Introduction
Endometrial cancer (EC) is the most commonly
occurring malignant neoplasm of the female reproductive tract in
developed countries (1). Typically,
EC is confined to the uterus and is characterized by a good
prognosis, with an overall 5-year survival rate of 75–80% (2). Endometrial endometrioid cancer (EEC), a
histological subtype of EC, represents 80% of all cases of EC
(2).
Adenomyosis uteri (AM) is defined by the presence of
endometrial mucosa within the myometrium. In hysterectomy specimens
obtained during the treatment of EEC, AM may be found in 16–42%
(3–6).
A more favorable prognosis and a lower histopathological grade in
cases EEC with coexisting AM has been reported in a number of
studies (3,4,7,8). However the significance of the presence
of AM in cases of EEC remains unclear. Despite early predictions,
the collective evidence suggests that estrogen receptor β (ERβ)
serves an important role in normal endometrial tissue (9–11) and also
in most, if not all, benign and malignant endometrial diseases
(12–14). In ectopic endometrial lesions and AM,
it has been reported that high levels of ERβ expression, 100-times
higher than that in eutopic endometrium, may be detected (15). In EEC, it has been reported that ERβ
mRNA and protein levels are decreased compared with adjacent normal
endometrium or normal proliferative endometrium from healthy
premenopausal women (16,17). However, a potential oncogenic role of
ERβ has also been proposed in other reports, which showed an
upregulation of the ERβ5 transcript in high-grade EEC (18,19). In
summary, ERβ appears to play a dual role: As a tumour suppressor in
healthy endometrium, and as a potential tumour promoter in
high-grade EC cells that have lost other receptor subtypes
(12).
The present study aimed to identify the differences
in the expression of several proteins, including ERβ, in cases of
EEC with and without AM, and to evaluate the potential
histopathological and prognostic distinctions between these two
groups.
Patients and methods
Patients and specimen
characteristics
The present retrospective observational cohort study
included 57 patients with EEC, whose records were obtained from the
archives of the Department of Obstetrics and Gynaecology,
Ludwig-Maximilian University of Munich (Munich, Germany). Group A
consisted of 22 patients with coexistent EEC and AM. Group B
comprised 35 patients with EEC alone. Patients had undergone
surgical resection of EEC between January 1990 and December 2002.
The patients' former tumour stages were corrected to the updated
FIGO classification from 2010 (20).
Study design
Patients were recruited between 1990 and 2002 as
described; follow-up continued until December 2015. Clinical and
follow-up data were retrieved retrospectively from patient charts
and from the Munich Cancer Registry (Munich, Germany). The overall
mean follow-up time of the cohort was 8 years (range, 4–15 years).
The outcomes assessed were overall survival time and progression
free survival. Overall survival is defined as the number of months
between the patient's initial diagnosis with endometrial carcinoma
until the last follow up or mortality. Progression free survival is
defined by the number of months between the initial diagnosis and
when the patient developed either a relapse or further progression
of the disease, including lymph node involvement or metastasis.
Ethical approval
All patient data were fully anonymized, and the
study was performed according to the standards set in the
Declaration of Helsinki, 1975. All tumour tissue used in the study
was leftover material that had initially been collected for
histopathological diagnostic assessments. All diagnostic procedures
had already been fully completed when samples were retrieved for
the study. The present study was approved by the Ethics Committee
of the Ludwig-Maximilian University of Munich (approval no.
449-14). Investigators were blinded to the patients' clinical
information during experimental analysis.
Assay methods
Formalin-fixed, paraffin-embedded tissue sections
were stored at room temperature. Tumour tissues and tissues with AM
were selected from hematoxylin-stained whole uterus slides.
Sections with a thickness of 2 µm of tumour or AM positive tissue
were prepared and used for the staining. Slides were dewaxed in
xylol and rehydrated in a descending series of alcohol
concentrations. The immunohistochemical staining of the
paraffin-embedded tissues for glycodelin (in Group B) and inhibin
βB was detected with the avidin-biotin complex method described by
Kricka and Wild (21) using the mouse
VECTASTAIN Elite ABC kit (Vector Laboratories, Inc., Burlingame,
CA, USA). For the detection of glycodelin (in Group A) and ERβ1,
the ZytoChem Plus HRP Polymer kit (Zytomed Systems GmbH, Berlin,
Germany) was used, according to the manufacturer's protocol. The
primary antibodies are described in Table
I, and were incubated with the tissue sections for 16 h at 4°C,
prior to detection with the aforementioned methods.
 | Table I.Primary antibodies used in the
study. |
Table I.
Primary antibodies used in the
study.
| Primary
antibody | Host | Clone | Cat. no. | Supplier | Dilution |
|---|
| Estrogen receptor β
1 | Mouse (IgG2a) | PPG5/10 | M7292 | Dako; Agilent
Technologies GmbH, Waldbronn, Germany | 1:200 in Dako
antibody diluent |
| Glycodelin | Mouse (IgG1κ) | 6F2 | 116–0646 | Zytomed Systems
GmbH, Berlin, Germany | 1:4,000 in PBS |
| Inhibin βB | Mouse (IgG2a) | C5 | MCA1661 | Serotec; Bio-Rad
Laboratories, Inc., Hercules, CA, USA | 1:70 in Dako
antibody diluent |
Immunoreactivity was quantified with Remmele's
semi-quantitative immunoreactivity score (IRS) (22) by two independent observers by
consensus. This scoring method quantifies immunoreactivity by
multiplication of the optical staining intensity score (0, none; 1,
weak; 2, moderate; or 3, strong) with a score representing the
percentage of positively stained cells (0, no staining; 1, ≤10% of
cells; 2, 11–50% of cells; 3, 51–80% of cells; or 4, ≥81% of
cells), resulting in the overall IRS, which can be subdivided as
follows: 0–2, negative; 3–4, weak-positive; 6–8, moderate-positive;
and 9–12, strong-positive.
Statistical analysis
The IBM statistical package SPSS (version 23; IBM,
Armonk, NY, USA) was used to test data for statistical
significance. A Mann-Whitney U-Test was performed for analyses of
differences in staining results and Spearman's rank correlation
analysis was performed for correlation analyses. Survival times
were compared by using Kaplan-Meier estimates, and the differences
in the patients' overall survival rates were tested for
significance by using the log-rank test. Data were considered to be
statistically significantly different where P<0.05.
Results
General patient features
The patients in Group A (AM and EEC) and Group B
(EEC only) were similar in age at time of EEC diagnosis (mean age,
63.9 vs. 63.2 years, respectively). With regard to the prevalence
of diabetes, hypertension or obesity in the patients' medical
histories, there were no significance differences between the
groups. The clinicopathological characteristics and follow-up data
of all included patients are reported in detail in Table II.
 | Table II.General features, histological
features and follow-up data of the patients in the two study
groups. |
Table II.
General features, histological
features and follow-up data of the patients in the two study
groups.
| Characteristic | All patients
(n=57) | Group
Aa (n=22) | Group
Bb (n=35) | P-value |
|---|
| Age at diagnosis,
years |
|
|
| 0.987 |
|
Mean | 63.5 | 63.9 | 63.2 |
|
|
Range | 36–83 | 52–82 | 36–83 |
|
| Obesity, n (%) |
|
|
| 0.583 |
|
Yes | 18 (31.6) | 6 (27.3) | 12 (34.3) |
|
| No | 39 (68.4) | 16 (72.7) | 23 (65.7) |
|
| Diabetes, n
(%) |
|
|
| 0.946 |
|
Yes | 8 (14.0) | 3 (13.6) | 5 (14.3) |
|
| No | 49 (86.0) | 19 (86.4) | 30 (85.7) |
|
| Hypertension, n
(%) |
|
|
| 0.703 |
|
Yes | 19 (33.3) | 8 (36.4) | 11 (31.4) |
|
| No | 38 (66.7) | 14 (63.6) | 24 (68.6) |
|
| FIGO stage, n
(%) |
|
|
| 0.002 |
| I | 50 (87.7) | 22 (100) | 28 (80.0) |
|
| IA | 37 (64.9) | 19 (86.4) | 18 (51.4) |
|
| IB | 13 (22.8) | 3 (13.6) | 10 (28.6) |
|
| II | 3 (5.3) | 0 (0.0) | 3 (8.6) |
|
|
III | 4 (7.0) | 0 (0.0) | 4 (11.4) |
|
| IV | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| Lymph node
metastasis, n (%) | 2 (3.5) | 1 (4.5) | 1 (2.9) |
|
| Distant metastasis,
n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| Grade, n (%) |
|
|
| 0.001 |
| 1 | 36 (63.2) | 20 (90.9) | 16 (45.7) |
|
| 2 | 19 (33.3) | 1 (4.5) | 18 (51.4) |
|
| 3 | 1 (1.8) | 0 (0.0) | 1 (2.9) |
|
| Lymphatic invasion,
n (%) |
|
|
| 0.849 |
|
Yes | 3 (5.3) | 1 (4.5) | 2 (5.7) |
|
| No | 54 (94.7) | 21 (95.5) | 33 (94.3) |
|
| Vascular invasion,
n (%) |
|
|
| 0.428 |
|
Yes | 1 (1.8) | 0 (0.0) | 1 (2.9) |
|
| No | 56 (98.2) | 22 (100) | 34 (97.1) |
|
| Follow-up duration,
years |
|
|
|
|
|
Mean | – | 8.9 | 7.8 |
|
|
Range | – | 4–14 | 5–14 |
|
| Mortality during
follow-up, n (%) | 10 (17.5) | 2 (9.1) | 8 (22.9) |
|
| No follow-up, n
(%) | 2 (5.7) | 0 (0.0) | 2 (5.7) |
|
AM is associated with lower FIGO stage
in EEC
In the current cohort, the surgical FIGO stage
differed significantly between the two groups. In Group A (AM and
EEC), 22/22 patients (100%) were assigned to FIGO stage I, compared
with 28/35 patients (80%) in Group B (EEC only; P=0.002). Notably,
86.4% (19/22 patients) of Group A and 51.4% (18/35 patients) of
Group B were categorized as stage IA. In Group B, 3/35 patients
(8.6%) were assigned to FIGO stage II, and 4/35 patients (11.4%)
were assigned to FIGO stage III.
AM is associated with a lower grade in
EEC
With regard to the prevalence of lymphatic or
vascular invasion, no significant differences between the two
groups could be identified. Regarding tumour grade, a significant
difference was identified between Group A and Group B (G1, 90.9 vs.
45.7%; G2, 4.5 vs. 51.4%; G3, 0 vs. 2.9%, respectively; P=0.001;
Fig. 1). Thus, higher tumour grades,
indicating poor differentiation of the tumour cells, were more
common in the patients without AM.
AM is associated with increased
survival time in patients with EEC
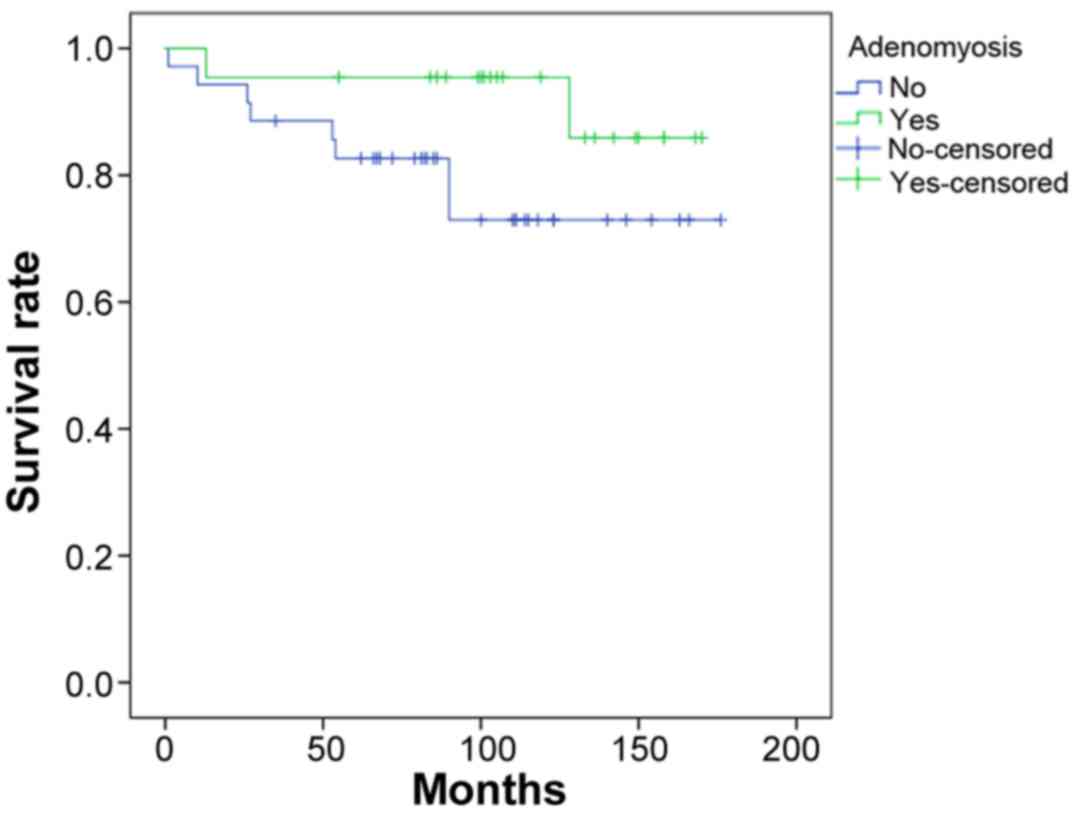
The survival curves of the patients in Groups A and
B are shown in Fig. 2. The 5-year
overall survival rate of Group A was 95%, which was significantly
higher than that of Group B (82%; P=0.024). The survival rate after
10 years remained the same for Group A, whereas the 10-year
survival rate of Group B decreased to 72%. The mean overall
survival time was higher in Group A than in Group B (159 months
(13.25 years) vs. 142 months (11.8 years), respectively). In
summary, patients with AM and EEC (Group A) had a significantly
increased mean overall survival times than patients with EEC alone
(Group B; P=0.024).
AM is associated with increased expression of ERβ
and decreased expression of glycodelin in EEC. Table III summarizes the results of the
different protein expression analyses. When comparing the
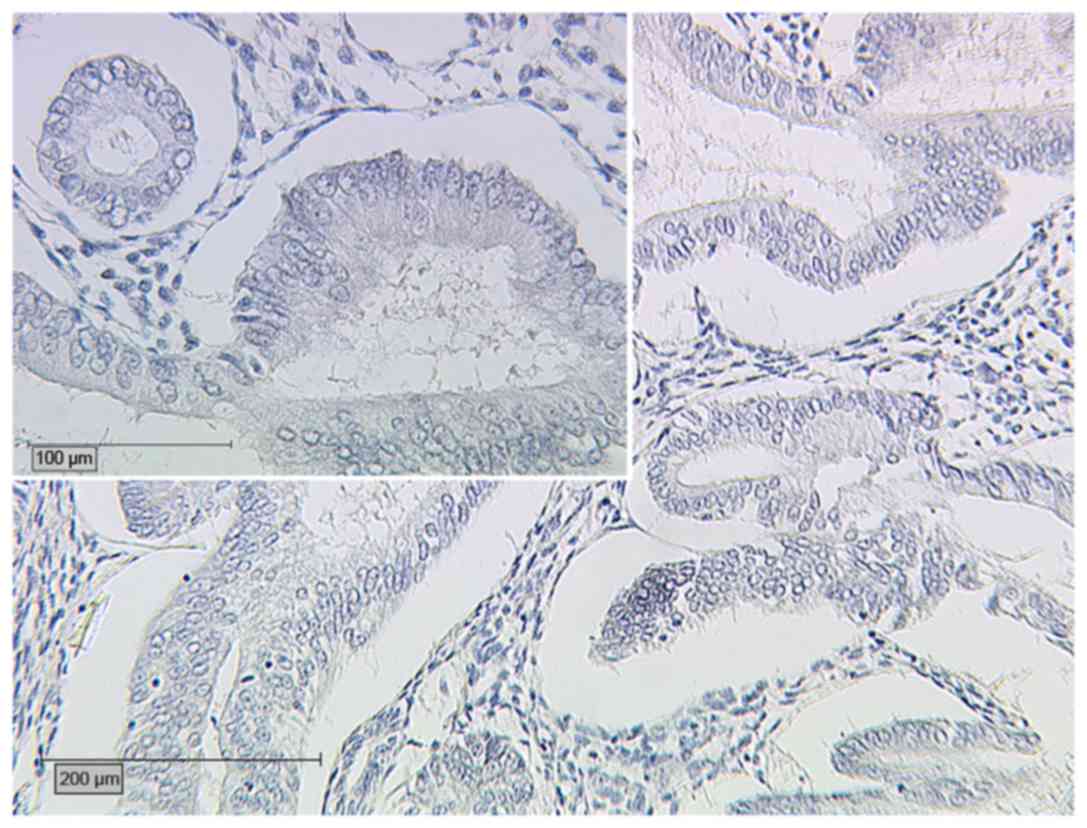
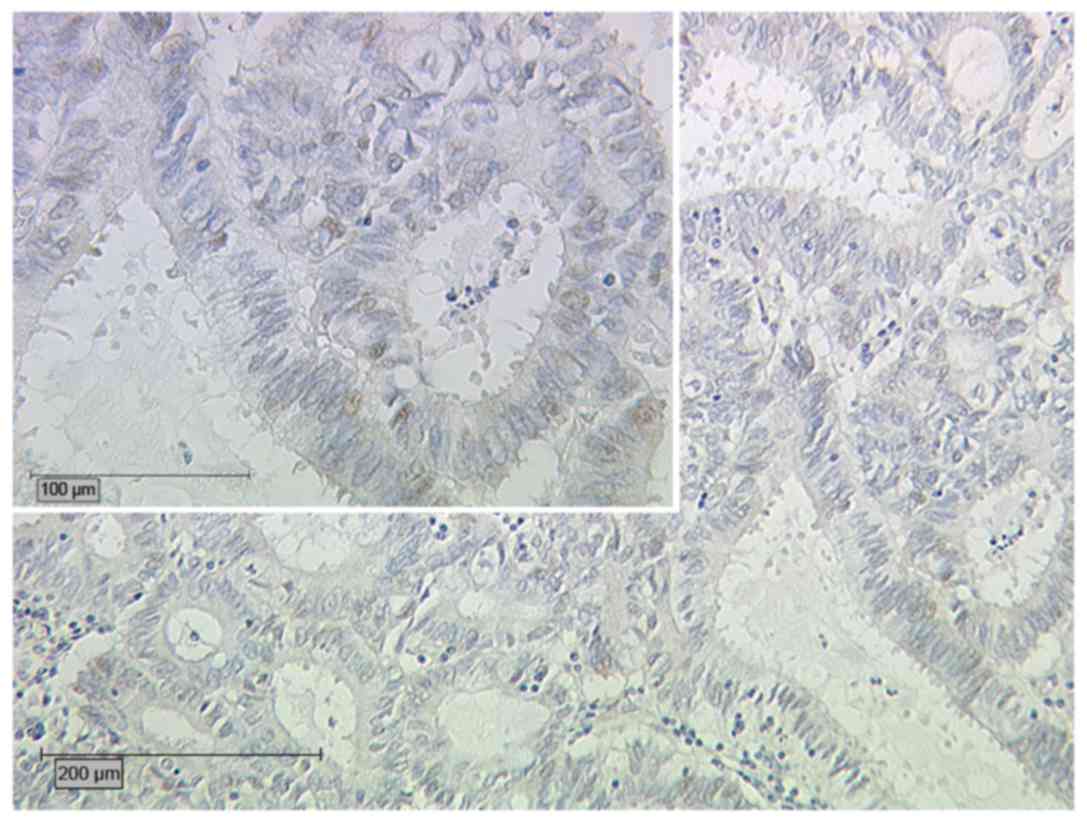
percentage of cases with an IRS >2 for ERβ, a significant
difference was identified between the two groups (P<0.001): In
Group B, ERβ expression was detected in 6/35 cases (17.1%), and
only 1 case (2.9%) was assigned an IRS >2 (Fig. 3); whereas, in Group A, 14/20 cases
assessed for ERβ expression (70%) exhibited expression of ERβ, with
6 cases (30%) assigned an IRS >2 (Fig.
4). Therefore, the presence of AM in cases of EEC is associated
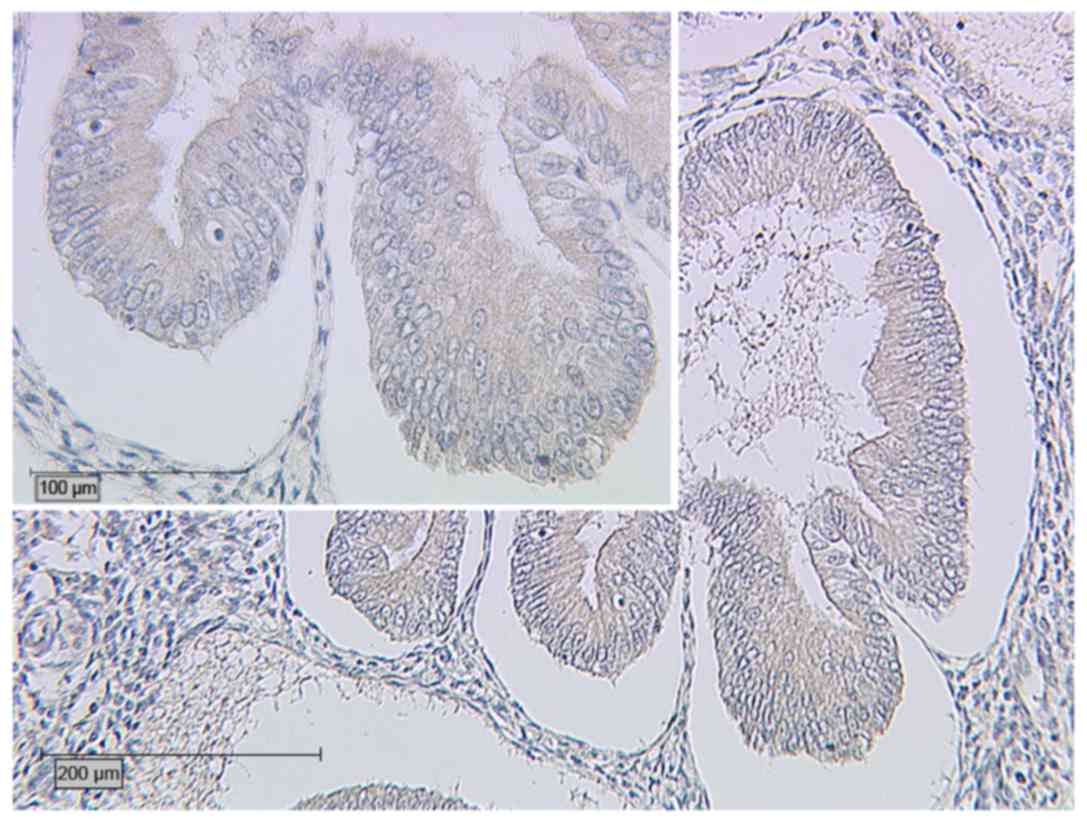
with higher expression of ERβ than that in cases of EEC alone. In
Group A, the prevalence of glycodelin expression with an IRS >2
was significantly lower (P=0.028; Fig.
5) than that in Group B (Fig. 6;
Table III). With regard to the
prevalence of inhibin βB expression (data not shown), no
significant differences between the two groups were identified
(P=0.077).
 | Table III.Expression of the different proteins
in the different groups. |
Table III.
Expression of the different proteins
in the different groups.
| Protein | All patients | Group
Aa | Group
Bb | P-value |
|---|
| Estrogen receptor β
(IRS >2), n (%) |
|
|
| <0.001 |
|
Yes | 7 (12.7) | 6 (30.0) | 1 (2.9) |
|
| No | 48 (87.3) | 14 (70.0) | 34 (97.1) |
|
| Glycodelin (IRS
>2), n (%) |
|
|
| 0.028 |
|
Yes | 38 (86.4) | 15 (78.9) | 23 (92.0) |
|
| No | 6 (13.6) | 4 (21.1) | 2 (8.0) |
|
| Inhibin βB (IRS
>2), n (%) |
|
|
| 0.077 |
|
Yes | 34 (94.4) | 14 (93.3) | 20 (95.2) |
|
| No | 2 (5.5) | 1 (6.7) | 1 (4.8) |
|
Discussion
The majority of cases of EC (80%) are of the
endometrioid subtype (EEC) (2). This
Type I EC is estrogen-dependent, is typically well-differentiated,
and is characterized by a good prognosis in general. By contrast,
Type II EC is estrogen-independent, predominantly comprises the
serous and clear cell histological subtypes, and has a worse
prognosis (23–26).
AM is a benign disease characterized by the presence
of endometrial mucosa within the myometrium. It is a
hormone-dependent disease and typically regresses subsequent to
menopause. In postmenopausal women with EEC, there is a higher
prevalence of AM and myoma uteri than in patients of the same age
with uterine prolapse (27). Certain
studies have reported a more favorable prognosis and a lower
histopathological grade in cases of EEC with coexisting AM
(3,4,7,8).
Regarding the tumour stage, all 22 patients in Group
A (AM and EEC; 100%) vs. only 28/35 patients (80%) in Group B (EEC
only) were assigned to FIGO stage I (P=0.02). Additionally, a
higher percentage of patients in Group A, compared with Group B,
were assigned to FIGO stage IA (86.4 vs. 51.4%). These findings are
in accord with the findings of studies by Musa et al
(28) and Gizzo et al
(7), which reported that concurrent
AM and EEC was associated with a lower tumour grade, with <50%
myometrial invasion, and with the absence of lymphovascular space
involvement and lymph node metastasis. Furthermore, the lower
histopathological grade (G1, 90.9 vs. 45.7%, respectively) in Group
A (with AM) vs. Group B (without AM) observed in the present study
was consistent with the report by Koshiyama et al (8). Thus, the present study confirmed that
patients with coexisting AM and EEC tend to have a lower tumour
stage and a higher differentiation grade. One possible explanation
for this finding could be that there is an adhesion mechanism
between AM foci and cancer cells; these adhesions may prevent
cancer cells from invading deeper in the myometrium (29–31).
Regarding the survival data, patients with EEC and coexistent AM
showed significantly more favorable outcomes than those with EEC
alone.
Although previous studies have investigated ERβ,
glycodelin and inhibin βB in cases of AM and EEC (31–35), to
the best of our knowledge, the present study is the first in which
these factors were measured and compared with one other in a single
study. Regarding the expression of ERβ, Group A (AM and EEC) showed
a significantly increased prevalence compared with Group B (EEC).
This result may be explained by the increased expression of ERβ
that has been shown to be present in AM (15). The decreased expression of ERβ in
patients with EEC alone was also consistent with previous studies
(16,17).
Glycodelin, also known as a progestogen-associated
endometrial protein, is a glycoprotein that has immunosuppressive
capacity, and is predominantly produced in reproductive tissues
(36). In a former study by our
group, intermediate and high expression levels of glycodelin were
associated with a prolonged survival time in patients with EC
(33). Notably, in the current study,
the prevalence of glycodelin expression in Group A (AM and EEC) was
significantly decreased compared with that in Group B (EEC;
P=0.028). This can be explained because women with endometriosis
exhibited a >50-fold downregulation of glycodelin in endometrial
tissue compared to normal controls during the window of
implantation (37). This decreased
glycodelin expression also seemed to persist during the
carcinogenesis of EC in the present study. In this context, women
with AM and EEC appear to have a survival advantage, which could be
explained by the angiogenic role of glycodelin during
tumorigenesis; Song et al (38) found that a synthetic peptide derived
from the sequence of glycodelin may serve an important role in
neovascularization during embryogenesis and tumour development. It
is notable that the inverse association between the presence of AM
and the expression of glycodelin in EEC has only been identified in
specimens that were stained with an antibody raised against a
peptide sequence of glycodelin, and not in those that were stained
with anti-glycodelin A antibody, which is specific to a particular
glycosylated form of glycodelin (39–41). The
measurement of glycodelin or ERβ was previously described in
endometrial tissue (42,43). However, no correlation between these
two parameters has been described.
Inhibins are heterodimers consisting of an α subunit
and a β subunit, and they belong to the transforming growth factor
β cytokine family (44). The α
subunit can dimerize with either βA or βB to form inhibin A (α-βA)
or B (α-βB) (45,46). In a previous study by our group,
hyperplastic endometrial tissue was found to exhibit more intense
staining for inhibins, particularly inhibin βA and βB, compared
with EC. The presence of inhibin βA and βB suggests that they have
an important function in endometrial pathogenesis and in
endometrial carcinogenesis (34). For
inhibin βB, more intense labelling was noted in atypical
hyperplasia compared with EC (34).
Thus, the present study investigated the differences in the
expression of inhibin βB between the two groups. However, no
significant differences were observed. In adenosquamous EC, the
absence of the expression of inhibin βB and ERβ indicates the
malignancy of these tumors (32).
One limitation of the present study was the
variation in immunostaining in each specimen due to varying
expression levels in different regions of the tumour. Other
molecular methods for determining expression levels, such as
western blotting, would be desirable to verify the results.
However, as the present study utilized formalin-fixed,
paraffin-embedded tissue specimens, this was not possible.
In conclusion, in cases of EEC, the presence of AM
is associated with a lower FIGO stage, lower tumour grade and an
increased survival rate. The expression of ERβ was more prevalent
in cases of AM and EEC than in cases of EEC alone, whereas
glycodelin expression was less prevalent when AM was present.
Future research should focus on the influence of estrogen on the AM
and EEC cases, and on prevention strategies in the development of
EEC.
Acknowledgements
The authors would like to thank Ms. Christina Kuhn,
Ms. Sandra Schulze and Ms. Simone Hofmann from the
Ludwig-Maximilian University of Munich for their technical support.
In addition, the authors thank Mr. Laurent Soussana from the
Ludwig-Maximilian University of Munich for language revision.
Glossary
Abbreviations
Abbreviations:
|
AM
|
adenomyosis uteri
|
|
EC
|
endometrial cancer
|
|
EEC
|
endometrioid endometrial cancer
|
|
ERβ
|
estrogen receptor β
|
|
IRS
|
immunoreactivity score
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prat J: Prognostic parameters of
endometrial carcinoma. Hum Pathol. 35:649–662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergeron C, Amant F and Ferenczy A:
Pathology and physiopathology of adenomyosis. Best Pract Res Clin
Obstet Gynaecol. 20:511–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kucera E, Hejda V, Dankovcik R, Valha P,
Dudas M and Feyereisl J: Malignant changes in adenomyosis in
patients with endometrioid adenocarcinoma. Eur J Gynaecol Oncol.
32:182–184. 2011.PubMed/NCBI
|
|
5
|
Ismiil N, Rasty G, Ghorab Z, Nofech-Mozes
S, Bernardini M, Ackerman I, Thomas G, Covens A and Khalifa MA:
Adenomyosis involved by endometrial adenocarcinoma is a significant
risk factor for deep myometrial invasion. Ann Diagn Pathol.
11:252–257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seidman JD and Kjerulff KH: Pathologic
findings from the Maryland Women's Health Study: Practice patterns
in the diagnosis of adenomyosis. Int J Gynecol Pathol. 15:217–221.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gizzo S, Patrelli TS, Dall'Asta A, Gangi
DIS, Giordano G, Migliavacca C, Monica M, Merisio C, Nardelli GB,
Quaranta M, et al: Coexistence of adenomyosis and endometrioid
endometrial cancer: Role in surgical guidance and prognosis
estimation. Oncol Lett. 11:1213–1219. 2016.PubMed/NCBI
|
|
8
|
Koshiyama M, Okamoto T and Ueta M: The
relationship between endometrial carcinoma and coexistent
adenomyosis uteri, endometriosis externa and myoma uteri. Cancer
Detect Prev. 28:94–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mylonas I, Jeschke U, Shabani N, Kuhn C,
Kriegel S, Kupka MS and Friese K: Normal and malignant human
endometrium express immunohistochemically estrogen receptor alpha
(ER-alpha), estrogen receptor beta (ER-beta) and progesterone
receptor (PR). Anticancer Res. 25:1679–1686. 2005.PubMed/NCBI
|
|
10
|
Mylonas I, Jeschke U, Shabani N, Kuhn C,
Balle A, Kriegel S, Kupka MS and Friese K: Immunohistochemical
analysis of estrogen receptor alpha, estrogen receptor beta and
progesterone receptor in normal human endometrium. Acta Histochem.
106:245–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brandenberger AW, Lebovic DI, Tee MK, Ryan
IP, Tseng JF, Jaffe RB and Taylor RN: Oestrogen receptor (ER)-alpha
and ER-beta isoforms in normal endometrial and
endometriosis-derived stromal cells. Mol Hum Reprod. 5:651–655.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hapangama DK, Kamal AM and Bulmer JN:
Estrogen receptor β: The guardian of the endometrium. Hum Reprod
Update. 21:174–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mylonas I, Speer R, Makovitzky J, Richter
DU, Briese V, Jeschke U and Friese K: Immunohistochemical analysis
of steroid receptors and glycodelin A (PP14) in isolated glandular
epithelial cells of normal human endometrium. Histochem Cell Biol.
114:405–411. 2000.PubMed/NCBI
|
|
14
|
Shabani N, Kuhn C, Kunze S, Schulze S,
Mayr D, Dian D, Gingelmaier A, Schindlbeck C, Willgeroth F, Sommer
H, Jeschke U, et al: Prognostic significance of oestrogen receptor
alpha (ERalpha) and beta (ERbeta), progesterone receptor A (PR-A)
and B (PR-B) in endometrial carcinomas. Eur J Cancer. 43:2434–2444.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bulun SE, Monsavais D, Pavone ME, Dyson M,
Xue Q, Attar E, Tokunaga H and Su EJ: Role of estrogen receptor-β
in endometriosis. Semin Reprod Med. 30:39–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paul M, Cholewa K, Mazurek U, Witek A and
Wilczok T: Estrogen receptor beta delta 6 (ER beta delta 6) isoform
in human endometrial hyperplasia and adenocarcinoma. Cancer Invest.
22:211–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smuc T and Rizner TL: Aberrant
pre-receptor regulation of estrogen and progesterone action in
endometrial cancer. Mol Cell Endocrinol. 301:74–82. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skrzypczak M, Bieche I, Szymczak S, Tozlu
S, Lewandowski S, Girault I, Radwanska K, Szczylik C, Jakowicki JA,
Lidereau R and Kaczmarek L: Evaluation of mRNA expression of
estrogen receptor beta and its isoforms in human normal and
neoplastic endometrium. Int J Cancer. 110:783–787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haring J, Schüler S, Lattrich C, Ortmann O
and Treeck O: Role of estrogen receptor β in gynecological cancer.
Gynecol Oncol. 127:673–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horn LC, Schierle K, Schmidt D, Ulrich U,
Liebmann A and Wittekind C: Current TNM/FIGO classification for
cervical and endometrial cancer as well as malignant mixed
mullerian tumors. Facts and background. Pathologe. 32:239–243.
2011.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kricka LJ and Wild D: Signal generation
and detection systems (excluding homogeneous assays)The Immunoassay
Handbook. 3rd. Amsterdam: Elsevier Publisher; pp. 2202005
|
|
22
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
23
|
Tabernero JM, Alonso MC, Ojeda B, Fuentes
J, Balart J, Badia J, Climent MA and Delgado E: Endometrial cancer
stages I and II. Analysis of survival and prognostic factors. Eur J
Gynaecol Oncol. 16:18–25. 1995.PubMed/NCBI
|
|
24
|
Reisinger SA, Staros EB and Mohiuddin M:
Survival and failure analysis in stage II endometrial cancer using
the revised 1988 FIGO staging system. Int J Radiat Oncol Biol Phys.
21:1027–1032. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ayhan A, Taskiran C, Celik C and Yuce K:
The long-term survival of women with surgical stage II endometrioid
type endometrial cancer. Gynecol Oncol. 93:9–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahdi H, Hou H, Kowk LL, Moslemi-Kebria M
and Michener C: Type II endometrial cancer in Hispanic women: Tumor
characteristics, treatment and survival compared to non-Hispanic
white women. Gynecol Oncol. 133:512–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koshiyama M, Morita Y, Fujii H, Kobashi Y
and Yoshida M: Gynecologic malignancies accompanied by benign
hormone-dependent diseases. Menopause. 8:149–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Musa F, Frey MK, Im HB, Chekmareva M,
Ellenson LH and Holcomb K: Does the presence of adenomyosis and
lymphovascular space invasion affect lymph node status in patients
with endometrioid adenocarcinoma of the endometrium? Am J Obstet
Gynecol. 207(417): e1–e6. 2012.
|
|
29
|
Matsuo K, Cahoon SS, Gualtieri M, Scannell
CA, Jung CE, Takano T, Paulson RJ, Muderspach LI and Roman LD:
Significance of adenomyosis on tumor progression and survival
outcome of endometrial cancer. Ann Surg Oncol. 21:4246–4255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuo K, Moeini A, Machida H, Scannell
CA, Casabar JK, Kakuda M, Adachi S, Garcia-Sayre J, Ueda Y and
Roman LD: Tumor characteristics and survival outcome of endometrial
cancer arising in adenomyosis: An exploratory analysis. Ann Surg
Oncol. 23:959–967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mehasseb MK, Panchal R, Taylor AH, Brown
L, Bell SC and Habiba M: Estrogen and progesterone receptor isoform
distribution through the menstrual cycle in uteri with and without
adenomyosis. Fertil Steril. 95:2228–2235, 2235.e1. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gingelmaier A, Gutsche S, Mylonas I,
Shabani N, Kuhn C, Kunze S, Jeschke U and Friese K: Expression of
HPV, steroid receptors (ERalpha, ERbeta, PR-A and PR-B) and
inhibin/activin subunits (alpha, betaA and betaB) in adenosquamous
endometrial carcinoma. Anticancer Res. 27:2011–2017.
2007.PubMed/NCBI
|
|
33
|
Lenhard M, Heublein S, Kunert-Keil C,
Vrekoussis T, Lomba I, Ditsch N, Mayr D, Friese K and Jeschke U:
Immunosuppressive Glycodelin A is an independent marker for poor
prognosis in endometrial cancer. BMC Cancer. 13:6162013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mylonas I, Makovitzky J, Hoeing A, Richter
DU, Vogl J, Schulze S, Jeschke U, Briese V and Friese K:
Inhibin/activin subunits beta-A (−betaA) and beta-B (−betaB) are
differentially localised in normal, hyperplastic and malignant
human endometrial tissue. Acta Histochem. 108:1–11. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Panoulis K, Christantoni E, Pliatsika P,
Anagnostis P, Goulis DG, Kondi-Pafiti A, Armeni E, Augoulea A,
Triantafyllou N, Creatsa M and Lambrinoudaki I: Expression of
gonadal steroid receptors in the ovaries of post-menopausal women
with malignant or benign endometrial pathology: A pilot study.
Gynecol Endocrinol. 31:613–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mazurkiewicz JE, Bank JF and Joshi SG:
Immunocytochemical localization of a progestagen-associated
endometrial protein in the human decidua. J Clin Endocrinol Metab.
52:1006–1068. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kao LC, Germeyer A, Tulac S, Lobo S, Yang
JP, Taylor RN, Osteen K, Lessey BA and Giudice LC: Expression
profiling of endometrium from women with endometriosis reveals
candidate genes for disease-based implantation failure and
infertility. Endocrinology. 144:2870–2881. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song M, Ramaswamy S, Ramachandran S,
Flowers LC, Horowitz IR, Rock JA and Parthasarathy S: Angiogenic
role for glycodelin in tumorigenesis. Proc Natl Acad Sci U S A.
98:9265–9270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jeschke U, Kuhn C, Mylonas I, Schulze S,
Friese K, Mayr D, Speer R, Briese V, Richter DU, Haase M and
Karsten U: Development and characterization of monoclonal
antibodies for the immunohistochemical detection of glycodelin A in
decidual, endometrial and gynaecological tumour tissues.
Histopathology. 48:394–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jeschke U, Bischof A, Speer R, Briese V,
Richter DU, Bergemann C, Mylonas I, Shabani N, Friese K and Karsten
U: Development of monoclonal and polyclonal antibodies and an ELISA
for the determination of glycodelin in human serum, amniotic fluid
and cystic fluid of benign and malignant ovarian tumors. Anticancer
Res. 25:1581–1589. 2005.PubMed/NCBI
|
|
41
|
Jeschke U, Mylonas I, Kunert-Keil C, Stahn
R, Scholz C, Janni W, Kuhn C, Schröder E, Mayr D and Friese K:
Immunohistochemistry, glycosylation and immunosuppression of
glycodelin in human ovarian cancer. Histochem Cell Biol.
131:283–295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fazleabas AT, Brudney A, Chai D, Langoi D
and Bulun SE: Steroid receptor and aromatase expression in baboon
endometriotic lesions. Fertil Steril. 80:(Suppl 2). 820–827. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fazleabas AT, Kim JJ, Srinivasan S,
Donnelly KM, Brudney A and Jaffe RC: Implantation in the baboon:
Endometrial responses. Semin Reprod Endocrinol. 17:257–265. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vale W, Rivier C, Hsueh A, Campen C,
Meunier H, Bicsak T, Vaughan J, Corrigan A, Bardin W, Sawchenko P,
et al: Chemical and biological characterization of the inhibin
family of protein hormones. Recent Prog Horm Res. 44:1–34.
1988.PubMed/NCBI
|
|
45
|
Mylonas I, Jeschke U, Winkler L,
Makovitzky J, Richter DU, Briese V and Friese K:
Immunohistochemical expression of inhibin-alpha in human
endometrium and the in vitro secretion of inhibin, estradiol and
cortisol in cultured human endometrial glandular cells. Arch
Gynecol Obstet. 268:142–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mylonas I, Winkler L, Jeschke U, Briese V
and Friese K: Investigations on isolation, purification and
cultivation of human endometrial cells and on the in vitro inhibin
expression in glandular epithelial cells. Zentralbl Gynakol.
125:415–423. 2003.(In German). PubMed/NCBI
|