Introduction
Glycans in glycoconjugates including glycoproteins
and glycolipids participate in a number of important biological
events, including cell-cell interactions, inflammation and tumor
progression (1).
Poly-N-acetyllactosamine (polylactosamine), carried on N- or
O-glycans, is an important glycan structure containing repeats of
the N-acetyllactosamine unit (Gal1-4GlcNAc1-3)n (2). The polylactosamine structure has key
roles in mediating molecular interactions during embryogenesis,
tumorigenesis and tumor metastasis (3), and is synthesized by members of the
β-1,3-N-acetylglucosaminyltransferase (β3GnT) family.
β3GnT8 is a member of the β3GnT family (4). When β3GnT8 was first cloned, it was
named β3GalT7 and mapped to chromosome 19q13.2 in our laboratory.
β3GnT8 was renamed β3GnT8 on the basis of subsequent enzymatic
study (2). β3GnT8 is a
polylactosamine synthase and transfers GlcNAc to the non-reducing
terminus of the tetra-antennary β1-6-branched N-glycans of
Galβ1-4GlcNAc (2). Previously, it was
reported that β3GnT8 is highly expressed in various types of tumor
tissues, including colon cancer, gastric cancer and laryngeal
carcinoma (2), which suggests a
possible role for β3GnT8 in tumor malignancy. Our recent study
demonstrated that β3GnT8 is able to regulate the metastasis of
colorectal cancer cells by altering the β1,6-branched
polylactosamine sugars of cluster of differentiation 147 (CD147)
(5). The extracellular region of
CD147 contains three Asn glycosylation sites, and the
N-glycosylation sites make similar contributions to both high and
low glycoforms of CD147 (HG-CD147 and LG-CD147, respectively)
(6). A number of studies have
confirmed that modulation of CD147 is associated with the
expression of matrix metallopeptidases (MMPs) in normal and tumor
tissues (7–9). High glycoforms of CD147 (HG-CD147)
stimulate the production of matrix metalloproteinase (6,7).
Additionally, increased HG-CD147 glycosylation has been attributed
to β1-6-branched N-glycan to form polylactosamine structures
(7,8).
Consistent with these results, our previous study demonstrated that
β3GnT8 may have an important role in the CD147 signal transduction
pathway as an upstream modulator of MMP2 production in tumor cells
(9). Although the functions of β3GnT8
in tumor invasion and metastasis are well documented, how β3GnT8
expression is regulated in tumor cells or tissues remains largely
unclear.
Transcription factor c-Jun (c-Jun) is a well-known
cellular transcription factor belonging to the activator protein 1
(AP-1) family that is able to promote cell cycle progression and
cell proliferation (10,11). c-Jun regulates the expression of a
number of genes that affect tumor invasion and metastasis by
binding to their promoters (12,13).
Considering the known associations between β3GnT8 and c-Jun in
tumor malignancy, the aim of the present study was to investigate
whether β3GnT8 acts as a downstream target gene of c-Jun to
regulate tumor cell invasion. In the present study, the
overexpression of c-Jun was demonstrated to be able to increase
β3GnT8 expression in colorectal carcinoma cell lines. By contrast,
knockdown of c-Jun resulted in a decrease in β3GnT8 expression.
Notably, c-Jun was able to bind with β3GnT8 gene promoters and
activate β3GnT8 transcription, which is consistent with the initial
hypothesis. The results of the present study indicate a novel
molecular mechanism underlying c-Jun-mediated colorectal carcinoma
cell invasion and metastasis.
Materials and methods
Cell culture
SW480 and LoVo cells were obtained from the American
Type Culture Collection (Manassas, VA, USA) and were cultured in
RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), supplemented with 10% fetal bovine serum (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) in a humidified
atmosphere with 5% CO2 at 37°C.
Cell transfection
The pIRES2-EGFR plasmid, used as a mock control
vector, was purchased from Suzhou GenePharma Co., Ltd. (Suzhou,
China); the c-Jun-pIRES2-EGFR plasmid was constructed in our
laboratory. The plasmids c-Jun-shRNA-pGPU6/GFP/Neo and negative
control-shRNA-pGPU6/GFP/Neo (mock control) were purchased from
Suzhou GenePharma Co., Ltd. Cells were seeded in 6-well plates at a
density of 8×105 cells/ml (2 ml/well). Following cell
attachment, c-Jun-pIRES2-EGFR and pIRES2-EGFR plasmids (5 µg per
well) were transfected into SW480 cells, and
c-Jun-shRNA-pGPU6/GFP/Neo and NC-shRNA-pGPU6/GFP/Neo plasmids (5 µg
per well) were transfected into LoVo cells, using Lipofectamine™
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The effects of c-Jun-pIRES2-EGFR and
c-Jun-shRNA-pGPU6/GFP/Neo transfection were confirmed by western
blot analysis of c-Jun expression.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
(Invitrogen, Carlsbad, CA, USA). A total of 1 µg RNA was reverse
transcribed with the ReverTra Ace qPCR RT kit (Toyobo Co., Ltd.,
Osaka, Japan). RT-qPCR was performed using SYBR Green Real-Time PCR
Master mix (Toyobo Co., Ltd.). The reaction mixture was heated to
95°C for 1 min, followed by 40 cycles of 95°C for 15 sec, 60°C for
1 min. The primers were as follows: GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′,
c-Jun forward, 5′-TCCAAGTGCCGAAAAAGGAAG-3′ and reverse,
5′-CGAGTTCTGAGCTTTCAAGGT-3′, β3GnT8 forward,
5′-GTCGCTACAGTGACCTGCTG-3′ and reverse, 5′-GTCTTTGAGCGTCTGGTTGA-3′,
CD147 forward, 5′-ACCGTAGAAGACCTTGGCTC-3′ and reverse,
5′-CGTCGGAGTCCACCTTGAAC-3′, MMP2 forward,
5′-TATGGCTTCTGCCCTGAGAC-3′ and reverse, 5′-CACACCACATCTTTCCGTCA-3′
and MMP15 forward, 5′-TACGAGTGAAAGCCAACCTG-3′ and reverse primer,
5′-TCTCCGTGTAGTTCTGGATGC-3′. The data was analyzed with the ABI
7500 software (version 2.0.3; Applied Biosystems; Thermo Fisher
Scientific, Inc.). GAPDH was used as an internal control, and the
data were analyzed using the 2−ΔΔCq method (14).
Western blot analysis
Cells were harvested and homogenized with lysis
buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.5, 1% NP-40, 1% sodium
deoxycholate, 0.1% SDS and protease inhibitor cocktail) (Roche
Applied Science, Madison, WI, USA). Proteins (30 µg/lane) were
resolved with SDS-PAGE (10% gel; Invitrogen; Thermo Fisher
Scientific, Inc.) and transferred onto nitrocellulose membranes
(EMD Millipore, Billerica, MA, USA). The membranes were blocked
with 5% skimmed milk or 1% bovine serum album (BSA) in
Tris-buffered saline (TBS; 10 mM Tris-HCl and 150 mM NaCl, pH 7.9)
containing 0.05% Tween-20 at room temperature for 2 h. The proteins
were analyzed using specific antibodies as indicated below. The
membranes were incubated with the appropriate primary antibodies at
4°C overnight. Following three washes in TBS containing Tween-20,
the membranes were incubated at room temperature for 2 h with the
appropriate peroxidase-conjugated secondary antibodies. Following
three washes in TBS containing Tween-20, the protein bands on the
membranes were visualized using an enhanced chemiluminescence kit
(GE Healthcare Life Sciences, Shanghai, China). The antibodies,
which were used at a dilution of 1:1,000, were as follows:
Anti-CD147 (cat. no., sc13976), anti-MMP2 (Cat. sc-6838),
anti-MMP15 (cat. no., sc-80213; all Santa Cruz, Dallas, TX, USA),
anti-GAPDH (cat. no., AG019), and horseradish peroxidase-conjugated
anti-rabbit (cat. no., A0208), anti-goat (cat. no., A0181) and
anti-mouse (cat. no., A0216, all Beyotime Institute of
Biotechnology, Haimen, China) secondary antibodies.
A rabbit anti-human β3GnT8 affinity polyclonal
antibody was also used, produced in an earlier study as previously
described (15). In brief, the
antibody was purified from rabbit antiserum with 50% saturated
ammonium sulfate and 33.3% saturated ammonium sulfate, followed by
immunizing protein affinity purification. The purity of the
antibody was determined by SDS-PAGE analysis. The specificity of
the antibody was confirmed previously via western blotting and/or
immunochemical analysis of β3GnT8 protein in tumor cells and
tissues (5,15,16).
Chromatin immunoprecipitation (ChIP)
assay
ChIP was performed using a ChIP assay kit (cat. no.,
P2078; Beyotime Institute of Biotechnology) according to the
manufacturer's protocol with a small number of modifications.
Chromatin solutions were sonicated and incubated with an anti-c-Jun
antibody (dilution, 1:2,000; cat. no., ab119944; Abcam, Cambridge,
MA, USA) or mouse control IgG (dilution, 1:2,000; cat. no., A7028;
Beyotime Institute of Biotechnology), and rotated overnight at 4°C.
The solution was washed for 3–5 min in each of the following from
the ChIP assay kit: Low salt immune complex wash buffer, high salt
immune complex, LiCl immune complex wash buffer and Tris-EDTA
buffer. DNA-protein cross-links were reversed, and chromatin DNA
was purified and subjected to PCR analysis with the Easy-Load PCR
Master mix (cat. no., D7251; Beyotime Institute of Biotechnology).
PCR was performed with 30 cycles of 95°C for 35 sec, 60°C for 45
sec and 72°C for 1 min, followed by 72°C for 10 min. Primers
5′-TGTACGCGTGAGGCACATGGCAAAGG-3′ (forward) and
5′-GTTCTCGAGAGTGGGGAGGAAGTGGT-3′ (reverse) were used to amplify the
β3GnT8 promoter sequence. Following amplification, PCR products
were resolved on a 1.5% agarose gel and visualized by ethidium
bromide staining.
Flow cytometric analysis
To detect polylactosamine structures of cell-surface
glycoproteins, biotin-labeled Solanum lycopersicum (tomato)
agglutinin lectin (LEA; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), which specifically binds polylactosamine residues, was
used. Cells were detached with 0.25% trypsin-EDTA solution and
subsequently washed three times with PBS. The cell density was
adjusted to 3×106 cells/ml, and the cells were stained
with 10 µg/ml LEA in PBS (containing 0.5% BSA and 0.05% sodium
azide) at 37°C for 1 h. The cells were subsequently washed three
times with PBST (PBS containing 0.05% Tween-20). Staining was
performed with 10 µg/ml PE-conjugated streptavidin (Sigma-Aldrich;
Merck KGaA) at 37°C for 1 h, and the cells were washed three times
with PBST. The fluorescence intensity of the stained cells was
measured using a flow cytometer and analyzed with CellQuest
software (version 5.2.1; BD Biosciences, Franklin Lakes, NJ,
USA).
Wound healing assay
SW480 or LoVo cells (1×105) were plated
in a 6-well plate and incubated overnight, yielding confluent
monolayers. Wounds were made using a pipette tip, and cell motility
was examined using a light microscope. Images were captured at 0
and 24 h after wounding. The plates were marked to ensure
consistent photo documentation. Using ImageJ software (version
1.49; National Institute of Health, Bethesda, MD, USA), the area of
each wound was calculated at each time point.
Transwell migration and invasion
assays
The invasion assay was performed in 24-well cell
culture chambers using Transwell inserts (Corning Life Sciences,
Corning, NY, USA) with porous membrane (pore size, 8 µm) precoated
with Matrigel (BD Biosciences). SW480 or LoVo cells
(1×105) were plated in 200 µl serum-free RPMI 1640
medium in the upper chamber, and 500 µl RPMI 1640 medium with 10%
FBS was added to the lower wells. After 48 h, the non-invading
cells with Matrigel matrix were removed from the upper surface of
the membrane by scraping with a cotton tipped swab. The cells on
the lower surface of the filter were fixed for 30 min in 4%
polyoxymethylene, air-dried briefly and stained with eosin staining
solution (Beyotime Institute of Biotechnology, Haimen, China) at
room temperature for 30 min. The number of invading cells was
manually counted from 5 randomly selected microscopic fields at
×100 magnification using a light microscope (IX-70, Olympus, Tokyo,
Japan).
A cell migration assay was similarly performed,
except without Matrigel. Cells were incubated at 37°C for 24 h.
Cells on the lower surface of the filter were stained and counted
as previously described.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 22.0; IBM SPSS, Armonk, NY, USA). Each assay was
performed ≥3 times. Results are presented as the mean ± standard
deviation. Student's t-test was used to evaluate the significance
of data. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of c-Jun on the expression of
the β3GnT8, CD147, MMP2 and MMP15
It is well known that the transcription factor c-Jun
regulates the expression of numerous tumor invasion-associated
genes (11,12). To determine the role of c-Jun in the
regulation of β3GnT8, which is also involved in tumor invasion
(5), the effects of c-Jun
overexpression and knockdown on β3GnT8 expression were examined.
Additionally, the effects of c-Jun overexpression and knockdown on
the expression of a number of tumor metastasis-associated genes
(CD147, MMP2 and MMP15) were investigated. As presented in Fig. 1A, overexpression of c-Jun in SW480
cells was able to significantly increase the mRNA expression of
β3GnT8, CD147, MMP2 and MMP15 (P<0.001). By contrast, knockdown
of c-Jun in LoVo cells resulted in a significant decrease in mRNA
expression of these genes (P<0.001; Fig. 1B). Additionally, western blot analysis
indicated that overexpression of c-Jun increased protein levels of
β3GnT8, HG-CD147, MMP2 and MMP15 in SW480 cells (Fig. 2A). Similarly, the levels of all these
proteins decreased when c-Jun was knocked down in LoVo cells
(Fig. 2B). However, expression of
LG-CD147 did not alter when c-Jun was overexpressed or knocked down
(Fig. 2A and B). These results
suggest that c-Jun may be one of the master regulators of
colorectal carcinoma cell metastasis, and the alterations in the
N-glycosylation level of CD147 may be due to the induction of
β3GnT8 by c-Jun.
 | Figure 1.mRNA expression of c-Jun, β3GnT8,
CD147, MMP2 and MMP15 using RT-qPCR. (A) Exogenous c-Jun plasmid
vector and the empty vector were transfected into SW480 colon
cancer cells with a low metastatic potential. RT-qPCR was performed
to detect mRNA expression. (B) Exogenous c-Jun short hairpin RNA
vector and the empty vector were transfected into LoVo colon cancer
cells with a high metastatic potential. RT-qPCR was performed to
detect mRNA expression. Results are the mean ± standard deviation
representative of 3 independent experiments. ***P<0.001 vs.
untreated control cells. c-Jun, transcription factor c-Jun; β3GnT8,
β-1,3-N-acetylglucosaminyltransferase 8; CD147, cluster of
differentiation 147; MMP, matrix metalloproteinase; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction;
si-c-jun, c-Jun short hairpin RNA. |
 | Figure 2.Western blot analysis of c-Jun,
β3GnT8, CD147, MMP2 and MMP15. (A) Exogenous c-Jun plasmid vector
and the empty vector were transfected into SW480 colon cancer cells
with a low metastatic potential, and western blotting was performed
to detect protein levels. (B) Exogenous c-Jun shRNA vector and the
control vector were transfected into LoVo colon cancer cells with a
high metastatic potential, and western blotting was performed to
detect protein levels. c-Jun, transcription factor c-Jun; β3GnT8,
β-1,3-N-acetylglucosaminyltransferase 8; CD147, cluster of
differentiation 147; MMP, matrix metalloproteinase; HG, high
glycoform; LG, low glycoform; si-c-jun, c-Jun short hairpin
RNA. |
Effects of c-Jun on the level of
polylactosamine
In order to determine whether c-Jun affects the
structure of polylactosamine chain in colorectal carcinoma cells, a
flow cytometric assay was performed to examine the level of
polylactosamine in SW480 and LoVo cells. The results indicated that
overexpression of c-Jun significantly promoted the polylactosamine
level in SW480 cells (3.78 vs. 1.93; Fig.
3A). By contrast, knockdown of c-Jun in LoVo cells decreased
the polylactosamine level (1.6 vs. 4.71; Fig. 3B). These results suggest that c-Jun
has a significant effect on the structure of polylactosamine, and
this may be mediated via β3GnT8, which is involved in biosynthesis
of polylactosamine chain.
c-Jun directly binds to the β3GnT8
promoter
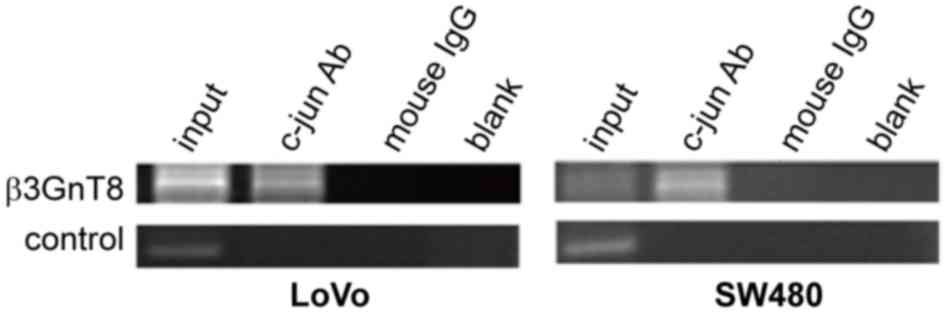
In order to determine whether there is interaction
between c-Jun and β3GnT8, a ChIP assay was performed in SW480 and
LoVo cells, and mouse IgG was used as a negative control.
Immunoprecipitated chromosomal DNA with anti-c-jun antibody or
mouse IgG was subjected to PCR. As presented in Fig. 4, compared to the mouse IgG control
group, the β3GnT8 promoter sequence was detected by PCR in
anti-c-Jun antibody-pulled down DNA. This result suggests that
c-Jun is able to bind to the promoter region of β3GnT8 gene and may
activate β3GnT8 transcription.
Effects of c-Jun expression on the
migratory response of SW480 and LoVo cells
c-Jun has a role in the migration of tumor cells. In
order to determine whether c-Jun affects the migration of SW480 and
LoVo cells, a wound healing assay was performed and images were
captured after 24 h. The results demonstrated that overexpression
of c-Jun markedly increased the migration of SW480 cells compared
with the control (Fig. 5A), whereas
c-Jun knockdown markedly decreased migration of LoVo cells compared
with the control (Fig. 5B). These
results suggest that c-Jun is able to affect the migratory response
of colorectal carcinoma cells in vitro.
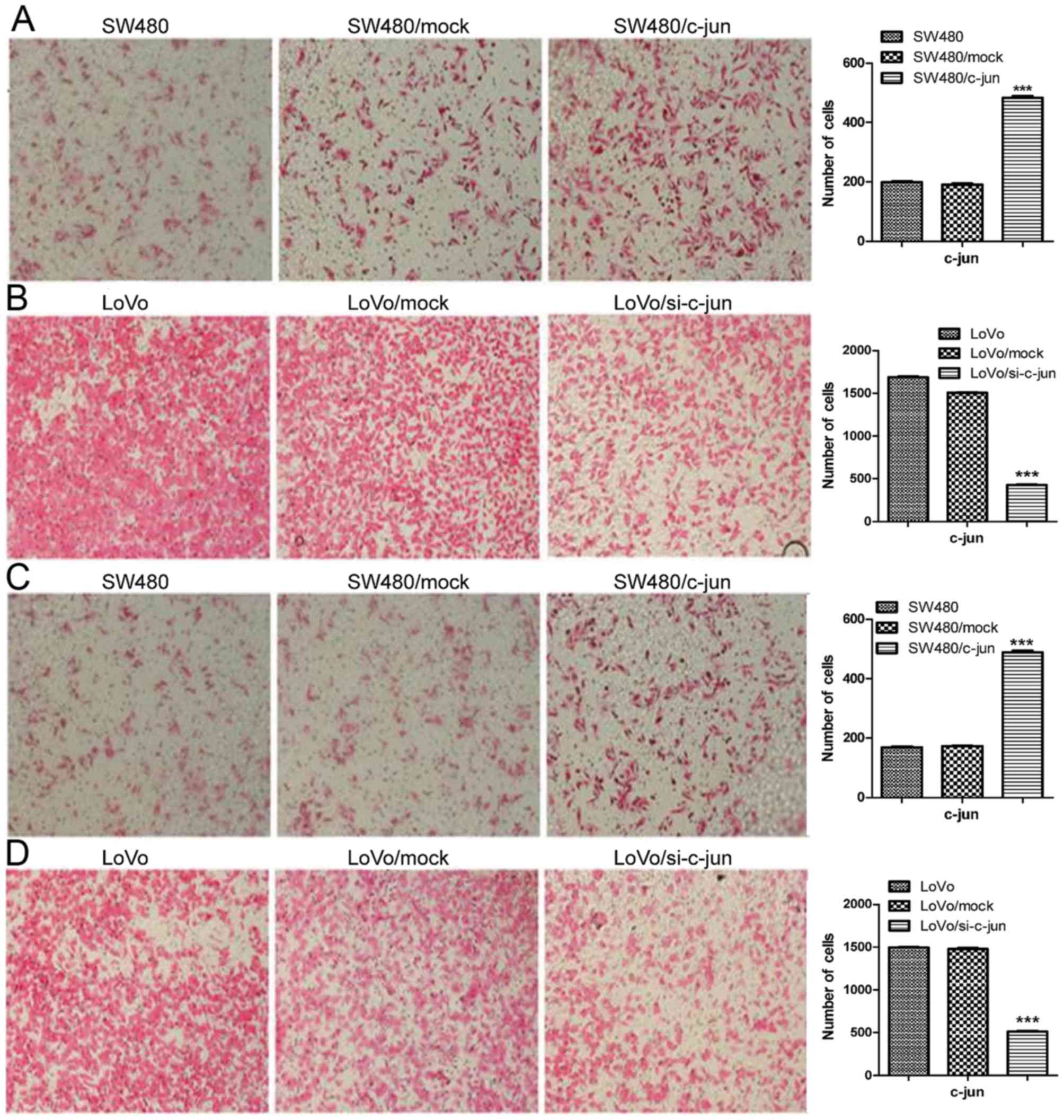
Effects of c-Jun expression on the
invasion and migration of SW480 and LoVo cells using a Transwell
assay
The effect of c-Jun on metastasis abilities of
colorectal carcinoma cells was assessed (Fig. 6). SW480 and LoVo cells were seeded
into the upper compartment of the Transwell chamber. SW480 cells
were incubated at 37°C for 48 h, LoVo cells were incubated at 37°C
for 24 h, and cell migration was assessed by counting the number of
cells that diffused through the membrane. As presented in Fig. 6A and C, overexpression of c-Jun in
SW480 significantly increased cell migration and invasion. By
contrast, c-Jun knockdown in LoVo cells inhibited cell migration
and invasion (Fig. 6B and D), which
suggests that c-Jun has an important role in tumor cell invasion
and metastasis.
Discussion
Glycosylation is one of the most common protein
post-translational modifications. Glycans have important roles in a
number of distinct cellular events, including cell migration,
cell-cell adhesion, cell signaling and growth (1,3). However,
aberrant glycosylation has been associated with various human
diseases and particularly with tumors; glycosylation is considered
a hallmark of cancer (3).
Colorectal cancer is one of the leading causes of
cancer-associated mortality (17). A
recent study has demonstrated associations between colorectal
cancer progression and changes in the pattern of expression of
N-glycans (18). The expression
patterns of β1,6-branched N-glycans (the most common structure of
N-glycans in colorectal cancer) are associated with increased
replicative potential, tissue invasion and metastasis,
characteristics of which are considered hallmarks of colorectal
cancer progression (2).
It has been well established that U937 (human
histiocytic lymphoma cells), ACHN (human kidney glandular cancer
cells), MKN45 (human gastric cancer cells), A549 (human lung cancer
cells) and Jurkat cells (acute T-cell leukemia) express a high
level of N-glycans with polylactosamine residues (19). β3GnT8 is an enzyme involved in the
biosynthesis of polyLac chains by transferring GlcNAc to the
non-reducing terminus of Galβ1-4GlcNAc on β1,6-branched N-glycan.
As overexpression of β3GnT8 in HCT15 colorectal cancer cells
resulted in an increase in L-phaseolus vulgaris erythroagglutinin
reactivity, the authors hypothesize that this enzyme may
participate in tumor malignancy by synthesizing polylactosamine on
β1,6-branched N-glycans (2). Our
previous study demonstrated that overexpression of β3GnT8 in
LS-174T cells increased the level of HG-CD147 and promoted tumor
cell invasion and migration, whereas knockdown of β3GnT8 in LoVo
cells had the opposite effect (5).
These results suggest that β3GnT8 regulates the
metastasis-associated behavior of colorectal cancer cells by
altering the glycosylated forms of CD147. We have also previously
demonstrated that β3GnT8 and polylactosamine residues on
β1,6-branched N-oligosaccharides are associated with the metastatic
potential of colorectal cancer cells and may promote the invasive
and migratory abilities by modulating the N-glycosylated forms of
CD147 (5). As a specific substrate of
β3GnT8, CD147 exists in the glycosylated form and serves key roles
in tumor invasion and metastasis. The glycosylated forms of CD147
are highly expressed on the cell surface of various types of tumor
cell, including oral, breast, lung, bladder, kidney, laryngeal,
pancreatic, gastric, colorectal cancer, glioma lymphoma and
melanoma (20–22). Additionally, the glycosylated forms of
CD147 are able to stimulate tumor cells to produce MMPs,
particularly MMP2 and MMP9 (7,8,23). CD147 is able to induce MMP expression
via phosphoinositide 3-kinase/protein kinase B (Akt)/inhibitor of
nuclear factor κB (NF-κB) (IκB) kinase-dependent IκB-α degradation,
which is mediated by Ras-related C3 botulinum toxin substrate 1,
NF-κB activation and by mitogen-activated protein kinase kinase
7/c-Jun N-terminal kinase-dependent AP-1 activation (20).
c-Jun is a protein encoded by the proto-oncogene
JUN. c-Jun in association with c-Fos forms the early response
transcription factor AP-1. AP-1 has been demonstrated to interact
with a number of genes (12,13) and has important functions in various
tumor types (11,24,25). In
the present study, it was demonstrated that c-Jun is able to
regulate the expression of β3GnT8, MMP2, MMP15, CD147 and
polylactosamine chains in the colorectal carcinoma cell lines SW480
and LoVo by using gain- and loss-of-function assays. Notably, our
previous studies revealed that β3GnT8 is able to regulate the
expression of HG-CD147, MMP2 and MMP15 (5,9).
Considering the results of these previous studies and those of the
present study, it is hypothesized that c-Jun is able to regulate
the expression of these genes, which is mediated partly through
CD147 glycosylation catalyzed by β3GnT8.
In order to demonstrate whether c-Jun protein and
β3GnT8 DNA interact, a ChIP assay was performed in SW480 and LoVo
cells. It was identified that c-Jun is able to directly bind to the
β3GnT8 gene promoter, which results in transcriptional activation
of β3GnT8 and in turn regulates expression of other tumor
invasion-associated genes including MMPs. In summary, the present
study, to the best of our knowledge, is the first report of the
functional and physical association between c-Jun and β3GnT8 and
therefore provides a novel clue for elucidation of the molecular
mechanisms regulating c-Jun-mediated tumor invasion and
metastasis.
Acknowledgements
The authors would like to thank Dr Ning Shi at the
Department of Physiology and Pharmacology, University of Georgia
(GA, USA) for helpful discussion. The present study was supported
by the National Natural Science Foundation of China (grant nos.
31170772 and 31400688) and the Suzhou Municipal Natural Science
Foundation (grant no. SY201208).
References
|
1
|
Fuster MM and Esko JD: The sweet and sour
of cancer: Glycans as novel therapeutic targets. Nat Rev Cancer.
5:526–542. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishida H, Togayachi A, Sakai T, Iwai T,
Hiruma T, Sato T, Okubo R, Inaba N, Kudo T, Gotoh M, et al: A novel
beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which
synthesizes poly-N-acetyllactosamine, is dramatically upregulated
in colon cancer. FEBS Lett. 579:71–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kannagi R, Izawa M, Koike T, Miyazaki K
and Kimura N: Carbohydrate-mediated cell adhesion in cancer
metastasis and angiogenesis. Cancer Sci. 95:377–384. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang C, Zhou J, Wu S, Shan Y, Teng S and
Yu L: Cloning and tissue distribution of the human B3GALT7 gene, a
member of the beta1, 3-Glycosyltransferase family. Glycoconj J.
21:267–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ni J, Jiang Z, Shen L, Gao L, Yu M, Xu X,
Zou S, Hua D and Wu S: β3GnT8 regulates the metastatic potential of
colorectal carcinoma cells by altering the glycosylation of CD147.
Oncol Rep. 31:1795–1801. 2014.PubMed/NCBI
|
|
6
|
Tang W, Chang SB and Hemler ME: Links
between CD147 function, glycosylation, and caveolin-1. Mol Biol
Cell. 15:4043–4050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang W, Luo WJ, Zhu P, Tang J, Yu XL, Cui
HY, Wang B, Zhang Y, Jiang JL, Chen ZN, et al: Modulation of
CD147-induced matrix metalloproteinase activity: Role of CD147
N-glycosylation. Biochem J. 449:437–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun J and Hemler ME: Regulation of MMP-1
and MMP-2 production through CD147/extracellular matrix
metalloproteinase inducer interactions. Cancer Res. 61:2276–2281.
2001.PubMed/NCBI
|
|
9
|
Jiang Z, Hu S, Hua D, Ni J, Xu L, Ge Y,
Zhou Y, Cheng Z and Wu S: β3GnT8 plays an important role in CD147
signal transduction as an upstream modulator of MMP production in
tumor cells. Oncol Rep. 32:1156–1162. 2014.PubMed/NCBI
|
|
10
|
Angel P and Karin M: The role of Jun, Fos
and the AP-1 complex in cell-proliferation and transformation.
Biochim Biophys Acta. 1072:129–157. 1991.PubMed/NCBI
|
|
11
|
Wisdom R, Johnson RS and Moore C: c-Jun
regulates cell cycle progression and apoptosis by distinct
mechanisms. EMBO J. 18:188–197. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vogt PK: Fortuitous convergences: The
beginnings of JUN. Nat Rev Cancer. 2:465–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wertz IE, O'Rourke KM, Zhang Z, Dornan D,
Arnott D, Deshaies RJ and Dixit VM: Human De-etiolated-1 regulates
c-Jun by assembling a CUL4A ubiquitin ligase. Science.
303:1371–1374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Z, Ge Y, Zhou J, Xu L and Wu SL:
Subcellular localization and tumor distribution of human
beta3-galactosyltransferase by beta3GalT7 antiserum. Hybridoma.
29:141–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Shen L, Yang L, Hu S, Xu L and Wu
S: High expression of β3GnT8 is associated with the metastatic
potential of human glioma. Int J Mol Med. 33:1459–1468.
2014.PubMed/NCBI
|
|
17
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Freitas Junior JC and Morgado-Díaz JA:
The role of N-glycans in colorectal cancer progression: Potential
biomarkers and therapeutic applications. Oncotarget. 7:19395–19413.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mitsui Y, Yamada K, Hara S, Kinoshita M,
Hayakawa T and Kakehi K: Comparative studies on glycoproteins
expressing polylactosamine-type N-glycans in cancer cells. J Pharm
Biomed Anal. 70:718–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sameshima T, Nabeshima K, Toole BP,
Yokogami K, Okada Y, Goya T, Koono M and Wakisaka S: Expression of
emmprin (CD147), a cell surface inducer of matrix
metalloproteinases, in normal human brain and gliomas. Int J
Cancer. 88:21–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu S, Chu D, Zhang Y, Wang X, Gong L, Han
X, Yao L, Lan M, Li Y and Zhang W: EMMPRIN/CD147 expression is
associated with disease-free survival of patients with colorectal
cancer. Med Oncol. 30:3692013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan Y, He B, Song G, Bao Q, Tang Z, Tian F
and Wang S: CD147 silencing via RNA interference reduces tumor cell
invasion, metastasis and increases chemosensitivity in pancreatic
cancer cells. Oncol Rep. 27:2003–2009. 2012.PubMed/NCBI
|
|
23
|
Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN
and Chan HC: The involvement of HAb18G/CD147 in regulation of
store-operated calcium entry and metastasis of human hepatoma
cells. J Biol Chem. 276:46870–46877. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karamouzis MV, Konstantinopoulos PA and
Papavassiliou AG: The activator protein-1 transcription factor in
respiratory epithelium carcinogenesis. Mol Cancer Res. 5:109–120.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lopez-Bergami P, Huang C, Goydos JS, Yip
D, Bar-Eli M, Herlyn M, Smalley KS, Mahale A, Eroshkin A, Aaronson
S and Ronai Z: Rewired ERK-JNK signaling pathways in melanoma.
Cancer Cell. 11:447–460. 2007. View Article : Google Scholar : PubMed/NCBI
|