Introduction
In recent years, histology-based chemotherapy
selection for advanced non-small cell lung cancer (NSCLC) has been
advocated. Specifically, the chemotherapeutic agent pemetrexed
(PEM) is predominantly restricted to treating patients with
non-squamous cell carcinoma (non-SCC) based on phase III trials
(1). Thus, there are less treatment
options available for SCC compared with for adenocarcinoma (AC).
The molecular basis underlying histology-specific chemotherapy
selection, and the predictive value of chemotherapy
sensitivity/resistance biomarkers in SCC remain unclear.
The clinical use of S-1, a chemotherapeutic agent
composed of tegafur, gimeracil, and oteracil potassium, for NSCLC
has been investigated in clinical trials (2). In the multicenter randomized phase III
Lung Cancer Evaluation of TS-1 (LETS) study, Okamoto et al
(3) reported that S-1/carboplatin
(CBDCA) was not inferior to CBDCA/paclitaxel as a first-line
treatment in terms of overall survival (OS) time in patients with
advanced NSCLC (3). In the updated
survival time data based on NSCLC histology, SCC patients in the
S-1/CBDCA group had a longer median OS time than those in the
CBDCA/paclitaxel group (4). According
to this analysis, S-1-based chemotherapy is now considered as the
major therapeutic option for lung SCC therapy among the limited
available options for chemotherapy regimens.
Several enzymes participate in the metabolic
pathways of 5-fluorouracil (5-FU) and folate, including thymidylate
synthase (TS), a target enzyme of 5-FU; dihydropyrimidine
dehydrogenase (DPD), which catalyzes 5-FU degradation; and orotate
phosphoribosyltransferase (OPRT), which activates 5-FU and produces
5-fluoroudine monophosphate. TS, DPD and OPRT expression levels
have been shown to be associated with 5-FU sensitivity in solid
tumors (5). A previous study
(6) has demonstrated that low TS and
DPD expression levels are predictive biomarkers for an improved
response to S-1/CBDCA in NSCLC patients, including an increased
survival time. TS and OPRT expression were significantly reduced in
tissue samples from NSCLC patients with AC compared with those
without, whereas DPD expression was higher in AC samples (7). A low TS expression level in lung SCC
tissue is associated with better response to 5-FU-based
chemotherapy (8). In addition, the
response to S-1-based chemotherapy was higher in head and neck SCC
patients with low TS activity than in those with high TS activity
(9,10). Thus, the evaluation of TS, OPRT and
DPD expression levels in histological subtypes may aid in
predicting the clinical response to chemotherapy, including S-1, in
SCC patients who have restricted chemotherapeutic options. However,
the clinical relevance of TS, OPRT and DPD has not been established
for lung SCC patients treated with S-1 alone or S-1 combination
chemotherapy. The aim of the present study was to evaluate the
predictive value of immunohistochemically detected TS, DPD and OPRT
expression for the response to S-1/CBDCA chemotherapy in patients
with lung SCC.
Materials and methods
Patients
The inclusion criteria for the present retrospective
study were as follows: i) Pathologically confirmed SCC; ii)
diagnosed with unresectable stage IIIA, IIIB or IV, or
postoperative recurrence without preoperative chemotherapy, or
radiation; and iii) an Eastern Cooperative Oncology Group
Performance status between 0 and 2. A total of 37 patients with
relapsed or advanced SCC who received CBDCA (Nippon Kayaku Co.,
Ltd., Tokyo, Japan) treatment at an area under the curve of 5 on
day 1, and S-1 (Taiho Pharmaceutical Co., Ltd., Tokyo, Japan) at 80
mg/m2 on days 1–14 at Juntendo University Hospitals
(Tokyo, Japan) between April 2011 and July 2014, were
retrospectively analyzed. Tumor response was examined using
computed tomography and evaluated according to the Response
Evaluation Criteria in Solid Tumors (version 1.1) (11). Comprehensive consent was obtained from
the patients, and the study protocol was approved by the Ethics
Committee of Juntendo University School of Medicine (no.
2013068).
Tissue samples
A total of 28 biopsy specimens and 9 resection
specimens (relapsed SCC, 6 specimens; incompletely resected SCC, 3
specimens) were fixed in 10% formalin for 48 h and embedded in
paraffin for evaluation by pathologists. Among the biopsy
specimens, 5 small specimens did not have sufficient tissue
available in paraffin blocks for immunohistochemical assessment.
The remaining 32 samples were investigated by immunohistochemical
analysis in the present study.
Immunohistochemistry and scoring of
protein expression
Tissue sections (thickness, 4 µm) were
deparaffinized in xylene and then rehydrated. Antigen retrieval was
conducted by microwaving at 750 W for 10 min in 10 mM citric acid
buffer (Ph 6.0) for TS and OPRT, and by boiling at 97°C for 40 min
in 1 mM EDTA/10 mM Tris buffer (pH 9.0) for DPD. Endogenous
peroxidase activity was deactivated by a 5-min incubation in 0.3%
H2O2/methanol. Following washing in
phosphate-buffered saline, the sections were incubated at room
temperature with primary polyclonal antibodies against TS
(dilution, 1:100; provided by Taiho Pharmaceutical Co., Ltd.) and
OPRT (dilution, 1:100; cat. no. 28135; Immuno-Biological
Laboratories Co., Ltd., Minneapolis, MN, USA) for 1 h, and against
DPD (dilution, 1:50; cat. no. 10411; Immuno-Biological Laboratories
Co., Ltd.) overnight. Ready-to-use peroxidase-based EnVision™
+ (cat. no. K5027; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) was applied as a secondary antibody for 30
min at room temperature. Peroxidase activity was visualized with
diaminobenzidine tetrahydrochloride solution (Dako; Agilent
Technologies, Inc.). Sections were counterstained with Mayer's
hematoxylin.
All immunostained sections were evaluated separately
by three observers (Y.H., S.T., and K.S.) without knowledge of the
patients' clinical data. Sections with discrepant results were
re-evaluated by the pathologists until a consensus was reached. TS,
OPRT and DPD cytoplasmic staining were scored in a semiquantitative
manner reflecting the staining intensity and percentage of area
with stained cells at each intensity, as previously described
(12). Staining intensity (I) was
classified as 0 (no staining), +1 (weak staining), +2 (intermediate
staining) or +3 (strong staining). The percentage of positively
stained cells (PC) was graded as 0 (0%), 0.1 (1–9%), 0.5 (10–49%)
or 1.0 (≥50%). H-scores were obtained by calculating the mean I ×
PC value as follows: Mean value of I × PC = Σ(I × PC) among all
fields/total number of fields evaluated.
Statistical analysis
TS, OPRT and DPD expression were compared between
groups using the Spearman rank-correlation coefficient. The
selection of clinically important cut-off scores for TS, OPRT and
DPD expression was based on median values. OS and progression-free
survival (PFS) times were assessed from the first day of
chemotherapy administration to the date of mortality due to any
cause, and the date of objective disease progression, respectively.
Patients without documented mortality at the time of the final
analysis were evaluated on the last date they were known to be
alive or the date of their last objective tumor assessment. The
Kaplan-Meier method was used to estimate the probability of
survival as a function of time, and differences in the survival of
patient subgroups were evaluated using the log-rank test. The
multivariate logistic regression models and Cox proportional
hazards regression models were used to assess the predictive value
of TS, OPRT and DPD for PFS time in lung SCC patients treated with
S-1/CBDCA as a first-line chemotherapy. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using JMP software (version 11.0.0; SAS
Institute Inc., Cary, NC, USA).
Results
Patient characteristics and S-1/CBDCA
response
The characteristics of the patients are summarized
in Table I. A total of 32 patients
were administered S-1/CBDCA as first-line chemotherapy. This
included 27 male and 5 female patients. The patients ranged in age
from 52 to 82 years (median, 69.5 years). There were 31 patients
who smoked, and 1 patient who had never smoked. Histological
differentiation was classed as differentiated in 21 patients and
undifferentiated in 11 patients. The clinical stage was diagnosed
as stage IIIA in 6 patients, stage IIIB in 6 patients, and stage IV
in 14 patients. Relapse occurred in 6 patients who had undergone
surgery without preoperative chemotherapy or radiation. The median
number of chemotherapy cycles was 4 (range, 1–6 cycles). Of the 32
patients, 2 received 6 cycles, 1 received 5 cycles, 17 received 4
cycles, 6 received 3 cycles, 3 received 2 cycles, and 3 received 1
cycle. Complete response (CR) was observed in 1 patient (3%),
partial response (PR) in 13 patients (41%), stable disease (SD) in
15 patients (47%) and progressive disease (PD) in 3 patients
(9%).
 | Table I.Characteristics of 32 patients with
lung squamous cell carcinoma. |
Table I.
Characteristics of 32 patients with
lung squamous cell carcinoma.
| Characteristics | Value |
|---|
| Age, years |
|
|
Median | 69.5 |
|
Range | 52–82 |
| Gender, n (%) |
|
|
Male | 27 (84) |
|
Female | 5 (16) |
| Performance status,
n (%) |
|
| 0 | 17 (53) |
| 1 | 11 (34) |
| 2 | 4 (13) |
| Stage, n (%) |
|
|
IIIA | 6 (19) |
|
IIIB | 6 (19) |
| IV | 14 (43) |
|
Relapsed | 6 (19) |
| Smoking status, n
(%) |
|
|
Non-smoker | 1 (3) |
|
Smoker | 31 (97) |
| Specimens, n
(%) |
|
|
Biopsy | 23 (72) |
|
Resection | 9 (28) |
| Histological
differentiation, n (%) |
|
|
Differentiated | 21 (66) |
|
Undifferentiated | 11 (34) |
| Treatment response,
n (%) |
|
|
Complete response | 1 (3) |
| Partial
response | 16 (50) |
| Stable
disease | 12 (38) |
|
Progressive disease | 3 (9) |
Immunohistochemical expression of TS,
OPRT and DPD in tumor tissues
Intratumoral TS, OPRT and DPD expression levels
(H-scores) ranged from 1.0 to 3.6 (median, 2.0), 0.4 to 2.5
(median, 1.0), and 0.5 to 2.7 (median, 1.1), respectively.
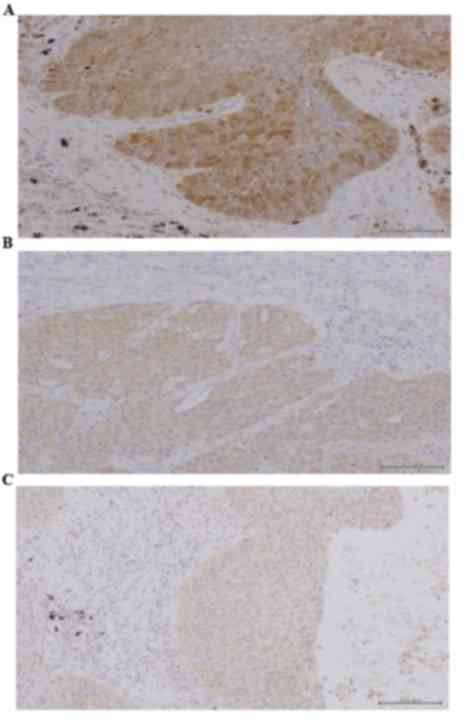
Representative tumor sections with high TS, OPRT and DPD expression
levels are shown in Fig. 1. TS
H-score was correlated with DPD H-score (R=0.509; P=0.023) and OPRT
H-score (R=0.343; P=0.042). Intratumoral TS, OPRT and DPD H-scores
did not significantly differ between tumor biopsy and resection
specimens (P=0.205, P=0.642 and P=0.267, respectively). Tumor
biopsy and resection specimen H-scores, respectively, were 2.12
[95% confidence interval (CI), 1.80–2.46] and 1.74 (95% CI,
1.31–2.18) for TS, 1.15 (95% CI, 0.93–1.37) and 1.11 (95% CI,
0.94–1.28) for OPRT, and 1.19 (95% CI, 1.01–1.37) and 0.95 (95% CI,
0.71–1.19) for DPD.
Association of TS, OPRT and DPD
expression levels with patient characteristics
TS, OPRT and DPD H-scores were not significantly
associated with patient demographics, including age, gender,
performance status, stage or differentiation (Table II).
 | Table II.Associations between H-scores of TS,
OPRT and DPD and various characteristics in 32 patients with lung
squamous cell carcinoma. |
Table II.
Associations between H-scores of TS,
OPRT and DPD and various characteristics in 32 patients with lung
squamous cell carcinoma.
|
|
| TS | OPRT | DPD |
|---|
|
|
|
|
|
|
|---|
| Variable | n | H-score, mean ±
SD | P-value | H-score, mean ±
SD | P-value | H-score, mean ±
SD | P-value |
|---|
| Age, years |
|
| 0.234 |
| 0.246 |
| 0.595 |
|
<75 | 22 | 2.14±0.78 |
| 1.13±0.35 |
| 1.18±0.45 |
|
|
≥75 | 10 | 1.76±0.55 |
| 1.17±0.63 |
| 1.03±0.21 |
|
| Gender |
|
| 0.513 |
| 0.476 |
| 0.524 |
|
Male | 27 | 1.99±0.76 |
| 1.16±0.48 |
| 1.10±0.37 |
|
|
Female | 5 | 2.16±0.61 |
| 1.04±0.09 |
| 1.30±0.47 |
|
| Performance
status |
|
| 0.17 |
| 0.623 |
| 0.268 |
|
0/1 | 28 | 1.94±0.67 |
| 1.15±0.48 |
| 1.05±0.19 |
|
| 2 | 4 | 2.58±1.02 |
| 1.05±0.10 |
| 1.68±0.83 |
|
| Stage |
|
| 0.295 |
| 0.624 |
| 0.704 |
|
IIIA/IIIB | 12 | 2.28±0.84 |
| 1.23±0.63 |
| 1.25±0.52 |
|
| IV | 14 | 1.93±0.69 |
| 1.10±0.34 |
| 1.09±0.31 |
|
|
Relapsed | 6 | 1.70±0.46 |
| 1.02±0.18 |
| 1.02±0.27 |
|
| Pathology |
|
| 0.412 |
| 0.6 |
| 0.204 |
|
Differentiated | 21 | 1.99±0.84 |
| 1.14±0.38 |
| 1.15±0.39 |
|
|
Undifferentiated | 11 | 2.07±0.50 |
| 1.14±0.57 |
| 1.08±0.39 |
|
Predictive relevance of TS, OPRT and DPD expression
levels for response to S-1/CBDCA. To evaluate the relationship
between TS, OPRT and DPD H-scores and S-1/CBDCA response, tumors
were categorized as either responding (CR or PR) or non-responding
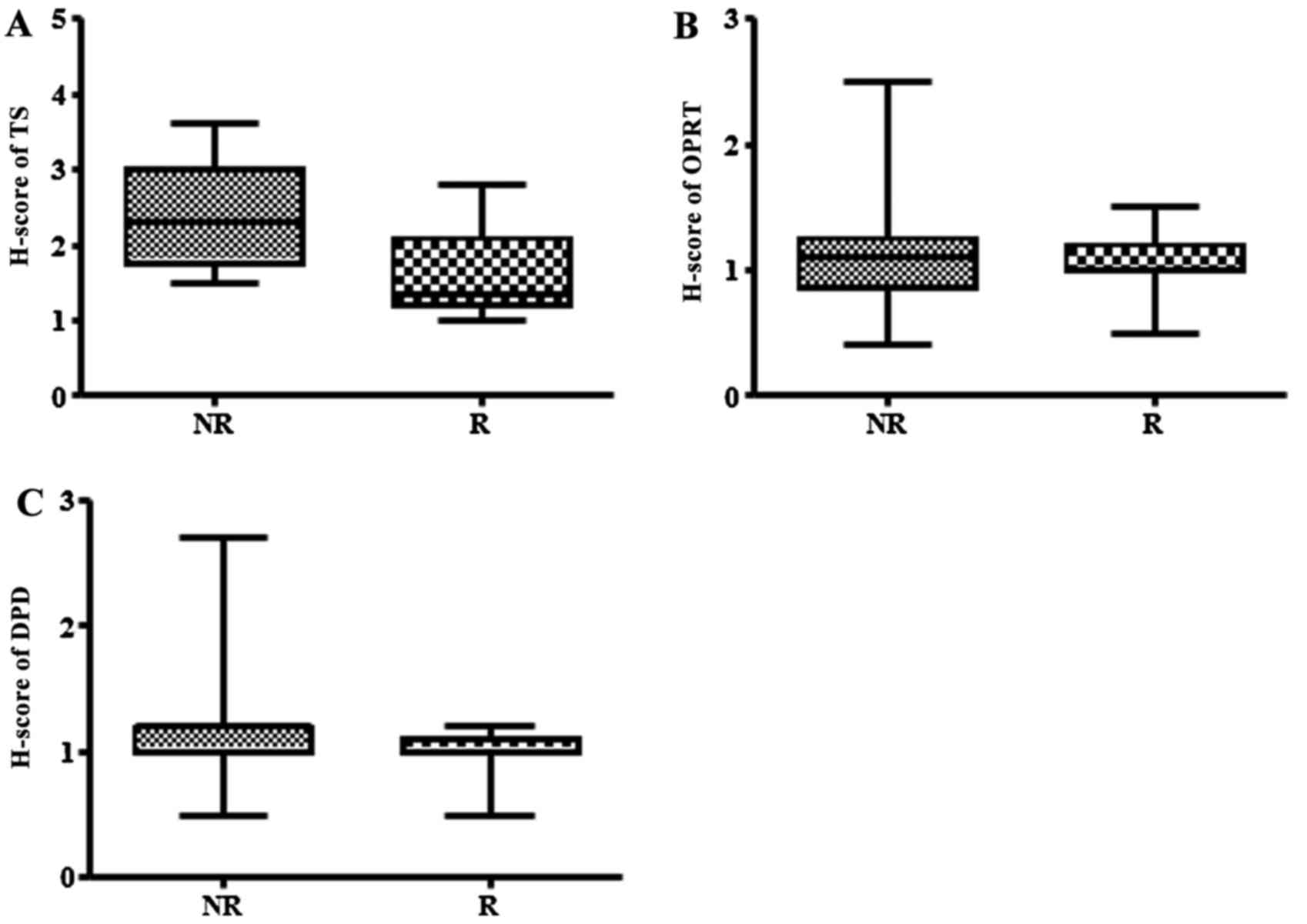
(SD or PD) (Fig. 2). TS H-scores were
significantly lower in responding tumors than in non-responding
tumors (P=0.002). High-TS tumors were defined as tumors with a TS
H-score >2.0. The proportion of low-TS tumors responding to
therapy was 71.4% (n=10/14) and the proportion of high-TS tumors
which did not respond was 83.3% (n=15/18). OPRT H-scores was not
significantly associated with tumor response to S-1/CBDCA therapy
(P=0.849). Furthermore, DPD H-scores demonstrated an association
with tumor response to S-1/CBDCA therapy; however this was
insignificant (P=0.086).
Association of TS, OPRT and DPD
expression and patient characteristics with PFS and OS
The median PFS and OS times were 137 (range, 25–455)
days and 348 (range 41–929) days, respectively. A cut-off value was
selected for each clinicopathological factor according to the
median value. Univariate Cox analysis identified factors that
significantly affected PFS (Table
III) and OS (Table IV) times. TS
expression was a prognostic factor for PFS (P=0.008) but not OS
(P=0.185) time. DPD and OPRT expression were not significant
prognostic factors for PFS time (P=0.772 and P=0.828, respectively)
or OS time (P=0.313 and P=0.650, respectively). Performance status
was significantly correlated with PFS (P=0.033) and OS (P=0.0001)
time.
 | Table III.Logistic analysis for response in
terms of progression-free survival in 32 patients with lung
squamous cell carcinoma. |
Table III.
Logistic analysis for response in
terms of progression-free survival in 32 patients with lung
squamous cell carcinoma.
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Variable | n | HR (95% CI) |
P-valuea | HR (95% CI) |
P-valueb |
|---|
| Age, years |
|
|
|
|
|
|
<75 | 22 | 1 | – | – | – |
|
≥75 | 10 | 1.17
(0.52–2.50) | 0.687 | – | – |
| Gender |
|
|
|
|
|
|
Male | 27 | 1 | – | – | – |
|
Female | 5 | 1.94
(0.62–5.14) | 0.230 | – | – |
| Performance
status |
|
|
|
|
|
|
0/1 | 28 | 1 | – | 1 | – |
| 2 | 4 | 4.16
(1.14–12.34) | 0.0327 | 2.96
(0.80–9.04) | 0.0975 |
| Stage |
|
|
|
|
|
|
IIIA/IIIB | 12 | 1 | – | – | – |
| IV | 14 | 0.73
(0.32–1.65) | 0.443 | – | – |
|
Relapsed | 6 | 0.77
(0.27–2.01) | 0.609 | – | – |
| Pathology |
|
|
|
|
|
|
Differentiated | 21 | 1 | – | – | – |
|
Undifferentiated | 11 | 1.61
(0.71–3.50) | 0.244 | – | – |
| TS H-score |
|
|
|
|
|
|
>2.0 | 14 | 1 | – | 1 | – |
|
≤2.0 | 18 | 0.35
(0.17–0.75) | 0.0076 | 0.40
(0.18–0.87) | 0.0213 |
| OPRT H-score |
|
|
|
|
|
|
>1.0 | 14 | 1 | – | – | – |
|
≤1.0 | 18 | 0.83
(0.40–1.78) | 0.828 | – | – |
| DPD H-score |
|
|
|
|
|
|
>1.0 | 13 | 1 | – | – | – |
|
≤1.0 | 17 | 1.12
(0.53–2.38) | 0.772 | – | – |
 | Table IV.Univariate logistic analysis for
response in terms of overall survival in 32 patients with lung
squamous cell carcinoma. |
Table IV.
Univariate logistic analysis for
response in terms of overall survival in 32 patients with lung
squamous cell carcinoma.
| Variable | n | HR (95% CI) |
P-valuea |
|---|
| Age, years |
|
|
|
|
<75 | 22 | 1 | – |
|
≥75 | 10 | 0.81
(0.34–1.79) | 0.610 |
| Gender |
|
|
|
|
Male | 27 | 1 | – |
|
Female | 5 | 0.95
(0.27–2.53) | 0.920 |
| Performance
status |
|
|
|
|
0/1 | 28 | 1 | – |
| 2 | 4 | 34.15
(6.30–255.06) | 0.0001 |
| Stage |
|
|
|
|
IIIA/IIIB | 12 | 1 | – |
| IV | 14 | 0.50
(0.21–1.18) | 0.500 |
|
Relapsed | 6 | 0.39
(0.12–1.12) | 0.082 |
| Pathology |
|
|
|
|
Differentiated | 21 | 1 | – |
|
Undifferentiated | 11 | 1.04
(0.44–2.27) | 0.919 |
| TS H-score |
|
|
|
|
>2.0 | 14 | 1 | – |
|
≤2.0 | 18 | 0.60
(0.28–1.29) | 0.185 |
| OPRT H-score |
|
|
|
|
>1.0 | 14 | 1 | – |
|
≤1.0 | 18 | 1.20
(0.55–2.76) | 0.650 |
| DPD H-score |
|
|
|
|
>1.1 | 13 | 1 | – |
|
≤1.1 | 17 | 0.66
(0.29–1.49) | 0.313 |
Multivariate analysis by Cox proportional hazards
model was performed to evaluate the influence of TS expression and
performance status on PFS time after adjusting for possible
confounding factors. TS expression was the only significant factor
associated with PFS time [hazard ratio (HR), 0.40; 95% CI,
0.18–0.87; P=0.021].
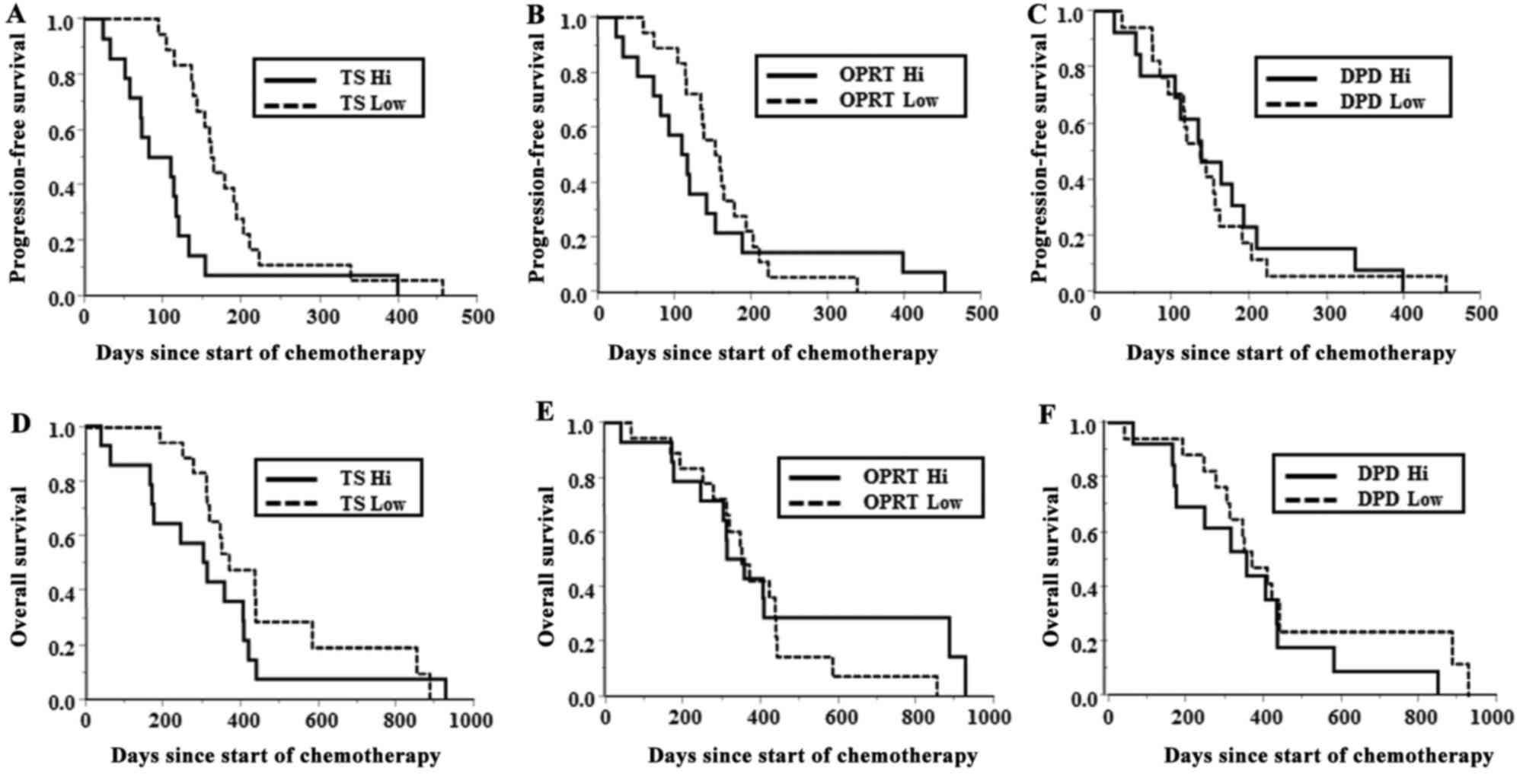
Kaplan-Meier survival curves of PFS and OS time for
patients in the high and low TS, DPD and OPRT expression groups are
presented in Fig. 3. Patients in the
low TS group had a significantly longer median PFS time compared
with that of patients in the high TS group (162.5 vs. 97 days;
P=0.004). OS time was not significantly different between TS
expression groups (370 vs. 309.5 days; P=0.177). DPD and OPRT
H-scores were not significantly associated with median PFS time
(P=0.772 and P=0.828, respectively) or OS time (P=0.313 and
P=0.650, respectively). Notably, 1 patient with a CR from S-1/CBDCA
treatment presented with the lowest TS H-score of 1.0. By contrast,
two patients with PD during treatment presented with the highest TS
H-scores of 3.6.
Discussion
The present study investigated the association
between immunohistochemical TS, OPRT and DPD expression levels and
clinical outcomes for SCC patients treated with combination
S-1/CBDCA therapy. Patients with a low TS expression level had a
significantly longer median PFS time, although not a significantly
longer OS time, when compared with patients with high TS
expression.
The majority of advanced NSCLC cases are diagnosed
by examination of small biopsy specimens, as it is difficult to
obtain resection specimens except from relapsed patients who
previously underwent surgical resection. Therefore, small tumor
biopsy specimens are often used to identify potential biomarkers.
In the use of such samples, the important issues are tumor cell
content, representativeness of the sample, and mode of biomarker
assessment. In the present study, H-scores of biopsy specimens and
resection specimens were observed to be equally evaluable. A
previous study also demonstrated that TS expression scores in
biopsy specimens largely reflect TS expression in the corresponding
resection specimens; the authors recommended a cut-off value of 10%
moderately to strongly stained tumor cells in order to obtain the
highest agreement between biopsy and resection specimens in NSCLC,
particularly in SCC (13). By
contrast, cut-off criterions for TS positivity in NSCLC range from
29.6–72.5% in previously published studies (14,15). The
variability in TS expression cut-offs may be attributed to the
heterogeneous histology of invasive AC subtypes and the lack of a
standardized immunohistochemistry scoring system. Therefore, the
present study quantified H-score by calculating the intensity and
percentage of stained tumor cells, as described in a previous
report (12). Several groups have
used immunohistochemistry and reverse transcription-polymerase
chain reaction (RT-PCR) to detect TS expression (15,16).
Shimizu et al (16) reported a
significant correlation between the two detection methods. TS gene
copy number by silver in situ hybridization was found to be
significantly correlated with the immunohistochemical expression of
TS (17). However, the contamination
of whole tumor tissue with non-neoplastic cells cannot be avoided
in mRNA extraction from tissue, requiring time-consuming
microdissection to ensure that only neoplastic tissue is obtained.
Immunohistochemistry is preferred over RT-PCR, as well-visualized
immunohistochemical staining can allow neoplastic tissues to be
distinguished from non-neoplastic tissues.
Several studies have analyzed TS expression in
patients with non-squamous cell lung cancer treated with PEM-based
chemotherapy. Low gene copy number and low protein levels of TS in
biopsy specimens have been associated with an improved response to
PEM-based chemotherapy in lung AC (18–20). In
the present study, the superior response to S-1-based chemotherapy
in SCCs with low TS expression is consistent with TS being a target
enzyme and a predictive biomarker of chemotherapy response for PEM.
Takeda et al (6) reported that
low TS and DPD immunohistochemical expression levels, according to
cut-off criteria for high or low expression, were associated with
improved response and longer survival time in 22 patients with
advanced NSCLC treated with S-1-based chemotherapy. However, only 1
of the 22 patients with NSCLC treated with S-1/CBDCA in this study
had histological SCC. In the present study, the immunohistochemical
expression levels of TS, OPRT and DPD were quantified in 32 SCC
patients, and low TS expression levels were demonstrated to be
strongly associated with longer PFS time in patients with lung SCC
treated with S-1/CBDCA.
Several studies have suggested that DPD and OPRT are
also predictors of S-1 response in NSCLC (6,21–23). DPD activity levels have been shown to
be higher in NSCLC tissues than in gastric, colorectal and breast
cancer tissues (21). S-1 contains
gimeracil, which acts as a DPD inhibitor; gimeracil is a stronger
DPD inhibitor than uracil when it is used in combination with
tegafur. Therefore, NSCLC exhibits a different response to S-1 when
compared with other solid tumors (21–23), and a
high DPD expression level predicts resistance to S-1-based
chemotherapy (6). Patients with AC
with low intratumoral DPD mRNA expression who received 5-FU
subsequent to surgery had a significantly better prognosis than
those who received surgery alone (24). The present study demonstrated that low
DPD expression levels, compared with high DPD levels, were only
weakly associated with an improved response to S-1/CBDCA
(P<0.1).
OPRT, a key enzyme that catalyzes the first step in
nucleic acid-mediated 5-FU phosphorylation, is hypothesized to have
significant associations with the antitumor activity of 5-FU.
Ichikawa et al (22) reported
that low TS expression and high OPRT expression are predictors of
S-1 response in gastric cancer. Nakano et al (25) found that, in surgically resected NSCLC
specimens, TS and OPRT immunohistochemical expression are higher in
SCC than in AC. High OPRT expression in SCC tissues may account for
the increased response and longer median OS time in patients
treated with S-1/CBDCA compared with those treated with
CBDCA/paclitaxel combination therapy (4,26).
However, in the H-score analysis of the present study, OPRT
expression level was not associated with treatment response or PFS
and OS times in patients with lung SCC treated with S-1/CBDCA.
The present study demonstrated that TS, OPRT and DPD
H-scores were not associated with OS in SCC patients. However, low
TS expression is significantly associated with a higher rate of
response and longer PFS in SCC patients treated with S-1/CBDCA.
Hence, the evaluation of TS expression level may be a sensitive
biomarker to predict the response to S-1-based chemotherapy of
patients with advanced SCC, and may be a more useful biomarker for
decision-making regarding further S-1 maintenance therapy
subsequent to S-1-based induction therapy and adjuvant S-1-based
chemotherapy following curative resection in future clinical
studies.
The sample size in the present study was limited,
and the majority of the available information regarding the
predictive value of TS has been derived from retrospective studies.
Large-scale prospective clinical trials using appropriate biomarker
evaluation methodology are required to validate the prospective
utility of TS in clinical decision-making. However, the present
study has demonstrated that patients with lung SCC with low tumor
TS expression can significantly benefit from S-1-based
chemotherapy, and indicates that TS expression level is an
independent predictive biomarker of response to S-1-based
chemotherapy in patients with lung SCC.
Acknowledgements
A part of this work was supported by the Leading
Center for the Development and Research of Cancer Medicine,
Juntendo University Graduate School of Medicine. The authors thank
their colleagues who assisted in sample collection and Taiho
Pharmaceutical Co., Ltd. for the gift of the anti-TS polyclonal
antibodies.
Glossary
Abbreviations
Abbreviations:
|
AC
|
adenocarcinoma
|
|
CBDCA
|
carboplatin
|
|
CI
|
confidence interval
|
|
CR
|
complete response
|
|
DPD
|
dihydropyrimidine dehydrogenase
|
|
5-FU
|
5-fluorouracil
|
|
I
|
intensity of staining
|
|
NSCLC
|
non-small cell lung cancer
|
|
OPRT
|
orotate phosphoribosyltransferase
|
|
OS
|
overall survival
|
|
PC
|
percentage of positively-stained
cells
|
|
PD
|
progressive disease
|
|
PEM
|
pemetrexed
|
|
PFS
|
progression-free survival
|
|
PR
|
partial response
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
SCC
|
squamous cell carcinoma
|
|
SD
|
stable disease
|
|
TS
|
thymidylate synthase
|
References
|
1
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ichinose Y, Yoshimori K, Sakai H, Nakai Y,
Sugiura T, Kawahara M and Niitani H: S-1 plus cisplatin combination
chemotherapy in patients with advanced non-small cell lung cancer:
A multi-institutional phase II trial. Clin Cancer Res.
10:7860–7864. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okamoto I, Yoshioka H, Morita S, Ando M,
Takeda K, Seto T, Yamamoto N, Saka H, Asami K, Hirashima T, et al:
Phase III trial comparing oral S-1 plus carboplatin with paclitaxel
plus carboplatin in chemotherapy-naïve patients with advanced
non-small-cell lung cancer: Results of a west Japan oncology group
study. J Clin Oncol. 28:5240–5246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshioka H, Okamoto I, Morita S, Ando M,
Takeda K, Seto T, Yamamoto N, Saka H, Atagi S, Hirashima T, et al:
Efficacy and safety analysis according to histology for S-1 in
combination with carboplatin as first-line chemotherapy in patients
with advanced non-small-cell lung cancer: Updated results of the
West Japan Oncology Group LETS study. Ann Oncol. 24:1326–1331.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maring JG, Groen HJ, Wachters FM, Uges DR
and de Vries EG: Genetic factors influencing pyrimidine-antagonist
chemotherapy. Pharmacogenomics J. 5:226–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takeda M, Okamoto I, Hirabayashi N, Kitano
M and Nakagawa K: Thymidylate synthase and dihydropyrimidine
dehydrogenase expression levels are associated with response to S-1
plus carboplatin in advanced non-small cell lung cancer. Lung
Cancer. 73:103–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaira K, Ohde Y, Nakagawa K, Okumura T,
Murakami H, Takahashi T, Kondo H, Nakajima T, Endo M and Yamamoto
N: Thymidylate synthase expression is closely associated with
outcome in patients with pulmonary adenocarcinoma. Med Oncol.
29:1663–1672. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishihama H, Chida M, Araki O, Karube Y,
Seki N, Tamura M, Umezu H, Honma K, Masawa N and Miyoshi S:
Comparison of 5-fluorouracil-related gene expression levels between
adenocarcinomas and squamous cell carcinomas of the lung. Jpn J
Clin Oncol. 39:33–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harada K, Kawashima Y, Yoshida H and Sato
M: Thymidylate synthase expression in oral squamous cell carcinoma
predicts response to S-1. Oncol Rep. 15:1417–1423. 2006.PubMed/NCBI
|
|
10
|
Koga M, Anegawa E, Yoh J, Tsuyama H,
Sakaino H, Iwamoto O, Koga C and Kusukawa J: Clinical relevance of
thymidylate synthase (TS) activity for S-1-based chemotherapy in
squamous cell carcinoma of the oral cavity. Br J Oral Maxillofac
Surg. 48:88–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria is solid tumours:
Revised RECIST guideline (Version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vilmar A, Garcia-Foncillas J, Huarriz M,
Santoni-Rugiu E and Sorensen JB: RT-PCR versus immunohistochemistry
for correlation and quantification of ERCC1, BRCA1, TUBB3 and RRM1
in NSCLC. Lung Cancer. 75:306–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herpel E, Schnabel PA, Steins M, Dienemann
H, Herth FJ, Thomas M, Schirmacher P and Warth A: Assessment of
thymidylate synthase expression in biopsy specimens and
corresponding resection specimens of non-small-cell lung cancer.
Histopathology. 61:465–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicolson MC, Fennell DA, Ferry D, O'Byrne
K, Shah R, Potter V, Skailes G, Upadhyay S, Taylor P, André V, et
al: Thymidylate synthase expression and outcome of patients
receiving pemetrexed for advanced nonsquamous non-small-cell lung
cancer in a prospective blinded assessment phase II clinical trial.
J Thorac Oncol. 8:930–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang T, Chuan Pan C, Rui Yu J, Long Y,
Hong Cai X, De Yin X, Qiong Hao L and Li Luo L: Association between
TYMS expression and efficacy of pemetrexed-based chemotherapy in
advanced non-small cell lung cancer: A meta-analysis. PLoS One.
8:e742842013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimizu T, Nakanishi Y, Nakagawa Y,
Tsujino I, Takahashi N, Nemoto N and Hashimoto S: Association
between expression of thymidylate synthase, dihydrofolate
reductase, and glycinamide ribonucleotide formyltransferase and
efficacy of pemetrexed in advanced non-small cell lung cancer.
Anticancer Res. 32:4589–4596. 2012.PubMed/NCBI
|
|
17
|
Wynes MW, Konopa K, Singh S,
Reyna-Asuncion B, Ranger-Moore J, Sternau A, Christoph DC,
Dziadziuszko R, Jassem J and Hirsch FR: Thymidylate synthase
protein expression by IHC and gene copy number by SISH correlate
and show great variability in non-small cell lung cancer. J Thorac
Oncol. 7:982–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kasai D, Ozasa H, Oguri T, Miyazaki M,
Uemura T, Takakuwa O, Kunii E, Ohkubo H, Maeno K and Niimi A:
Thymidylate synthase gene copy number as a predictive marker for
response to pemetrexed treatment of lung adenocarcinoma. Anticancer
Res. 33:1935–1940. 2013.PubMed/NCBI
|
|
19
|
Lee SH, Noh KB, Lee JS, Lee EJ, Min KH,
Hur GY, Lee SH, Lee SY, Kim JH, Lee SY, et al: Thymidylate synthase
and ERCC1 as predictive markers in patients with pulmonary
adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer.
81:102–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buqué A, Aresti U, Calvo B, Muhialdin ShJ,
Muñoz A, Carrera S, Azkona E, Rubio I and López-Vivanco G:
Thymidylate synthase expression determines pemetrexed targets and
resistance development in tumour cells. PLoS One. 8:e633382013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukushima M, Morita M, Ikeda K and
Nagayama S: Population study of expression of thymidylate synthase
and dihydropyrimidine dehydrogenase in patients with solid tumors.
Int J Mol Med. 12:839–844. 2003.PubMed/NCBI
|
|
22
|
Ichikawa W, Takahashi T, Suto K, Shirota
Y, Nihei Z, Shimizu M, Sasaki Y and Hirayama R: Simple combinations
of 5-FU pathway genes predict the outcome of metastatic gastric
cancer patients treated by S-1. Int J Cancer. 119:1927–1933. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ichikawa W, Takahashi T, Suto K, Yamashita
T, Nihei Z, Shirota Y, Shimizu M, Sasaki Y and Hirayama R:
Thymidylate synthase predictive power is overcome by irinotecan
combination therapy with S-1 for gastric cancer. Br J Cancer.
91:1245–1250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shintani Y, Inoue M, Funakoshi Y,
Matsumura A, Ohta M, Maeda H and Okumura M: Low dihydropyrimidine
dehydrogenase crrelates with prolonged survival in patients with
lung adenocarcinoma treated with 5-fluorouracil. Anticancer Res.
31:4665–4671. 2011.PubMed/NCBI
|
|
25
|
Nakano J, Huang C, Liu D, Masuya D,
Nakashima T, Yokomise H, Ueno M, Wada H and Fukushima M:
Evaluations of biomarkers associated with 5-FU sensitivity for
non-small-cell lung cancer patients postoperatively treated with
UFT. Br J Cancer. 95:607–615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto N, Yamanaka T, Ichinose Y, Kubota
K, Sakai H, Gemma A, Saijo N, Fukuoka M and Niitani H: Pooled
analysis of S-1 trials in non-small cell lung cancer according to
histological type. Anticancer Res. 30:2985–2990. 2010.PubMed/NCBI
|