Introduction
Ultrasound (US) at 20 kHz is a special sound range
just above the threshold of human hearing (1). The application of low-frequency (20 kHz)
US has been demonstrated to be useful for increasing the efficiency
and consumer safety in food processing (2), removing heavy metal (lead, mercury and
arsenic) contamination in milk (3),
decreasing the viscosity and particle size of milk (4) and improving the functional properties of
dairy ingredients (5). US at a
frequency of 20 kHz has been used to degrade the antibiotic
ofloxacin in water (6). Low to
moderate levels of ofloxacin degradation were reported, and this
degradation was attributed to radical reactions in the liquid bulk
rather than thermal reactions in the vicinity of the cavitation
bubble (6).
US at 20 kHz can also increase membrane permeation
(7). Size measurements and the direct
visualization of vesicles demonstrate that US does not completely
rupture membranes into fragments but causes transient poration, as
leakage from the core is governed by acoustic cavitation (8). The extent of leakage inversely depends
on the membrane thickness and directly depends on the sonication
time and intensity (7).
US at 20 kHz is applied not only in industry but
also to improve the in vitro and in vivo bio-effects
in medicine (9–11). In vitro studies have
demonstrated that 20 kHz US enhances the permeation of diclofenac
sodium across EpiDerm™ 5-fold (9–11). It is a
unique and exciting theranostic modality that can be used to track
drug carriers, trigger drug release and improve drug deposition to
tumor cells with high spatial precision (9). Cavitation bubbles induced by 20 kHz US
may induce cell death or transient membrane permeabilization, which
is defined as sonoporation on a single cell level (10). Microscopy disclosed that the collapse
of a microbubble (MB) and generation of a jet produce small holes
within cell membranes (12). Pores
produced within the cell membrane may be transient (facilitating
successful therapeutic delivery) or permanent (resulting in cell
death) (13). The application of
sonoporation can be used to deliver genes into cells (14,15). In
vitro, a variety of cell lines, including fibroblast cells,
ovarian carcinoma cells and HeLa cells, have been successfully
transfected for gene therapy (14–16) with
concomitant cell death (17).
In vivo studies demonstrated a loss of
balance stability and reduced motor response time in humans due to
20 kHz airborne US up to 1 h with 120 dB (18) and temporary hearing loss for 5 min at
154 dB (19). Human subjects
complained of fatigue, headache, nausea and tinnitus as a result of
the airborne ultrasonic exposure at 20 kHz and 110 dB for 1 day
(20). Nude mice succumbed after 8–70
min of exposing the head to 20 kHz with a sound pressure of 162 dB
(21). Boucaud et al (22) researched the human skin bio-effect of
a 20-kHz contact US pulse wave with a 10% duty cycle (0.1 sec on,
0.9 sec off) and a 20-kHz continuous wave for 10 min with a varying
intensity of 0.25–7 W/cm2. They found that 20 kHz US
significantly increased skin permeabilization. The skin
permeabilization induced by 20 kHz US results primarily from the
direct mechanical impact of gas bubbles that collapse on the skin
surface (resulting in microjets and shockwaves) (22). Tang et al (23) studied pig skin permeabilization
effects of a coupling medium with 20 kHz US, with a 10% duty cycle
(0.1 sec on, 0.9 sec off) for 2 h at a power of 1.6–33.5
W/cm2. The results indicated that US-induced cavitation
in the coupling medium is the key mechanism of skin
permeabilization during low frequency sonophoresis. Transient
cavitation occurring on, or in the vicinity of, the skin membrane
is the central mechanism responsible for the observed enhancement
of skin permeability (23).
The industrial and medical applications of 20 kHz US
are diverse, ranging from high power industrial US equipment to
various therapeutic medical applications (24,25).
Previous studies have investigated the effects of low-frequency US
on small animals, including nude mice, and revealed that
low-frequency US could downregulate the expression of
vascular-related protein (25) and
inhibit tumor growth (26). However,
the cavitation effect on tumors in larger animals, including
rabbits, has not been thoroughly studied. When MBs are
intravenously injected, the acoustic pressures that facilitate the
therapeutic effects will decrease (27). Thus, the present study aimed to
explore the effects of 20 kHz US and MBs on rabbit liver tumors
using computed tomography (CT) and compare them with pathology as a
reference standard.
Materials and methods
Animal protocol and tumor
inoculation
The experiments were performed with 16 New Zealand
white rabbits that weighed 2.0–2.5 kg (median, 2.2 kg). All 16
rabbits were male, aged 90 days and were provided from the
Laboratory Animal Center of Nantong University (Nantong, China).
The experiments were approved by the Animal Care Committee of
Nantong University Medical School (Jiangsu, China) and were
performed in accordance with the institutional guidelines. The
animals were anesthetized with an intravenous injection of 30 mg/kg
pentobarbital. VX2 carcinoma cells (Department of Ultrasound in
Medicine, Shanghai Jiao Tong University Affiliated Sixth People's
Hospital, Shanghai, China) were used to establish a model of
hepatic tumors. The abdomens of the recipient rabbits were shaved
and prepared with povidone iodine, and a midline subxyphoid
incision was made. The anterior surface of the liver was exposed,
and prepared tumor tissue was implanted in the liver lobe using
forceps. The outlet of the inoculated area was blocked by a gelatin
sponge (Suzhong Medical Instrument Co., Ltd., Yangzhou, Jangsu,
China). Only one inoculation site was used per liver. Aseptic
technique was maintained during each inoculation. Following
surgery, the animals were returned to their cages, kept warm
(temperature 18°C; humidity, 65%; good ventilation; dim light;
quiet environment; and with sufficient food and clean water) and
monitored in the animal laboratory until they recovered from
anesthesia. An iU22 ultrasound system (Philips Medical Systems,
Inc., Bothell, WA, USA) with a 12–15 MHz broadband linear probe was
used to monitor the tumor growth every day following tumor
inoculation. VX2 carcinoma nodules reaching 0.8–0.9 cm in diameter
were considered appropriate for low-frequency US treatment. The
period for tumors to reach a size of 0.8 cm ranged from 12 to 13
days. The same investigator performed all inoculations and
inoculated specimens of the same tumor into all rabbits to minimize
inter-animal variations in the tumor growth rate.
Experimental protocol
In the present study, 16 tumor-bearing rabbits were
randomly divided into four groups, with 4 rabbits per group: Group
A, negative control (sham treatment); group B, MB; group C,
low-frequency US; and group D, US + MB. The MBs used were an US
contrast agent (SonoVue; Bracco SpA, Milan, Italy). The rabbits
were anesthetized via an auricular vein injection of 30 mg/kg
pentobarbital, which is a routine site for vein injection in
rabbits (28).
Subsequent to successful anesthesia, an iU22
ultrasound system (Philips Medical Systems, Inc.) was used to
locate the liver tumor prior to insonication. The hepatic tumors
were subsequently sonicated using a focused low-frequency US
transducer manufactured by Taizhou Research Institute of Ultrasound
Technology (Taizhou, Jiangsu, China). The probe was placed on the
shaved abdominal skin of rabbits covered with a US transmission gel
(a Lotus medical ultrasonic coupling agent; Yi Jie Guangzhou
Pharmaceutical Technology Co., Ltd., Guangzhou, China) to ensure US
propagation. The diameter of the therapeutic US transducer was ~24
mm. The low-frequency US parameters were set to 20 kHz, 2
W/cm2, duty cycle 40% (on 2 sec, off 3 sec) and a
duration of 5 min once every other day for 2 weeks. The US contrast
agent was simultaneously and continuously infused with US wave
irradiation via the auricular vein of rabbits (at a flow rate of
0.2 ml/min). The dose of the contrast agent administered was 1 ml
(0.4 ml/kg) per rabbit for each treatment. The concentration of the
contrast agent was 1.8×109 MBs/ml.
CT scanning
The rabbits were fasted for 8 h before scanning and
anesthetized with an intramuscular injection of 0.2 ml/kg sumianxin
(1 ml, including 4 µg dihydroetorphine and 2.5 mg aloperidin;
Nantong University Animal Center, Nantong, China). CT perfusion
scans were performed before and at 1 and 2 weeks after tumor
treatment. CT imaging was completed on a multi-slice spiral CT
(LightSpeed 16-Slice Spiral CT; GE Healthcare, Chicago, IL, USA).
To select the scanning range, a plain CT scan of the liver was
performed prior to beginning perfusion scanning. The CT scanning
parameters were defined as follows: 80 kVp; 120 mAs; slice
thickness, 3 mm; matrix, 512×512; field of view, 15 cm; and
contrast medium IV injection in marginal ear vein, 1 ml/sec
(1.0–1.5 ml/kg body weight). The tumor size on imaging studies was
recorded as the median of the maximum transverse and
anteroposterior dimensions. Following treatment, the tumor area was
determined each week by measuring the transversal and
anteroposterior tumor diameters. The tumor growth rate (%) =
(transversal × anteroposterior diameters) after therapy -
(transversal × anteroposterior diameters) before
therapy/(transversal × anteroposterior diameters) before therapy
×100.
Histological examination
At the end of the therapy experiment, 16 rabbits
with 4 rabbits per group were sacrificed by established technique,
and the maximum cross-section areas of the hepatic tumors were
calculated based on the anatomical measurements and compared with
the CT results. The tumor specimens were then collected and cut
into two sections for histological examination (thickness, 1 cm)
and transmission electron microscopy (TEM; thickness, 1 mm). The
tumor tissues intended for histology examination were fixed with
10% neutral formaldehyde solution for 24 h at 18°C and embedded in
paraffin, and each tumor was stained using hematoxylin and eosin
(H&E) for 2 h at 18°C. Subsequently, the structure of the tumor
cells was observed using an Olympus microscope (BX50;
magnification, ×200; Olympus Corporation, Tokyo, Japan). A
histopathologist blind to the study evaluated the findings using
microscopy.
TEM
Each tumor sample intended for TEM was ~1 mm3 in
volume and fixed in 2% glutaraldehyde and PBS for 2 h at 4°C,
followed by two washes in PBS buffer for 10 min. Following
treatment with 1% osmium tetroxide in PBS, the specimens were fixed
with 1% osmic acid at 4°C for 2 h and dehydrated via immersion in
30% ethanol, followed by 50 and 70% ethanol three times each for 10
min. The samples were then embedded in propylene oxide at 18°C for
2 h and stained with lead citrate E for 12 h at 18°C. Finally, the
specimens were examined after sectioning using TEM (Philips CM-120;
Philips Molecular Systems, Inc.).
Statistical analysis
The data were presented as median ± standard
deviation. Statistical analysis was performed using SPSS version
11.0 (SPSS, Inc., Chicago, IL, USA). The tumor sizes were subjected
to Student's t-test to statistically compare groups. Multiple
groups were compared using a one-way analysis of variance (ANOVA),
and the two groups were compared using the Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of CT and pathology
measurements
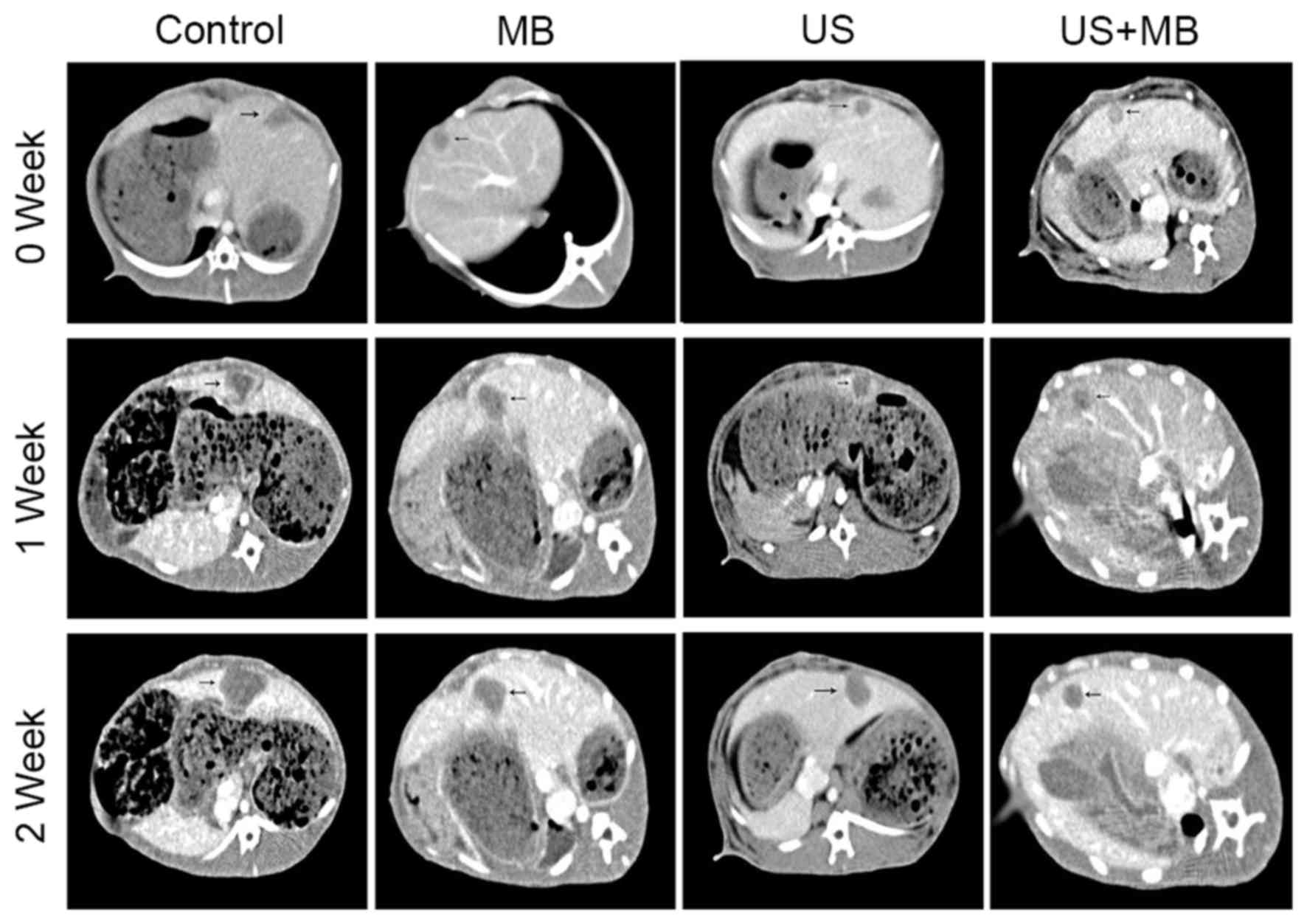
The CT images of 16 hepatic tumors in rabbits were
obtained (Fig. 1). A basic rabbit
liver scan showed a low density of tumors, while the tumors were
markedly enhanced in the arterial phase, with no enhancement in the
necrotic tissue within the tumor and in the surrounding normal
liver parenchyma, which manifested a clear demarcation. The portal
venous phase scan indicated a low density of tumors and clear
enhancement of the surrounding normal liver parenchyma, which best
clarified the tumor margins (Fig. 1).
The tumor size and area calculated based on CT data and pathology
measurements did not significantly differ at 2 weeks (P>0.05;
Table I).
 | Table I.Comparison of tumor area on CT and
pathology after 2 weeks. |
Table I.
Comparison of tumor area on CT and
pathology after 2 weeks.
| Group | CT (D1 × D2),
mm | Anatomy (D1 × D2),
mm | t value | P-value |
|---|
| Control | 22±2.2×21±1.8 | 20±1.6×21±1.6 | −0.03 | 0.9782 |
| MB | 19±2.2×18±2.4 | 19±1.4×19±1.8 | −0.66 | 0.5763 |
| US | 18±2.2×17±2.6 | 18±2.3×18±2.4 | −1.87 | 0.1579 |
| US + MB | 14±2.3×14±2.2 | 15±1.4×15±1.4 | 1.27 | 0.2936 |
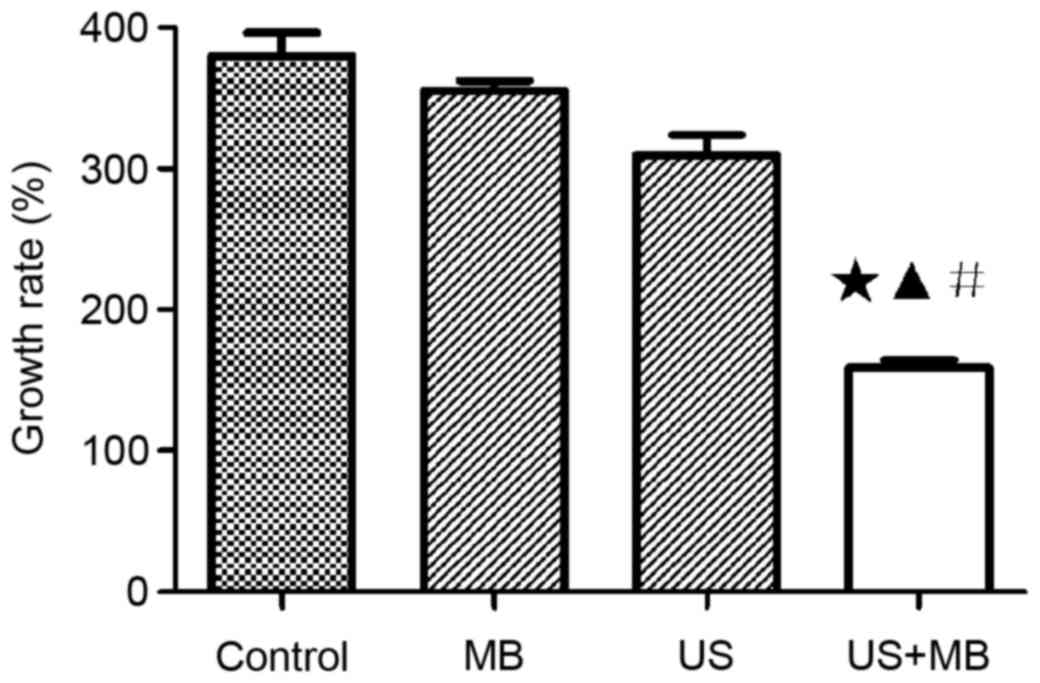
Tumor growth result with CT
As shown in Table II,
the calculation of tumor size of the maximum transverse and
anteroposterior dimensions. The tumor growth rates at 1 and 2 weeks
based on CT are also shown in Table
II and Fig. 2. The mean tumor
growth rates after 2 weeks in the control, MB, US and US + MB
groups were 385±21, 353±12, 302±14 and 154±9%, respectively. The
tumor growth rate significantly differed among the four groups and
was determined using the ANOVA test, with F=87.53 and P<0.0001.
A Newman-Keuls multiple comparison test identified a significant
difference between the US + MB group and the other three groups
(P<0.05), but the control, MB and US groups did not
significantly differ (P>0.05) (Table
II and Fig. 2). The mean tumor
growth rates in the control, MB, US and US + MB groups after 1 week
of treatment were 101±9, 92±6, 77±4 and 73±6%, respectively. The
tumor growth rate did not significantly differ between the US + MB
group and the other three groups after 1 week (P>0.05; Table II).
 | Table II.Growth rate of rabbit hepatic tumor
area calculated by computed tomography in the four groups. |
Table II.
Growth rate of rabbit hepatic tumor
area calculated by computed tomography in the four groups.
| Groups | Pre (D1 × D2),
mm | 1 week (D1 × D2),
mm | 2 week (D1 × D2),
mm | GR 1 week, % | GR 2 weeks, % |
|---|
| Control | 9±0.1×9±0.1 | 13±1.2×13±2.3 | 22±2.2×21±1.8 | 101±9 | 385±21 |
| MB | 9±0.2×8±0.1 | 12±2.3×12±1.4 | 19±2.2×18±2.4 |
92±6 | 353±12 |
| US | 9±0.1×8±0.2 | 11±2.1×12±2.4 | 18±2.2×17±2.6 |
77±4 | 302±14 |
| US + MB | 9±0.2×8±0.1 | 11±2.2×11±1.8 | 14±2.3×14±2.2 |
73±6 | 154±9 |
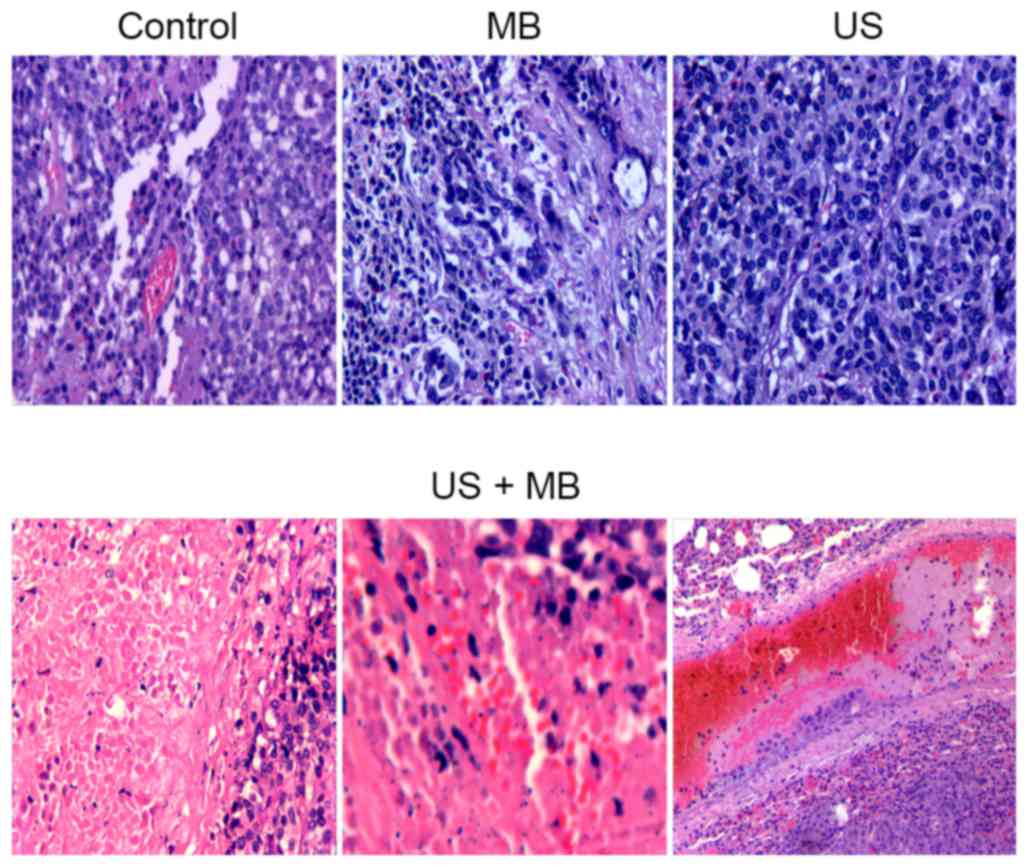
Histological findings
The US + MB group showed tumor coagulation necrosis
(magnification, ×200). Diffused interstitial hemorrhage and
vascular thrombus were also observed 2 weeks after treatment.
Residual liver neoplastic cells could be found only at the
peripheral border area. In the control, MB and US groups, intact
liver tumor tissues grew in a solid pattern without evident
vascular rupture and necrosis (Fig.
3).
TEM results
TEM revealed vascular endothelial cell wall rupture,
widened endothelial cell gaps, interstitial erythrocyte leakage and
vascular lumen thrombosis in the US + MB group. The majority of
tumor cells in the other three groups appeared normal. Intact
vascular lumen and normal erythrocytes in the tumor vessels were
observed in the control, MB and US groups (Fig. 4).
 | Figure 4.Microvessels of transmission electron
microscopy in the control (scale bar, 1 µm), MB (scale bar, 1 µm)
and US (scale bar, 2 µm) groups had an intact vascular lumen and
normal erythrocytes in the vessels (arrow head); ruptured vascular
endothelial cells (scale bar, 2 µm), a widened endothelial cell gap
(scale bar, 2 µm) (slim arrow), erythrocyte exudation (scale bar, 1
µm) (slim arrow) and microvascular thrombosis (scale bar, 5 µm)
(wider arrow) were observed in the US + MB group. |
Discussion
The present study did not indicate significant
differences between the tumor area calculated based on CT and
pathology data after 2 weeks of treatment in the four groups, with
P>0.05 in all cases. CT is an accurate method to evaluate the
curative effect of liver cancer treatments (29). Enhanced CT can accurately detect the
tumor vasculature. It detected not only the border of the tumors
but also internal necrosis due to the non-enhancement of the
tumors. Thus, CT is a useful tool to evaluate liver cancer therapy
and tumor necrosis, and to measure the exact area of a tumor.
The present study used 20 kHz US, which is the
lowest frequency point on the US wave (30). This frequency was selected as higher
sound frequencies accelerate the vibration of the MB in the sound
field while reducing the amplitude (31). The time interval of the sonic pressure
half cycle between the acoustic positive and negative range is
short, which decreased the expansion of MBs; the attraction between
the liquid molecules is not easily broken, which hinders the
formation of a cavitation bubble (31). Therefore, high frequency US requires
higher sound pressures to generate cavitation nuclei. The intensity
threshold of collapse cavitation positively correlates with the
frequency of US (32). The onset of
collapse cavitation at 20 kHz occurs at ~0.015 W/cm2,
while this threshold increases to 0.38 W/cm2 at 70 kHz
(33). By contrast, when MB was
irradiated with lower frequency US, such as 20 kHz, the amplitude
of MB increased (25). The vibration
time at negative pressure is longer; thus, the liquid-phase
intermolecular attraction breaks more easily and more cavitation
nuclei form (34). Furthermore, the
longer time gap between the compression and expansion of the liquid
affords the cavitation bubble with more time to grow prior to
bursting, and the volume of the cavitation bubble is positively
associated with the intensity of the cavitation effects when the
bubble collapses (35). Thus,
selecting an appropriate ultrasonic frequency is crucial to the
induced cavitation effect.
The present study revealed that the tumor growth
rate in the US + MB group was lower than those in the control, MB
and US groups. In addition, vascularization damage was another
important result. The tumor cannot grow without angiogenesis and
malignant tumors are always rich in vasculature (36). The present results demonstrated that
the vascularization of rabbit liver tumors significantly changed in
the US + MB group compared with the other three groups. H&E
staining revealed tumor microvascular internal thrombosis in
certain areas, the interstitial exudation of red blood cell (RBC)
and tumor cell necrosis. US combined with MBs can effectively
destroy the tumor blood vessels and inhibit the growth of the tumor
(26). Previous studies have reported
that MB destruction during US exposure ruptures microvessels and
causes RBCs to extravasate (37,38).
TEM in the present study revealed tumor capillary
vessel wall fracture, cavity thrombosis and interstitial exudation
of RBCs in the US + MB group, and these findings were consistent
with the results of the H&E staining. US combined with MBs
caused significant vascular changes, which may be due to the
following. Sonovue, a second-generation contrast agent, is a
phospholipid coated, sulfur hexafluoride gas-containing MB, whose
diameter is much smaller than that of room air-filled bubbles (~2.5
mm) (39). This smaller diameter
improves the passage into the pulmonary capillary bed and allows
the bubbles to reach the micro-circulation of rabbit hepatic tumors
(39). MBs expand and contract in
size in response to the oscillating pressure and energy accumulates
when the MBs are constricted by sound pressure (40). Ultimately, the energy carried by
cavitation bubbles is released when the MBs collapse and sonication
cavitation occurs and during the cavitation, the MBs undergo
volumetric oscillations, thereby changing the local mechanical
condition of the tissue (40). When
MBs collapse, they can create shockwaves, increasing the local
pressure by 100 atm and the local temperature by several thousands
of degrees (41). Shockwaves can
cause substantial cell damage and possible cell lysis nearby
(42). The destruction of MBs may
cause high-energy microstreams or microjets that will result in
shear stress on the membrane of an endothelial cell and increase
its permeability (43).
During US sonication in the presence of MBs, the
oscillation, collapse and cavitation of MBs in the acoustic beam
produced vascular pores and disrupted the vessel wall to
significantly increase the vascular permeability in the sonicated
areas (35). This process may have
led to the formation of smaller bubbles, which interacted with the
US beam and caused the cellular bio-effects (44).
Other bio-effects include the free radicals
generated during MB collapse (41).
The formation of highly reactive species, including OH, H,
HO2 and H2O2, due to the transient
collapse of cavitation bubbles is the primary mechanism of the
sonochemical reaction (41), which
can also damage the vessel wall.
Notably, microvessel rupture was not observed in
response to US exposure. Similarly, ruptures were also absent when
MBs were infused in the absence of US. When MB infusion and US
exposure were performed simultaneously, the destruction of MBs was
evident in the microvessels of rabbit hepatic tumors. The
cumulative effects of 2 weeks of US sonication may have ruptured
tumor vessels and induced hemorrhage, which would have destroyed
the normal structural support for the capillaries. In general,
tumor vascular rupture, interstitial erythrocyte leakage and
continual injury induced by MB cavitation result in tumor ischemia,
which may explain the tumor growth inhibition in the MB + US group
(P<0.05 compared with the other three groups), as presented in
Fig. 2.
However, the disruption of blood vessels in the
sonicated tumors can enhance tumor inhibition, while it may also
facilitate the intravasation of tumor cells into circulation to
increase metastasis. Future studies should investigate the
possibility of metastasis increase in response to MB + US
treatment.
Acknowledgements
The present study was supported in part by the
Nantong Municipal Science and Technology Project (grant no.
MS12016033 and HS149072), the Nantong Municipal Youth Fund Project
(grant no. WQ2015054) and the Six Talent Peaks Project in Jiangsu
Province (grant no. WSW-081).
References
|
1
|
Fausti SA, Erickson DA, Frey RH, Rappaport
BZ and Schechter MA: The effects of noise upon human hearing
sensitivity from 8000 to 20 000 Hz. J Acoust Soc Am. 69:1343–1347.
1981. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miano AC, Ibarz A and Augusto PE:
Mechanisms for improving mass transfer in food with ultrasound
technology: Describing the phenomena in two model cases. Ultrason
Sonochem. 29:413–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porova N, Botvinnikova V, Krasulya O,
Cherepanov P and Potoroko I: Effect of ultrasonic treatment on
heavy metal decontamination in milk. Ultrason Sonochem.
21:2107–2111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao S, Hemar Y, Lewis GD and Ashokkumar M:
Inactivation of enterobacter aerogenes in reconstituted skim milk
by high- and low-frequency ultrasound. Ultrason Sonochem.
21:2099–2106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zisu B, Bhaskaracharya R, Kentish S and
Ashokkumar M: Ultrasonic processing of dairy systems in large scale
reactors. Ultrason Sonochem. 17:1075–1081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hapeshi E, Achilleos A, Papaioannou A,
Valanidou L, Xekoukoulotakis NP, Mantzavinos D and Fatta-Kassinos
D: Sonochemical degradation of ofloxacin in aqueous solutions.
Water Sci Technol. 61:3141–3146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pangu GD, Davis KP, Bates FS and Hammer
DA: Ultrasonically induced release from nanosized polymer vesicles.
Macromol Biosci. 10:546–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu H, Comer J, Cai W, Cai W and Chipot C:
Sonoporation at small and large length scales: Effect of cavitation
bubble collapse on membranes. J Phys Chem Lett. 6:413–418. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sirsi SR and Borden MA: State-of-the-art
materials for ultrasound-triggered drug delivery. Adv Drug Deliv
Rev. 72:3–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forbes MM, Steinberg RL and O'Brien WD Jr:
Frequency-dependent evaluation of the role of definity in producing
sonoporation of Chinese hamster ovary cells. J Ultrasound Med.
30:61–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aldwaikat M and Alarjah M: Investigating
the sonophoresis effect on the permeation of diclofenac sodium
using 3D skin equivalent. Ultrason Sonochem. 22:580–587. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohl CD, Arora M, Ikink R, de Jong N,
Versluis M, Delius M and Lohse D: Sonoporation from jetting
cavitation bubbles. Biophys J. 91:4285–4295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ward M, Wu J and Chiu JF:
Ultrasound-induced cell lysis and sonoporation enhanced by contrast
agents. J Acoust Soc Am. 105:2951–2957. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Domenici F, Giliberti C, Bedini A, Palomba
R, Luongo F, Sennato S, Olmati C, Pozzi D, Morrone S, Congiu
Castellano A and Bordi F: Ultrasound well below the intensity
threshold of cavitation can promote efficient uptake of small drug
model molecules in fibroblast cells. Drug Deliv. 20:285–295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Florinas S, Kim J, Nam K, Janát-Amsbury MM
and Kim SW: Ultrasound-assisted siRNA delivery via arginine-grafted
bioreducible polymer and microbubbles targeting VEGF for ovarian
cancer treatment. J Control Release. 183:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Javadi M, Pitt WG, Tracy CM, Barrow JR,
Willardson BM, Hartley JM and Tsosie NH: Ultrasonic gene and drug
delivery using eLiposomes. J Control Release. 167:92–100. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller DL, Pislaru SV and Greenleaf JE:
Sonoporation: Mechanical DNA delivery by ultrasonic cavitation.
Somat Cell Mol Genet. 27:115–134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dobroserdov VK: On the effect of low
frequency ultrasonic waves and high frequency sound waves on the
organism of workers. Gig Sanit. 32:17–21. 1967.(In Russian).
PubMed/NCBI
|
|
19
|
Davis H, Parrack HO and Eldredge DH:
Hazards of intense sound and ultrasound. Ann Otol Rhinol Laryngol.
58:732–738. 1949. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Acton WI and Carson MB: Auditory and
subjective effects of airborne noise from industrial ultrasonic
sources. Br J Ind Med. 24:297–304. 1967.PubMed/NCBI
|
|
21
|
Acton WI: The effects of industrial
airborne ultrasound on humans. Ultrasonics. 12:124–128. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boucaud A, Montharu J, Machet L, Arbeille
B, Machet MC, Patat F and Vaillant L: Clinical, histologic and
electron microscopy study of skin exposed to low-frequency
ultrasound. Anat Rec. 264:114–119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang H, Wang CC, Blankschtein D and Langer
R: An investigation of the role of cavitation in low-frequency
ultrasound-mediated transdermal drug transport. Pharm Res.
19:1160–1169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mawson R, Rout M, Ripoll G, Swiergon P,
Singh T, Knoerzer K and Juliano P: Production of particulates from
transducer erosion: Implications on food safety. Ultrason Sonochem.
21:2122–2130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen ZY, Shen E, Zhang JZ, Bai WK, Wang Y,
Yang SL, Nan SL, Lin YD, Li Y and Hu B: Effects of low-frequency
ultrasound and microbubbles on angiogenesis-associated proteins in
subcutaneous tumors of nude mice. Oncol Rep. 30:842–850.
2013.PubMed/NCBI
|
|
26
|
Shen ZY, Shen E, Diao XH, Bai WK, Zeng MX,
Luan YY, Nan SL, Lin YD, Wei C, Chen L, et al: Inhibitory effects
of subcutaneous tumors in nude mice mediated by low-frequency
ultrasound and microbubbles. Oncol Lett. 7:1385–1390.
2014.PubMed/NCBI
|
|
27
|
Ferrara KW: Driving delivery vehicles with
ultrasound. Adv Drug Deliv Rev. 60:1097–1102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen ZY, Xia GL, Wu MF, Ji LY and Li YJ:
The effects of percutaneous ethanol injection followed by 20-kHz
ultrasound and microbubbles on rabbit hepatic tumors. J Cancer Res
Clin Oncol. 142:373–378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen G, Ma DQ, He W, Zhang BF and Zhao LQ:
Computed tomography perfusion in evaluating the therapeutic effect
of transarterial chemoembolization for hepatocellular carcinoma.
World J Gastroenterol. 14:5738–5743. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohl SW, Klaseboer E and Khoo BC: Bubbles
with shock waves and ultrasound: A review. Interface Focus.
5:201500192015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Izadifar Z, Babyn P and Chapman D:
Mechanical and biological effects of ultrasound: A review of
present knowledge. Ultrasound Med Biol. 43:1085–1104. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vlaisavljevich E, Lin KW, Warnez MT, Singh
R, Mancia L, Putnam AJ, Johnsen E, Cain C and Xu Z: Effects of
tissue stiffness, ultrasound frequency and pressure on
histotripsy-induced cavitation bubble behavior. Phys Med Biol.
60:2271–2292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Husseini GA, Abdel-Jabbar NM, Mjalli FS
and Pitt WG: Modeling and sensitivity analysis of acoustic release
of doxorubicin from unstabilized pluronic P105 using an artificial
neural network model. Technol Cancer Res Treat. 6:49–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shneidman VA: Time-dependent cavitation in
a viscous fluid. Phys Rev E. 94:0621012016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen ZY, Xia GL, Wu MF, Shi MX, Qiang FL,
Shen E and Hu B: The effects of low-frequency ultrasound and
microbubbles on rabbit hepatic tumors. Exp Biol Med (Maywood).
239:747–757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Verdelli C, Avagliano L, Creo P, Guarnieri
V, Scillitani A, Vicentini L, Steffano GB, Beretta E, Soldati L,
Costa E, et al: Tumour-associated fibroblasts contribute to
neoangiogenesis in human parathyroid neoplasia. Endocr Relat
Cancer. 22:87–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Skyba DM, Price RJ, Linka AZ, Skalak TC
and Kaul S: Direct in vivo visualization of intravascular
destruction of microbubbles by ultrasound and its local effects on
tissue. Circulation. 98:290–293. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Price RJ, Skyba DM, Kaul S and Skalak TC:
Delivery of colloidal particles and red blood cells to tissue
through microvessel ruptures created by targeted microbubble
destruction with ultrasound. Circulation. 98:1264–1267. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Y, Ren W, Liu C, Huang K, Feng Y, Wang
X and Tong Y: Contrast-enhanced ultrasonography of the rabbit VX2
tumor model: Analysis of vascular pathology. Oncol Lett. 4:685–690.
2012.PubMed/NCBI
|
|
40
|
Kollath A, Brezhneva N, Skorb EV and
Andreeva DV: Microbubbles trigger oscillation of crystal size in
solids. Phys Chem Chem Phys. 19:6286–6291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Merouani S, Hamdaoui O, Rezgui Y and
Guemini M: Theoretical estimation of the temperature and pressure
within collapsing acoustical bubbles. Ultrason Sonochem. 21:53–59.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Coralic V and Colonius T: Shock-induced
collapse of a bubble inside a deformable vessel. Eur J Mech B
Fluids. 40:64–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suzuki R, Oda Y, Utoguchi N and Maruyama
K: Progress in the development of ultrasound-mediated gene delivery
systems utilizing nano- and microbubbles. J Control Release.
149:36–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin CY, Tseng HC, Shiu HR, Wu MF, Chou CY
and Lin WL: Ultrasound sonication with microbubbles disrupts blood
vessels and enhances tumor treatments of anticancer nanodrug. Int J
Nanomedicine. 7:2143–2152. 2012. View Article : Google Scholar : PubMed/NCBI
|