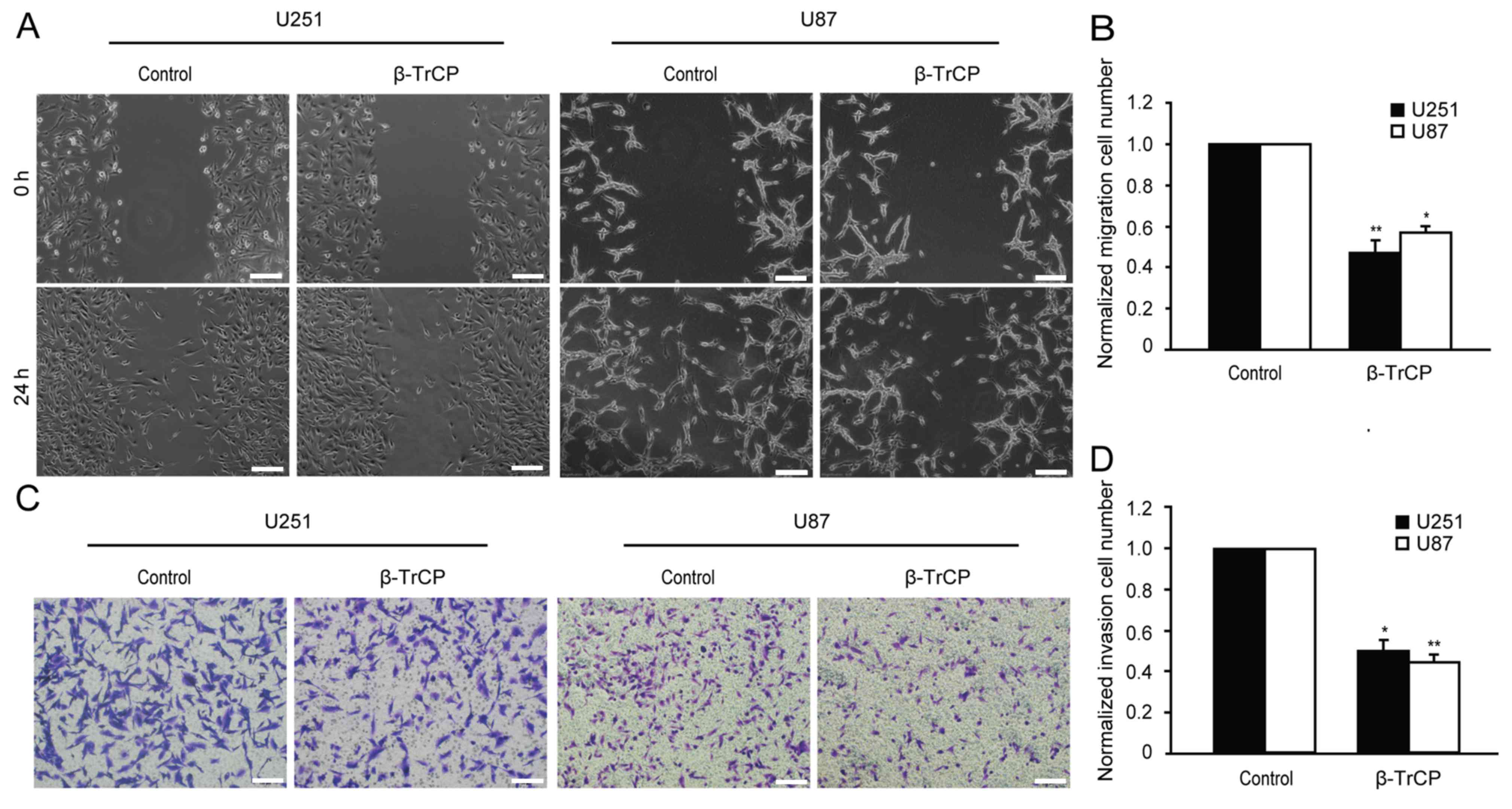
migration of glioma cells was examined using wound healing assay.
As shown in Fig. 2A, 48 h after being
scratched, the wound of the control group had clearly healed, and
exhibited a tendency to fuse, but any evidence of healing was not
observed in the β-TrCP-overexpressing group. Compared with the
control group, the migratory cell numbers of the β-TrCP
overexpressing group in U251 and U87 cells were decreased to
48.26±3.64 and 58.70±2.31%, respectively (Fig. 2B).
Effect of β-TrCP on glioma cell
invasion
Migration and invasion are widely considered to be
two closely interrelated processes. The role of β-TrCP in invasion
of glioma cells was investigated using Matrigel precoated Transwell
chambers. As demonstrated in Fig. 2C,
the overexpression of β-TrCP produced a significant reduction in
the number of invasive cells. Compared with the control group, the
number of invasive cells was reduced to 50.08±3.51 and 42.15±2.43%
in U251 and U87 cells, respectively, following β-TrCP
overexpression (Fig. 2D). These
results demonstrate that β-TrCP is directly involved in suppressing
cell migration and invasion.
Effect of β-TrCP on glioma
proliferation
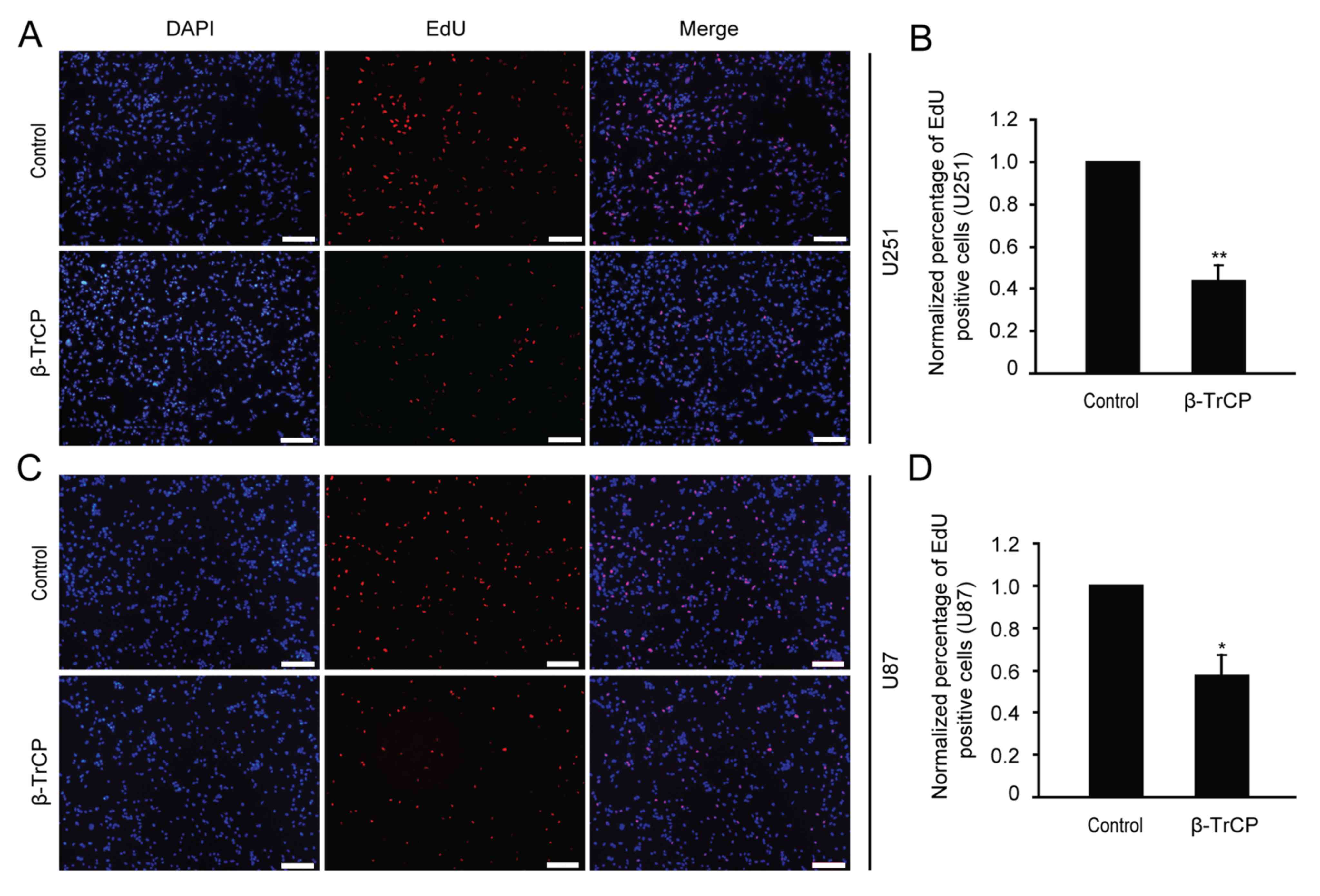
Cell proliferation is an important factor in the
progression of tumors, so possible changes in cell proliferation
following β-TrCP overexpression were next detected by EdU assay.
Following 24 h of transfection with β-TrCP plasmid, the U251 and
U87 glioma cells were treated with the EdU reagent (Fig. 3). The numbers of positive EdU cells
were counted, and the differences between different groups were
analyzed. Compared with the control group, the number of
proliferative cells was reduced to 44.08±6.12 and 57.6±9.07% in
U251 and U87 cells following β-TrCP overexpression, respectively
(Fig. 3B and D). The results of the
present study demonstrate that overexpressing β-TrCP may suppress
glioma cell proliferation.
Discussion
β-TrCP is a well-characterized E3 ubiquitin ligase
that is involved in the degradation of a number of proteins
involved in cell signaling and cell cycle regulation (8–10). Owing
to the diversity in its substrates, β-TrCP was suggested to be
responsible for oncogenesis or inhibiting tumorigenesis. In colon
cancer cells, β-TrCP has been demonstrated to promote
ubiquitination and degradation of PHLPP1, which negatively regulate
Akt signaling and promote colon cell growth (33). β-TrCP inhibition reduced prostate
cancer cell growth via upregulation of the aryl hydrocarbon
receptor (34). However, in lung
cancer cell lines, it has been shown that the loss of β-TrCP
resulted in the promotion of cell growth and invasion, possibly
through the regulation of the levels of CDC25A and the matrix
metalloproteinase 11 (31). β-TrCP
inhibited the activity of transforming growth factor-β in
pancreatic cancer cells by decreasing Smad4 stability (35). β-TrCP suppressed angiogenesis and
thyroid cancer cell migration by promoting ubiquitination and
destruction of VEGFR2 (27).
However, little is known about the role of β-TrCP in
the aggressive behavior of glioma. In a previous study by the
authors (32), the protein expression
level of β-TrCP in brain glioma tissue was detected by western blot
analysis and immunohistochemistry. It was identified that the
expression level of β-TrCP protein in human brain glioma samples
was significantly lower compared with non-tumor tissues, and the
expression level of β-TrCP in high-grade gliomas (grade III and IV)
was significantly lower compared with low-grade gliomas (grade I
and II). These results demonstrate that β-TrCP probably serves a
role in inhibiting the growth of glioma.
Migration, invasion and proliferation are basic
features of malignancies. These characteristics reflect the degree
of malignancy of tumor cells. Therefore the present study
investigated how β-TrCP affects migratory, invasive and
proliferative abilities of glioma cells. As the previous study
demonstrated that β-TrCP was expressed at low levels in glioma
tissues (32). In the present study,
β-TrCP was overexpressed by β-TrCP plasmid transfection prior to
subsequent experiments.
The effect of β-TrCP plasmid transfection was
validated in glioma cells. Exogenous β-TrCP and endogenous β-TrCP
were well-expressed, and this formed the basis of subsequent
experiments. Subsequently, wound healing assay was used to
investigate the effect of β-TrCP on glioma cell migration. It was
observed that the ability of wound healing in the β-TrCP
transfection cells group was weaker compared with the control
cells. This suggests that the overexpression of β-TrCP inhibits the
migration of glioma cells. Invasion is also the main manner of
tumor cell movement (36). In the
present study, Transwell assay demonstrated that the invasive
ability of glioma cells in the β-TrCP transfection group was
decreased compared with the control group. Proliferation is the
most important feature of tumor cells. Therefore in the present
study, the effect of β-TrCP on glioma cell proliferation was also
observed using EdU assay. The result demonstrated that the
proliferative ability of glioma cells was also significantly
decreased following β-TrCP overexpression.
From the above results, it may be hypothesized that
β-TrCP protein probably serves as a tumor suppressor to inhibit the
growth of glioma. The mechanism may be that β-TrCP acts on
different substrates in glioma cells, but this will require
additional investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81072072) and a
grant from Xuzhou Medical College (grant no. 09KJZ18).
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buonerba C, Di Lorenzo G, Marinelli A,
Federico P, Palmieri G, Imbimbo M, Conti P, Peluso G, De Placido S
and Sampson JH: A comprehensive outlook on intracerebral therapy of
malignant gliomas. Crit Rev OncolHematol. 80:54–68. 2011.
View Article : Google Scholar
|
|
3
|
Sherman JH, Hoes K, Marcus J, Komotar RJ,
Brennan CW and Gutin PH: Neurosurgery for brain tumors: Update on
recent technical advances. Curr Neurol Neurosci Rep. 11:313–319.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onishi M, Ichikawa T, Kurozumi K and Date
I: Angiogenesis and invasion in glioma. Brain Tumor Pathol.
28:13–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim CS, Jung S, Jung TY, Jang WY, Sun HS
and Ryu HH: Characterization of invading glioma cells using
molecular analysis of leading-edge tissue. J Korean Neurosurg Soc.
50:157–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates, and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rutka JT, Taylor M, Mainprize T, Langlois
A, Ivanchuk S, Mondal S and Dirks P: Molecular biology and
neurosurgery in the third millennium. Neurosurgery. 46:1034–1051.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Kato Y, Zhang Z, Do VM, Yankner BA
and He X: beta-Trcp couples beta-catenin
phosphorylation-degradation and regulates Xenopus axis formation.
Proc Natl Acad Sci USA. 96:6273–6278. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Busino L, Donzelli M, Chiesa M,
Guardavaccaro D, Ganoth D, Dorrello NV, Hershko A, Pagano M and
Draetta GF: Degradation of Cdc25A by beta-TrCP during S phase and
in response to DNA damage. Nature. 426:87–91. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe N, Arai H, Nishihara Y, Taniguchi
M, Watanabe N, Hunter T and Osada H: M-phase kinases induce
phospho-dependent ubiquitination of somatic Wee1 by SCF beta-TrCP.
Proc Natl Acad Sci USA. 101:4419–4424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiaur DS, Murthy S, Cenciarelli C, Parks
W, Loda M, Inghirami G, Demetrick D and Pagano M: Five human genes
encoding F-box proteins: Chromosome mapping and analysis in human
tumors. Cytogenet Cell Genet. 88:255–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koike J, Sagara N, Kirikoshi H, Takagi A,
Miwa T, Hirai M and Katoh M: Molecular cloning and genomic
structure of the betaTRCP2 gene on chromosome 5q35.1. Biochem
Biophys Res Commun. 269:103–109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ballarino M, Marchioni M and Carnevali F:
The Xenopus laevis beta TrCP gene: Genomic organization,
alternative splicing, 5′ and 3′ region characterization and
comparison of its structure with that of human beta TrCP genes.
Biochim Biophys Acta. 1577:81–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deshaies RJ: SCF and Cullin/Ring H2-based
ubiquitin ligases. Annu Rev Cell Dev Biol. 15:435–467. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuchs SY, Spiegelman VS and Kumar KG: The
many faces of beta-TrCP E3 ubiquitin ligases: Reflections in the
magic mirror of cancer. Oncogene. 23:2028–2036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cenciarelli C, Chiaur DS, Guardavaccaro D,
Parks W, Vidal M and Pagano M: Identification of a family of human
F-box proteins. Curr Biol. 9:1177–1179. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DeSalle LM and Pagano M: Regulation of the
G1 to S transition by the ubiquitin pathway. FEBS Lett.
490:179–189. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pickart CM: Mechanisms underlying
ubiquitination. Annu Rev Biochem. 70:503–533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ciechanover A, Orian A and Schwartz AL:
Ubiquitin-mediated proteolysis: Biological regulation via
destruction. Bioessays. 22:442–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou P: Targeted protein degradation. Curr
Opin Chem Biol. 9:51–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maniatis T: A ubiquitin ligase complex
essential for the NF-kappaB, Wnt/Wingless and Hedgehog signaling
pathways. Genes Dev. 13:505–510. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sahasrabuddhe AA, Dimri M, Bommi PV and
Dimri GP: βTrCP regulates BMI1 protein turnover via ubiquitination
and degradation. Cell Cycle. 10:1322–1330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fuchs SY, Chen A, Xiong Y, Pan ZQ and
Ronai Z: HOS, a human homolog of Slimb, forms an SCF complex with
Skp1 and Cullin1 and targets the phosphorylation-dependent
degradation of IkappaB and beta-catenin. Oncogene. 18:2039–2046.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hart M, Concordet JP, Lassot I, Albert I,
del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F,
Benarous R and Polakis P: The F-box protein beta-TrCP associates
with phosphorylated beta-catenin and regulates its activity in the
cell. Curr Biol. 9:207–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Latres E, Chiaur DS and Pagano M: The
human F box protein beta-Trcp associates with the Cul1/Skp1 complex
and regulates the stability of beta-catenin. Oncogene. 18:849–854.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shaik S, Nucera C, Inuzuka H, Gao D,
Garnaas M, Frechette G, Harris L, Wan L, Fukushima H, Husain A, et
al: SCF (β-TRCP) suppresses angiogenesis and thyroid cancer cell
migration by promoting ubiquitination and destruction of VEGF
receptor 2. J Exp Med. 209:1289–1307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong J, Shaik S, Wan L, Tron AE, Wang Z,
Sun L, Inuzuka H and Wei W: SCF β-TRCP targets MTSS1 for
ubiquitination-mediated destruction to regulate cancer cell
proliferation and migration. Oncotarget. 4:2339–2353. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Margottin-Goguet F, Hsu JY, Loktev A,
Hsieh HM, Reimann JD and Jackson PK: Prophase destruction of Emi1
by the SCF (betaTrCP/Slimb) ubiquitin ligase activates the anaphase
promoting complex to allow progression beyond prometaphase. Dev
Cell. 4:813–826. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Y, Lee SH, Kim HS, Kim NH, Piao S, Park
SH, Jung YS, Yook JI, Park BJ and Ha NC: Role of CK1 in
GSK3beta-mediated phosphorylation and degradation of snail.
Oncogene. 29:3124–3133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He N, Li C, Zhang X, Sheng T, Chi S, Chen
K, Wang Q, Vertrees R, Logrono R and Xie J: Regulation of lung
cancer cell growth and invasiveness by beta-TRCP. Mol Carcinog.
42:18–28. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang J, Wang WF, Xie S, Zhang XL, Qi WF,
Zhou XP, Hu JX, Shi Q and Yu RT: Expression of β-transducin
repeat-containing E3 ubiquitin protein ligase in human glioma and
its correlation with prognosis. Oncol Lett. 9:2651–2656.
2015.PubMed/NCBI
|
|
33
|
Li X, Liu J and Gao T: beta-TrCP-mediated
ubiquitination and degradation of PHLPP1 are negatively regulated
by Akt. Mol Cell Biol. 29:6192–6205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gluschnaider U, Hidas G, Cojocaru G,
Yutkin V, Ben-Neriah Y and Pikarsky E: beta-TrCP inhibition reduces
prostate cancer cell growth via upregulation of the aryl
hydrocarbon receptor. PLoS One. 5:e90602010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wan M, Huang J, Jhala NC, Tytler EM, Yang
L, Vickers SM, Tang Y, Lu C, Wang N and Cao X: SCF(beta-TrCP1)
controls Smad4 protein stability in pancreatic cancer cells. Am J
Pathol. 166:1379–1392. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruiz P and Günthert U: The cellular basis
of metastasis. World J Urol. 14:141–150. 1996. View Article : Google Scholar : PubMed/NCBI
|