Introduction
Breast cancer is a lethal disease with a high global
incidence. For a long time, tumor cells were the only focus of
cancer studies. However, it has become clear that the extracellular
matrix (ECM) performs an important function in carcinogenesis. The
ECM, particularly the basement membrane (BM), acts as a barrier
separating the tumor cells from the vessels which cancer
metastasizes through (1–3). The procedure of tumor invasion and
metastasis involves complex molecular mechanisms in cell-cell,
cell-matrix and matrix-matrix interactions, which are reflected in
variable up- and downregulation of multiple macromolecules
(4). One of these is fibulin-2, which
is associated with progression of multiple types of cancer
(5,6).
The loss of fibulin-2 expression results in abnormal cell adhesion
and migration ability, which may facilitate cancer cell invasion
and metastasis during breast cancer progression (7). In addition, previous studies have also
demonstrated that fibulin-2 stabilizes the ECM in lung cancer
(8,9).
These findings may also be due to the association of fibulin-2
expression with the progression of disease. Although fibulin-2
expression had been studied in breast cancer tissue, to the best of
our knowledge there are no direct studies on the association
between fibulin-2 and collagen IV, which are expressed primarily in
the BM. In the present study, immunohistochemical analysis was
performed to define the localization of fibulin-2 and its
association with collagen IV, and the involvement of fibulin-2 and
collagen IV in carcinogenesis, as well as potential therapeutic
targets, were explored.
Patients and methods
Patients and tissues
Necessary consent from all patients involved in the
present study was obtained, including consent to be involved in the
study and consent to publish. The present study was approved by the
Ethics Committees of the Clinical Medical School, Yangzhou
University (Yangzhou, China). Between September 2014 and March
2015, 46 samples were collected from 23 female patients, aged
35–63. These patients included 8 ductal carcinoma in situ
(DCIS) and 15 invasive ductal carcinoma (IDC). A total of two
samples were collected from every patient; normal and cancer (DCIS
or IDC) sections. All tissue sections were selected by an
experienced pathologist from Subei People's Hospital of Jiangsu
Province, Yangzhou University (Yangzhou, China), based on diagnosis
and microscopic morphology. All tissues were obtained during
surgical resection, and the size was ~1.5×0.5×0.3 cm. Antibodies
used in the present study were as follows: Rabbit-anti-human
fibulin-2 antibody (cat. no. sc-30176; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA); mouse-anti-human collagen IV monoclonal
antibody (cat. no. MS-375; Maixin-Bio Corporation, Fuzhou, China);
and MaxVison enzyme labeled goat-anti-mouse/rabbit immunoglobulin G
(IgG) antibody (cat. no. KIT-5010; Maixin-Bio Corporation).
Immunohistochemical staining of
fibulin-2 and collagen IV
Breast tissue samples were selected by an
experienced pathologist based on diagnosis and microscopic
morphology. Tissue sections (1.5×0.5×0.3 cm) were deparaffinized
with xylene, and then rehydrated in ethanol washes (100, 90, 80 and
70%). Tissues were then washed with PBS, followed by antigen
retrieval through a microwave oven for 20 min (collagen IV only).
Tissues were washed again with PBS and treated with 3%
H2O2 for 15 min to block endogenous
peroxidase activity. Subsequent to washing with PBS, tissues were
incubated at 24°C with 10% goat serum (cat. no. G9023;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h to block
nonspecific bindings. Samples were subsequently incubated with
rabbit-anti-human fibulin-2 polyclonal antibody (at a range of
dilutions, including 1:50, 1:100, 1:200 and 1:400) and
mouse-anti-human collagen IV monoclonal antibody (1:400) separately
overnight at 4°C in a humidified chamber, rinsed with PBS, and then
incubated with MaxVison enzyme labeled goat-anti-mouse/rabbit IgG
antibody (1:100) for 1 h at room temperature. Subsequent to washing
with PBS, tissues were incubated for 3–5 min with
3,3′-diaminobenzidine substrate and 3-amino-9-ethylcarbazole
substrate separately, followed by counterstaining with Meyer's
hematoxylin for 30 sec. Tissue sections (3-µm thick) were mounted
on coverslips, examined, and 5 fields of view were captured and
assessed from every section under a light microscope (magnification
range, ×50–200) with a digital camera (DP70; Olympus Corporation,
Tokyo, Japan).
Results
Expression of fibulin-2 at different
antibody dilutions
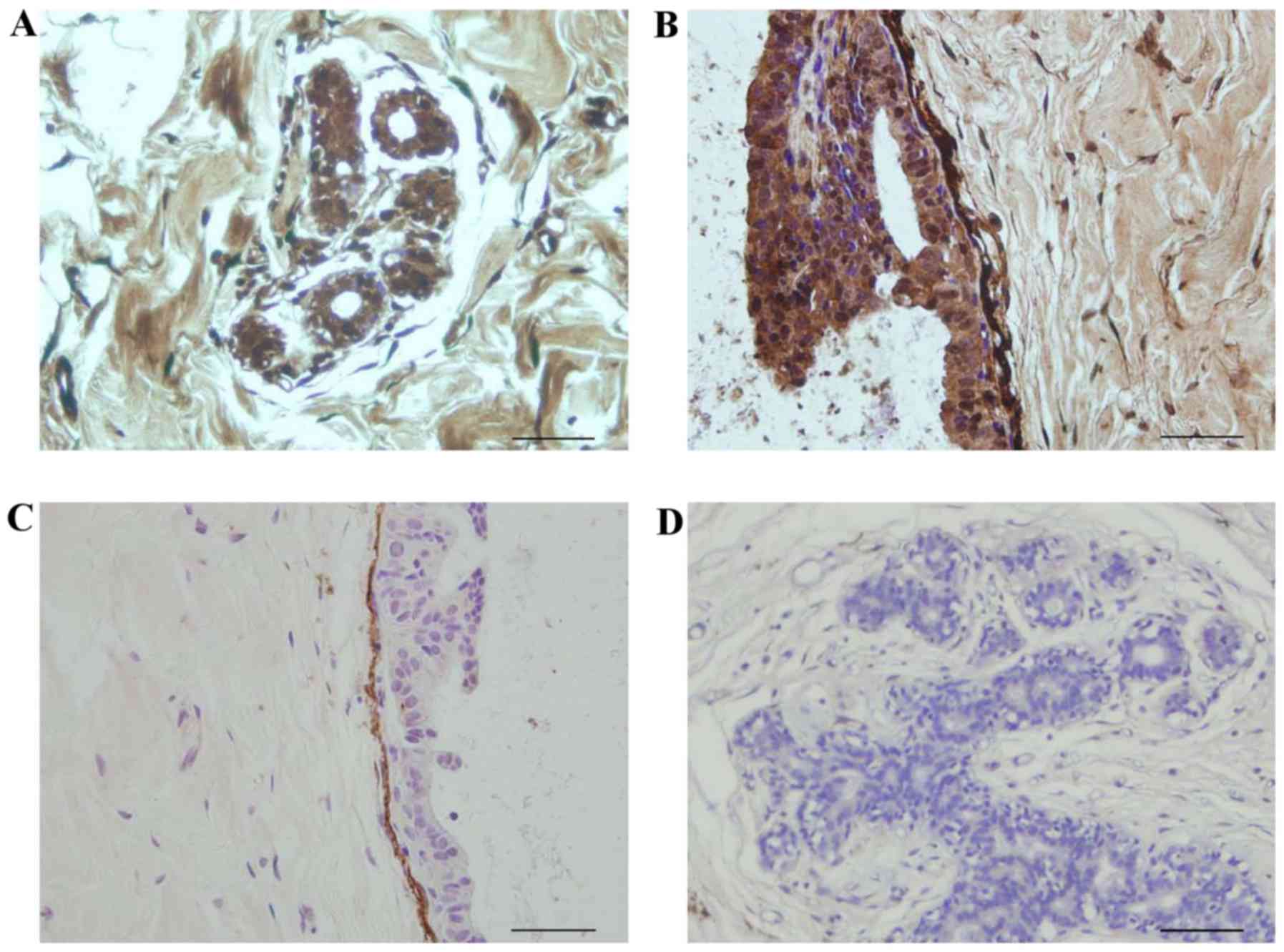
When the dilution of fibulin-2 antibody was 1:100,
fibulin-2 appeared to be expressed in the acinus (Fig. 1A) as well as around the BM (Fig. 1B). However, when the dilution was
1:400, fibulin-2 expression was only observed around the BM
(Fig. 1C) and not in the acinus
(Fig. 1D).
Expression of fibulin-2 and collagen
IV around large breast ducts and blood vessels
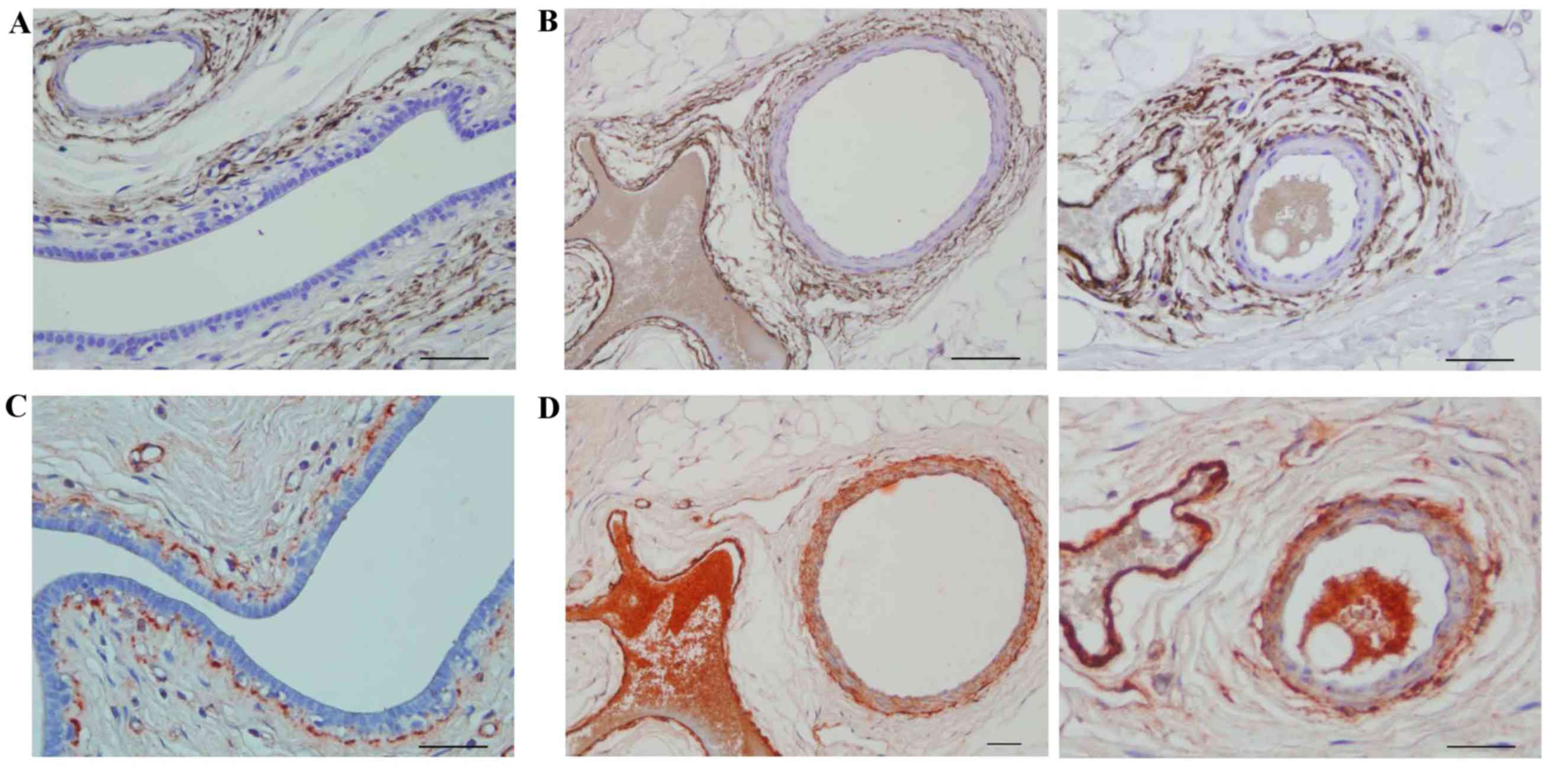
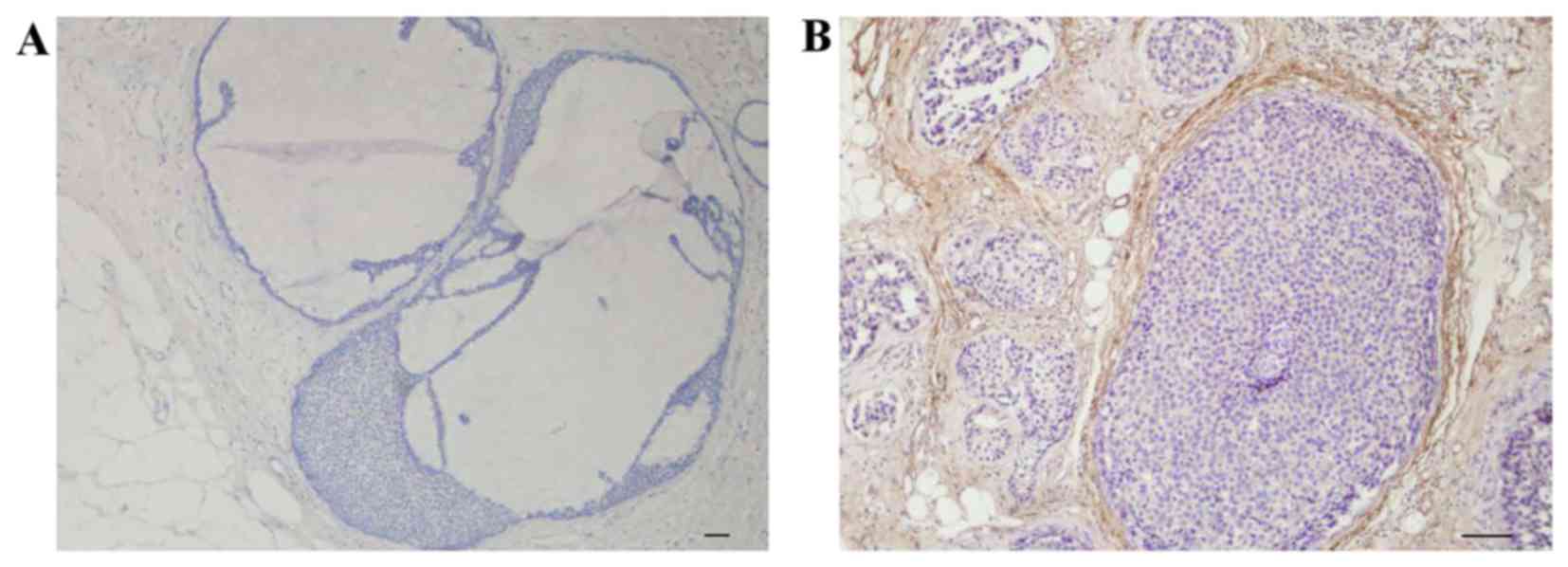
Fibulin-2 was expressed around the BM outside large
breast ducts (Fig. 2A) and blood
vessels (Fig. 2B), while collagen IV
was expressed inside the fibulin-2 layer around large breast ducts
(Fig. 2C) and blood vessels (Fig. 2D).
Expression of fibulin-2 and collagen
IV around medium breast ducts
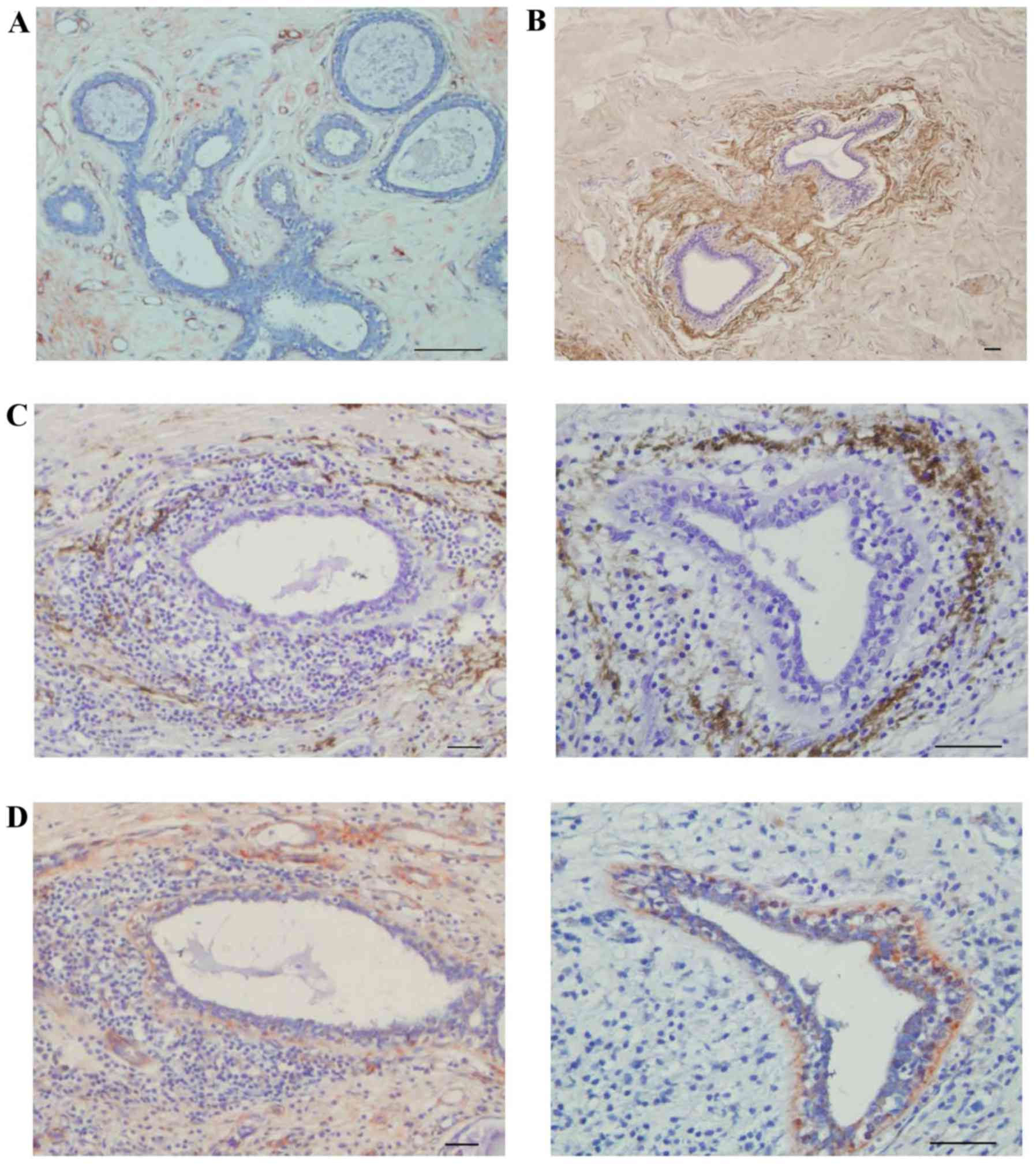
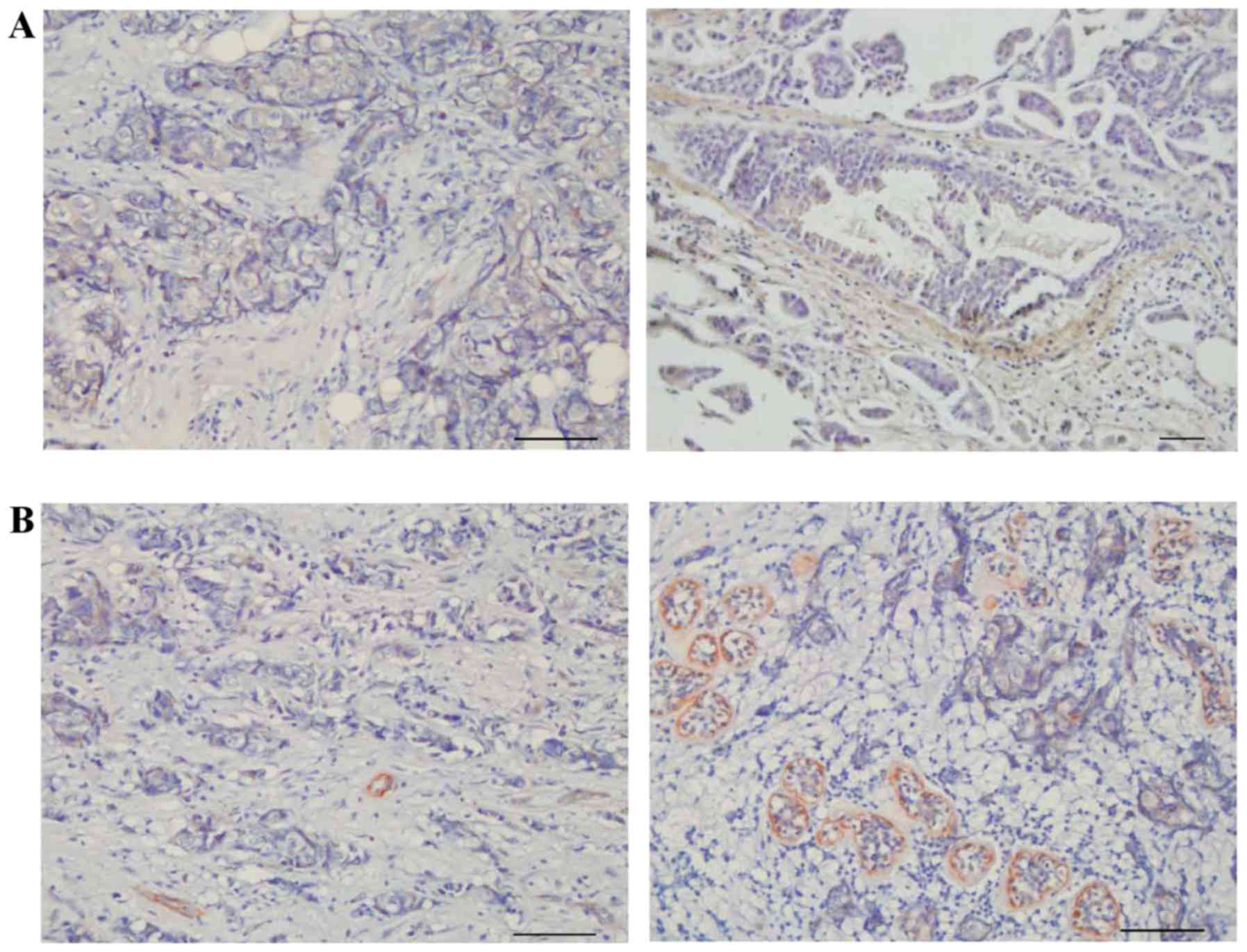
Fibulin-2 was not expressed around all medium breast
ducts. No fibulin-2 expression was observed around certain medium
breast ducts (Fig. 3A), but fibulin-2
was expressed completely around other medium breast ducts (Fig. 3B). In adjacent breast tissue that was
invaded by cancer cells, fibulin-2 was partially degraded (Fig. 3C), while collagen IV remained
integrated (Fig. 3D).
Expression of fibulin-2 and collagen
IV in the terminal duct-lobular unit (TDLU)
In the TDLU, collagen IV was expressed incompletely
around the acinus (Fig. 4A), however,
occasionally expression was completely absent (Fig. 4B). Fibulin-2 was expressed around the
breast ducts, but not in the acinus (Fig.
4C and D).
Expression of fibulin-2 and collagen
IV in IDC
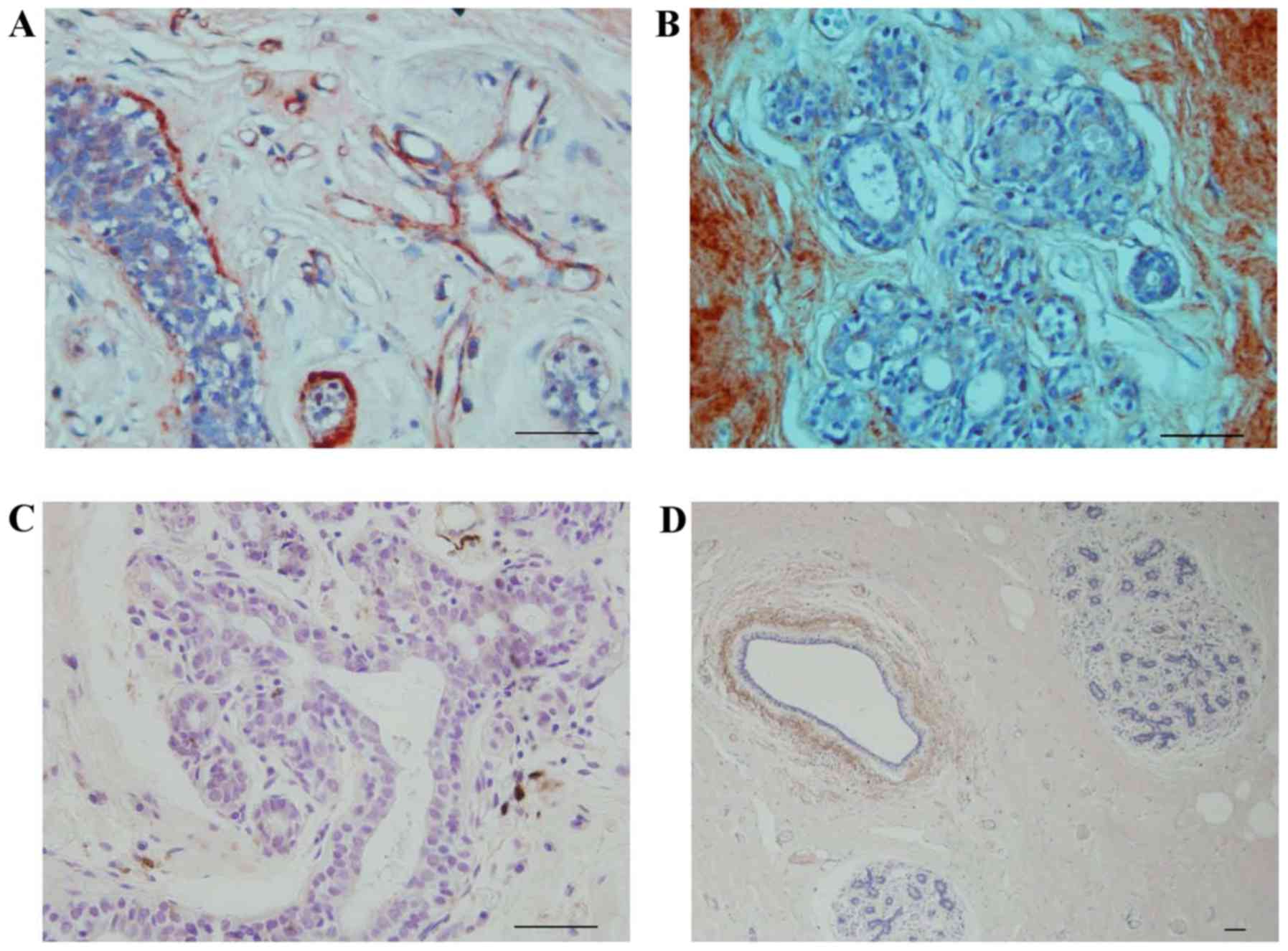
In IDC, fibulin-2 was not expressed (Fig. 5A). The expression level of collagen IV
was also low (Fig. 5B).
Expression of fibulin-2 in DCIS
Fibulin-2 was expressed around certain ducts in DCIS
(Fig. 6A) but not expressed around
others (Fig. 6B).
Discussion
BM is widely accepted to be the first barrier of the
body against cancer cells (10,11).
Invasion and metastasis of malignancy is a multi-step process
involving multiple macromolecules. ECM contains proteins and
polysaccharides synthesized and secreted by cells. The substances
in the ECM form thin, dynamic sheet-like structures, involved in
building tissue scaffolds and regulating embryonic development,
cell migration and signal transduction (1,2). A number
of ECM substances regulate body functions, as well as affect the
biological characteristics of tumor cells. The ECM includes BM and
intercellular substances. Integrated BM is one of the main defense
barriers inhibiting tumor invasion and metastasis. The maintenance
of its normal architecture may be disrupted when the BM is invaded
by tumor cells, facilitating the invasion and metastasis of these
tumor cells.
Fibulin-2, which belongs to a seven-member family of
extracellular glycoproteins, was first identified by Kluge et
al in 1990 (12). It contains a
diverse array of protein ligands, facilitating its interaction with
collagen IV, fibronectin, laminin and integrin (13,14). Yi
et al (7) reported that
fibulin-2 was expressed in the cytosol and on the cell surface of
normal ductal epithelial cells, particularly in the apical side of
the cells. At the beginning of the immunohistochemical studies,
fibulin-2 appeared to not only be expressed in mammary glandular
cells and fibroblasts, but also around vessels and large breast
ducts (fibulin-2 dilution was 1:100). However, when the antibody
dilution was changed to 1:400, no fibulin-2 expression was observed
in the cytosol and cell surface. Gu et al (15) also identified that fibulin-2 was
expressed in ECM, but not in epithelial cells. Therefore, it was
inferred that the immunostaining in the previous study by Yi et
al (7) was non-specific.
Additional innumohistochemical analysis performed in the present
study demonstrated that fibulin-2 was expressed around the outside
of collagen IV, with the two of them expressed around vessels and
large breast ducts. In one breast tissue observed during the
present study, in which ducts were invaded by cancer cells,
fibulin-2 was partially degraded while collagen IV remained
integrated. These findings indicated that fibulin-2 may form a new
barrier outside the BM to defend cancer cells. In addition,
fibulin-2 was only expressed partially around ducts in DCIS, with
total absence of fibulin-2 observed in IDC. Yue et al
(16) identified that fibulin-5
suppresses lung cancer invasion by inhibiting matrix
metalloproteinase-7 expression. Therefore, it was inferred that
fibulin-2 may be involved in passive defense, in which it may be
degraded by matrix metalloproteinases. These results indicated that
fibulin-2 is a negative regulator of invasiveness in breast cancer,
and additional studies are required for its therapeutic
applications in the treatment of breast cancer.
The present findings revealed that fibulin-2 is
involved in breast cancer invasion and that it collapsed prior to
the infiltration of the BM, indicating that it forms a barrier
similar to the traditional BM. Therefore, it was hypothesized that
fibulin-2 was part of the general BM, which differs from the
traditional BM. These findings provide novel insight into
extracellular matrix components, elucidating the involvement of
fibulin-2 in tumor invasion and metastasis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172508).
Glossary
Abbreviations
Abbreviations:
|
BM
|
basement membrane
|
|
DCIS
|
ductal carcinoma in situ
|
|
ECM
|
extracellular matrix
|
|
IDC
|
invasive ductal carcinoma
|
|
TDLU
|
terminal duct-lobular unit
|
References
|
1
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pickup MW, Mouw JK and Weaver VM: The
extracellular matrix modulates the hallmarks of cancer. EMBO Rep.
15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salvatore V, Focaroli S, Teti G, Mazzotti
A and Falconi M: Changes in the gene expression of co-cultured
human fibroblast cells and osteosarcoma cells: The role of
microenvironment. Oncotarget. 6:28988–28998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brady-Kalnay SM: Molecular mechanisms of
cancer cell-cell interactions: Cell-cell adhesion-dependent
signaling in the tumor microenvironment. Cell Adh Migr. 6:344–345.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Law EW, Cheung AK, Kashuba VI, Pavlova TV,
Zabarovsky ER, Lung HL, Cheng Y, Chua D, Lai-Wan Kwong D, Tsao SW,
et al: Anti-angiogenic and tumor-suppressive roles of candidate
tumor-suppressor gene, Fibulin-2, in nasopharyngeal carcinoma.
Oncogene. 31:728–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alcendor DJ, Knobel S, Desai P, Zhu WQ and
Hayward GS: KSHV regulation of fibulin-2 in Kaposi's sarcoma:
Implications for tumorigenesis. Am J Pathol. 179:1443–1454. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi CH, Smith DJ, West WW and Hollingsworth
MA: Loss of fibulin-2 expression is associated with breast cancer
progression. Am J Pathol. 170:1535–1545. 2008. View Article : Google Scholar
|
|
8
|
Baird BN: Fibulin-2 stabilizes tumor
extracellular matrix and drives malignant progression of lung
adenocarcinoma (unpublished PhD dissertation). The University of
Texas. 2972012.
|
|
9
|
Baird BN, Schliekelman MJ, Ahn YH, Chen Y,
Roybal JD, Gill BJ, Mishra DK, Erez B, O'Reilly M, Yang Y, et al:
Fibulin-2 is a driver of malignant progression in lung
adenocarcinoma. PLoS One. 8:e670542013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hasengaowa Kodama J, Kusumoto T, Shinyo Y,
Seki N, Nakamura K, Hongo A and Hiramatsu Y: Loss of basement
membrane heparan sulfate expression is associated with tumor
progression in endometrial cancer. Eur J Gynaecol Oncol.
26:403–406. 2005.PubMed/NCBI
|
|
11
|
Wilson DF, Jiang DJ, Pierce AM and Wiebkin
OW: Oral cancer: Role of the basement membrane in invasion. Aust
Dent J. 44:93–97. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kluge M, Mann K, Dziadek M and Timpl R:
Characterization of a novel calcium-binding 90-kDa glycoprotein
(BM-90) shared by basement membranes and serum. Eur J Biochem.
193:651–659. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olijnyk D, Ibrahim AM, Ferrier RK, Tsuda
T, Chu ML, Gusterson BA, Stein T and Morris JS: Fibulin-2 is
involved in early extracellular matrix development of the
outgrowing mouse mammary epithelium. Cell Mol Life Sci.
71:3811–3828. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Longmate WM, Monichan R, Chu ML, Tsuda T,
Mahoney MG and DiPersio CM: Reduced fibulin-2 contributes to loss
of basement membrane integrity and skin blistering in mice lacking
integrin α3β1 in the epidermis. J Invest Dermatol. 134:1609–1617.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu YC, Nilsson K, Eng H and Ekblom M:
Association of extracellular matrix proteins fibulin-1 and
fibulin-2 with fibronectin in bone marrow stroma. Br J Haematol.
109:305–313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yue W, Sun Q, Landreneau R, Wu C,
Siegfried JM, Yu J and Zhang L: Fibulin-5 suppresses lung cancer
invasion by inhibiting matrix metalloproteinase-7 expression.
Cancer Res. 69:6339–6346. 2009. View Article : Google Scholar : PubMed/NCBI
|