Introduction
Carotid body tumor (CBT) is a slow-growing
neuroendocrine neoplasm that is usually localized at the
bifurcation of the common carotid artery (1,2). CBT is an
infrequent neoplasm in clinical practice, rarely malignant, which
accounts for ≤0.5% of all head and neck tumors with no obvious
gender predominance. Approximately 10% of affected cases may be
bilateral (3). The diagnosis of CBT
remains a challenge, as its manifestations are not typical.
Generally, surgical resection is regarded as the first line of
treatment for CBT (4,5). It is challenging to treat as the carotid
body is a highly vascular structure situated in the adventitia of
the posteriomedial aspect of the carotid bifurcation (6). Therefore, clinicians understanding of
the vascular anatomy of this region is critical for the diagnosis
and treatment of CBT, and important factors affecting the success
of surgical resection include the ability and experience of the
surgeon. The present study, through a review of 58 patients (62
lesions) with CBT managed by the same surgeon, aimed to investigate
the diagnosis and treatment of CBT.
Materials and methods
Patients
A retrospective analysis was performed on patients
with CBT who were surgically treated in the Department of
Otolaryngology Head and Neck Surgery, Renmin Hospital of Wuhan
University (Wuhan, China) between October 2003 and October 2013. A
total of 58 patients (26 male and 32 female) were included, with an
age range of 31–65 years, an average age of 42.8 years and a
disease duration ranging from 3 to 84 months. The protocols used in
the present study were approved by the Ethics Committee of Renmin
Hospital of Wuhan University. Written informed consent was obtained
from all patients subsequent to a detailed explanation of the
study.
Imaging techniques
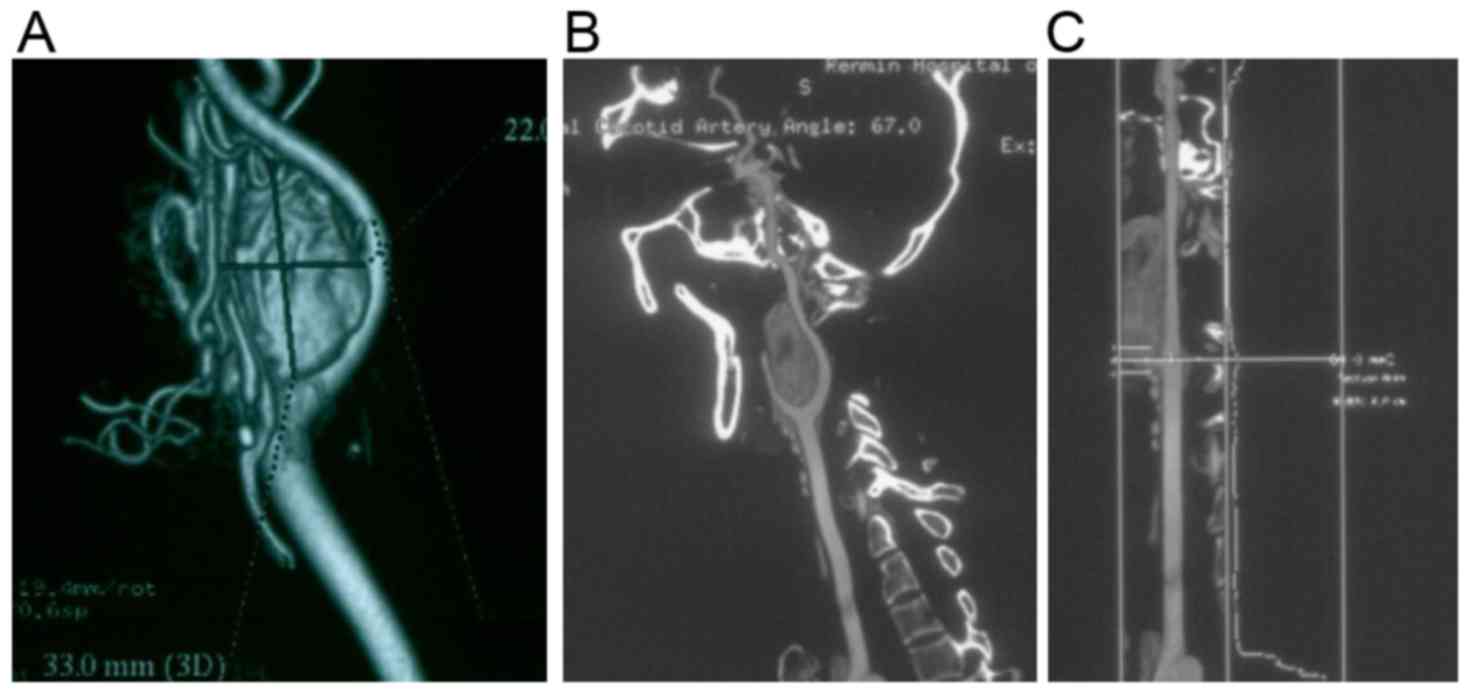
All the patients received computed tomography (CT)
and magnetic resonance imaging (MRI) scans. CT was performed to
localize the position of the tumor mass, and to display the
adherence of tumor to the peripheral tissues. CT angiography was
performed in 25 cases, and MRI angiography was performed in 15
cases to visualize the morphology of the blood vessels. Digital
subtraction angiography (DSA) was performed in 20 patients
(Figs. 1 and 2).
The brain ischemic tolerance of each patient was
monitored using a rheoencephalogram (REG). Approximately 2 weeks
prior to the surgery, carotid compression was performed on the
affected side(s). At 1 day prior to the surgery, DSA examination
was performed in 12 patients with Shamblin grade III tumors to
determine the establishment of cerebral collateral circulation.
Surgical technique
Laterally inclined incisions were made along the
anterior border of the sternomastoid muscle under general
anesthesia (7). On visualizing the
common carotid artery, a series of anatomical structures were
separated in order, including the cranial nerve, accessory nerve,
the common carotid artery and the internal and external carotid
veins. On this basis, the common carotid artery and the proximal
end of the tumor artery were blocked using sterilized bands to stop
the blood flow. Subsequently, sole resection of CBT, resection of
CBT and external carotid artery, and resection of CBT and common
carotid artery were performed using bipolar electrocoagulation
under a surgical microscope according to the adherence of the tumor
mass to the artery. Reconstruction of the common carotid and
external carotid arteries was performed in patients who underwent
resection of these arteries (Fig.
3).
Results
Patient characteristics
The 58 patients included 26 men and 32 women (mean
age, 42.8 years; age range, 31–65 years), and disease duration
ranged from 3 to 84 months. Unilateral lesions were identified in
54 patients, with 26 on the left side, and 28 on the right side,
and bilateral lesions were identified in 4 female patients. No
family history of CBT was reported for any of the patients.
Among the patients, 38 (65.5%) presented to the
Department of Otolaryngology Head and Neck Surgery at Renmin
Hospital of Wuhan University, due to a tumor mass in the neck, 12
(20.7%) presented with headache and a tumor mass in the neck, and 6
patients (10.3%) presented with hoarseness combined with headache
and a tumor mass in the neck. The remaining 2 patients (3.4%) were
transferred to our department due to massive bleeding caused by
resection of a tumor mass in the neck. Of the 62 lesions, 17 were
categorized as Shamblin I, 33 were categorized as Shamblin II, and
12 were categorized as Shamblin III (Table I).
 | Table I.Summary of tumor removal and vascular
injury. |
Table I.
Summary of tumor removal and vascular
injury.
| Variable | Shamblin I (17
sides) | Shamblin II (33
sides) | Shamblin III (12
sides) |
|---|
| Tumor removal |
|
|
|
| Complete removal of
tumor | 17 | 33 | 12 |
| Vascular injury |
|
|
|
| Ligation of external
carotid artery | – | 4 | 6 |
| Ligation of internal
carotid artery | – | – | 1 |
| Ligation of common
carotid artery | – | – | 1 |
Complete resection of CBT was performed in all 58
patients (62 lesions). The tumor masses ranged in size from 3 to 12
cm, with a median of 5.6 cm. Surgical duration was 3–8 h. The
average bleeding volume of patients with lesions of Shamblin grade
I and II was 300–600 ml, with a median of 485 ml. Patients with
Shamblin grade III lesions had an average bleeding volume of
400–1,200 ml, with a median of 780 ml. No tracheostomies were
performed. Pathological analysis indicated that the CBTs were
benign. Hospitalization lasted for 10–14 days (median, 12.4 days;
Table II).
 | Table II.Perioperative conditions of the
patients. |
Table II.
Perioperative conditions of the
patients.
| Variable | Shamblin I or II
grade (50 sides) | Shamblin III (12
sides) |
|---|
| Tumor size (cm) | 3–6 | 5–12 |
| Hemorrhage (ml) | 300–600 | 400–1200 |
| Duration of operation
(min) | 180–240 | 240–480 |
| Hospital stay
(days) | 10–12 | 10–14 |
Postoperative complications
No hemiplegia was observed in any of the patients.
Hoarseness and bucking were observed in 2 patients with Shamblin
III lesions (Table III). These
complications were eliminated 1 month subsequent to the
administration of hormone therapy and nerve-nurturing strategies
(including dexamethasone and mecobalamine). No relapse or mortality
was observed during the 6–84 months of follow-up.
 | Table III.Postoperative complications of each
group. |
Table III.
Postoperative complications of each
group.
| Variable | Shamblin I (17
sides) | Shamblin II (33
sides) | Shamblin III (12
sides) |
|---|
| Injury of central
nerve |
|
|
|
| Hemiplegia | – | – | – |
| Cranial nerve
injury |
|
|
|
| Glossopharyngeal
nerve | – | – | – |
| Vagus nerve | – | – | 2 |
| Accessory nerve | – | – | – |
| Hypoglossal
nerve | – | – | – |
Discussion
DSA is considered to be an important technique for
the diagnosis of CBT as it can define the profile of the tumor,
including location, size and the presence of intratumoral blood
vessels. In addition, DSA is usually used before CBT surgery with
the aims of aiding transarterial embolization therapy and reducing
the occurrence of intraoperative hemorrhage (8–10).
However, there are limitations (11).
For example, the adherence of the tumor mass to the peripheral
tissues is poorly displayed by DSA prior to surgery. Also, ectopic
embolism and vascular rupture are usually reported as the technique
is invasive. Additionally, a high risk of cardiac arrest was
observed in patients with carotid body hypersensitivity (9).
Currently, CT, CT angiography (CTA) and MRI are used
for the diagnosis of CBT as these techniques are able to define the
size and margins of the tumor mass, and the adherence of the tumor
mass to the peripheral tissues (12,13). In
particular, a previous study showed that the hemodynamic analysis
obtained using CTA contributed to the accurate evaluation of the
morphology of the vascular wall and the identification of tumor
invasion to the middle layer and tunica intima of the carotid wall
(14). According to our previous
clinical experience, we recommend complete evaluation of tumor
invasion to the carotid artery prior to the surgery and,
concomitantly, appropriate evaluation of brain ischemic tolerance
in order to evaluate whether the cerebral collateral circulation
has been established. In the present study, preoperative DSA was
beneficial to the establishment of cerebral collateral circulation.
In addition, hemodynamic observation of the CBT segment may
contribute to the identification of vascular wall invasion. Besides
the aforementioned disadvantages of DSA, the bleeding of patients
undergoing transarterial embolization prior to the surgery was not
obviously decreased in the present study. According to our clinical
experiences, preoperative DSA and transarterial embolization are
not recommended for patients with CBT.
A higher incidence of massive bleeding was observed
during the preoperative biopsy as the blood vessels in the CBT were
abundant. In the present study, 2 patients were transferred to the
Department of Otolaryngology Head and Neck Surgery, Renmin Hospital
of Wuhan University, following resection of the carotid mass at
local hospitals. On this basis, we recommend that biopsy and
surgery should not be performed for patients with tumor masses in
the carotid triangle, unless a confirmed diagnosis has been
achieved.
Currently, surgery and radiotherapy are considered
the primary treatment choices for CBT. According to our clinical
experiences, surgery is recommended once CBT is diagnosed.
Currently, the surgical procedures of CBT are mainly based on the
Shamblin classification (10),
including tumor resection, tumor and external carotid artery
resection, tumor resection together with vascular reconstruction
(internal carotid artery) and tumor resection together with
ligation of the common carotid artery. According to our clinical
practice, tumor resection is the desired procedure suitable for
cases that belong to Shamblin I or those exhibiting tumors with a
small size and a limited blood supply. For Shamblin II and III
cases, if the carotid artery lumen is well shaped, the wall
boundary is clear and the blood flow is also uniform, tumor
resection may be selected; however, preparation for vascular
reconstruction is required. Special attention should be paid during
the separation of the tumor body and blood vessels; for example,
bipolar electrocoagulation should be modulated to a refined
pattern, and the energy should be set at a suitable number and
assisted by washing using fresh water in order to prevent thermal
injury of the vascular intima. For the patients with Shamblin I and
II lesions with abundant blood supply, surgical resection of CBT
and external carotid artery is recommended. Tumor resection and
vascular reconstruction is suitable for the patients with Shamblin
grade II and III lesions, those with tumors of diameter >5 cm,
or those with abundant blood supply in the tumor mass (10). According to our clinical practice,
prior to surgery, preoperative evaluation of the affected region is
important in order to avoid ligation of blood vessels and
interruption of cerebral blood supply as far as possible, due to
reduce postoperative hemiplegia.
In the present study, surgical resection of the
tumor mass was performed in the majority of patients with Shamblin
grade III lesions. Blood vessel transplantation or ligation was
only performed in those with tumor cells invading into the middle
layer of the artery and tunica intima. Resection was performed in
62 lesions, among which 52 (83.87%) underwent resection of the CBT.
The internal carotid artery was well preserved in 11 lesions of
Shamblin grade III lesions, with no postoperative complications.
Ligation of the tumor mass and common carotid artery was performed
in 1 patient with a Shamblin grade III lesion, and no ischemia of
brain tissues was observed subsequent to the surgery. For the
patients with bilateral CBT, staging surgery is proposed in order
to guarantee cerebral blood supply. Generally, the lesion with the
larger tumor mass should be treated first, and then the
contralateral tumor mass should be removed subsequent to healing of
the wound of the previous surgical site.
At present, surgery is preferred for the treatment
of CBT; however, this remains a challenge as this type of tumor is
a highly vascular mass that is often densely adherent to the vagus
nerve (15). According to our
clinical experience, it is recommended that the surgery is
performed under microscopy in order to define the boundary of tumor
mass and the vascular wall. On this basis, the incidence of
vascular rupture and neural injury may be avoided. Concurrently,
bipolar electrocoagulation may be used to treat the thermal injury
to the vascular wall and peripheral tissues. Among the 58 patients
included in the present study, 1 patient exhibited vascular
rupture, and no severe complications, such as postoperative
hemiplegia or permanent injury of the cranial nerves, were
observed. Injury of the cranial nerves, which most of presents as
injury of the vagus nerve and hypoglossal nerve, is the most common
type of postoperative complication of CBT surgery. It is mainly
caused by intraoperative dragging, and may be healed subsequent to
conservative therapy unless the injury was permanent. In clinical
practice, a deep understanding of the anatomical structure of the
neck is required for the surgery. Therefore, surgery using
microscopy is recommended, which is beneficial for the
identification of the vascular wall and nerves. Additionally,
special care should be taken to obtain a clear surgical field, and
to prevent the occurrence of bleeding and injury of the cranial
nerves.
Radiotherapy is an alternative management for those
patients unfit for surgery, those who may not tolerate the surgery,
and those who exhibit metastasis subsequent to surgery (16,17).
Although the outcome of radiotherapy for CBT is satisfactory,
complications subsequent to chemotherapy are usually reported, such
as otitis externa, otitis media, osteoradionecrosis, cranial nerve
lesion and brain injury.
In summary, surgical resection is the preferred
treatment for CBT. The present study suggests that surgical
resection should be performed once CBT is confirmed. The evaluation
of imaging features and cerebral collateral circulation is critical
for the selection of treatment methods. Injuries to the cranial
nerves may be reduced with the development of microsurgery and
cardiovascular surgery. Tumor resection, without reconstruction and
ligation of the common carotid artery may be required for patients
with Shamblin grade III tumors. Reconstruction or ligation causes
potential damage to the nervous system, and is only required in
patients with tumor cells invading the middle layer and tunica
intima of carotid artery.
References
|
1
|
Unlu Y, Becit N, Ceviz M and Kocak H:
Management of carotid body tumors and familial paragangliomas:
Review of 30 years' experience. Ann Vasc Surg. 23:616–620. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martinelli O, Irace L, Massa R, Savelli S,
Giannoni F, Gattuso R, Gossetti B, Benedetti-Valentini F and Izzo
L: Carotid body tumors: Radioguided surgical approach. J Exp Clin
Cancer Res. 28:1482009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanghvi VD and Chandawarkar RY: Carotid
body tumors. J Surg Oncol. 54:190–192. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davidovic LB, Djukic VB, Vasic DM,
Sindjelic RP and Duvnjak SN: Diagnosis and treatment of carotid
body paraganglioma: 21 years of experience at a clinical center of
Serbia. World J Surg Oncol. 3:102005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rabl H, Friehs I, Gutschi S, Pascher O and
Koch G: Diagnosis and treatment of carotid body tumors. Thorac
Cardiovasc Surg. 41:340–343. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tong Y: Role of duplex ultrasound in the
diagnosis and assessment of carotid body tumour: A literature
review. Intractable Rare Dis Res. 1:129–133. 2012.PubMed/NCBI
|
|
7
|
Ma D, Liu M, Yang H, Ma X and Zhang C:
Diagnosis and surgical treatment of carotid body tumor: A report of
18 cases. J Cardiovasc Dis Res. 1:122–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Telischak N, Gross BA, Zeng Y, Reddy AS,
Frankenthaler R, Ogilvy CS and Thomas AJ: The glomic artery supply
of carotid body tumors and implications for embolization. J Clin
Neurosci. 21:1176–1179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Power AH, Bower TC, Kasperbauer J, Link
MJ, Oderich G, Cloft H, Young WF Jr and Gloviczki P: Impact of
preoperative embolization on outcomes of carotid body tumor
resections. J Vasc Surg. 56:979–989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bauer AM, Smith RB and Thorell WE:
Implications of carotid sinus hypersensitivity following
preoperative embolization of a carotid body tumor. An indication
for prophylactic intraoperative cardiac pacing. JAMA Otolaryngol
Head Neck Surg. 140:459–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim JY, Kim J, Kim SH, Lee S, Lim YC, Kim
JW and Choi EC: Surgical treatment of carotid body paragangliomas:
Outcomes and complications according to the shamblin
classification. Clin Exp Otorhinolaryngol. 3:91–95. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang F, Han D, Qu S, Liang J, Liu B and
Huang Y: Diagnosis and management of jugulare glomus tumor and
carotid body tumor. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
28:612–617. 2014.(In Chinese). PubMed/NCBI
|
|
13
|
Derchi LE, Serafini G, Rabbia C, De
Albertis P, Solbiati L, Candiani F, Musante F, Bertoglio C and
Rizzatto G: Carotid body tumors: US evaluation. Radiology.
182:457–459. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pacheco-Ojeda LA and Martínez-Viteri MA:
Preoperative imaging diagnosis of carotid body tumors. Int Surg.
95:242–246. 2010.PubMed/NCBI
|
|
15
|
Suárez C, Rodrigo JP, Mendenhall WM,
Hamoir M, Silver CE, Grégoire V, Strojan P, Neumann HP, Obholzer R,
Offergeld C, et al: Carotid body paragangliomas: A systematic study
on management with surgery and radiotherapy. Eur Arch
Otorhinolaryngol. 271:23–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Künzel J, Koch M, Brase C, Fietkau R, Iro
H and Zenk J: Treatment of cervical paragangliomas: Is surgery the
only way? Am J Otolaryngol. 35:186–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sur RK, Krawitz HE, Malas S, Donde B and
Levin CV: Carotid body tumour-a case for radiotherapy? S Afr J
Surg. 33:106–109. 1995.PubMed/NCBI
|