Introduction
Bevacizumab (BEV; Avastin®) is a
monoclonal humanized antibody that targets vascular endothelial
growth factor (VEGF). BEV-based chemotherapy has resulted in an
objective radiological response of 19.6–37.8% and a median response
duration of 3.0–11.0 months in high-grade gliomas (1–4). Even with
the high radiological response rates to anti-angiogenic therapy,
the majority of patients develop progressive disease within a year
of treatment. Following treatment, alternative VEGF-independent
pro-angiogenic growth factors may be activated, resulting in tumor
resistance and aggressive tumor characteristics (5,6).
Following anti-angiogenic treatment, the blood-brain
barrier may be restored (5). Contrast
enhancement in T1-weighted magnetic resonance images (MRI) and
hyperintensity in T2/fluid attenuation inversion recovery (FLAIR)
images may be reduced, which causes the early detection of tumor
progression to be challenging. In order to evaluate responses to an
anti-angiogenic treatment such as BEV, MRI results, including
T1-weighted images with contrast enhancement and T2/FLAIR images,
and clinical status must be considered according to the Response
Assessment in Neuro-Oncology (RANO) criteria (7). However, there are certain vague aspects
in determining tumor progression that only use the RANO criteria as
this criteria does not assess the degree of T2/FLAIR
alteration.
Tumor progression is difficult to identify in BEV
responders with recurrent malignant glioma. Certain patients
exhibit clinical deterioration without definitive radiological
progression, and other patients exhibit progressive enlargement of
non-enhancing lesions without neurological deterioration.
Therefore, the present study reviewed clinical and radiological
results following BEV-based chemotherapy, and evaluated patterns of
disease progression in the BEV responders.
Materials and methods
Patient clinical and tumor
characteristics
Between August 2011 and November 2015, surgery was
performed on 6 patients with anaplastic astrocytoma and 18 patients
with glioblastoma, which were classified according to the World
Health Organization criteria 2007 (8). The Institutional Review Board of Chonnam
National University Hwasun Hospital (CNUHH-2016-052; Chonnam,
Korea) approved the current study. Patient characteristics are
summarized in Table I. The
male-female ratio was 10:14 and the median age was 47.5 years
(range, 29–69).
 | Table I.Clinical and radiological
characteristics of patients who received BEV-based
chemotherapy. |
Table I.
Clinical and radiological
characteristics of patients who received BEV-based
chemotherapy.
| A. Characteristics of
all patients |
|---|
|
|---|
| Variable | n=24 (%) |
|---|
| Gender |
|
| Male | 10 (41.7) |
|
Female | 14 (58.3) |
| Median age | 47.5 years (range,
29–69) |
| Pathology |
|
|
Glioblastoma | 18 (75.0) |
|
Anaplastic astrocytoma | 6 (25.0) |
| ECOG PS |
|
| 1, 2 | 3, 9 (50.0) |
| 3, 4 | 9, 3 (50.0) |
| BEV chemotherapy |
|
|
Alone | 6 (25.0) |
| Combined
with Irinotecan | 18 (75.0) |
| Treatment-associated
complications (CTCAE 4.0) |
|
| Neutrophil loss |
|
| Grade
4 | 2 |
| Grade
3 | 1 |
| Grade
2 | 2 |
| Hypertension |
|
| Grade
3 | 1 |
| Platelet loss |
|
| Grade
2 | 1 |
| Hemorrhoid
bleeding |
|
| Grade
2 | 1 |
| Epistaxis |
|
| Grade
1 | 2 |
| General muscle
weakness |
|
| Grade
1 | 3 |
| Urticaria |
|
| Grade
1 | 1 |
|
| B. Response
evaluation |
|
| Variable | n=22 (%) |
|
| Best response (RANO
criteria) |
|
| Complete
response | 3 (13.6) |
| Partial
response | 10 (45.5) |
| Stable
response | 3 (13.6) |
|
Progressive disease | 6 (27.3) |
| Progression-free
survival, months | Median, 2.8 (range,
0.6–10.1) |
|
| C. Disease
progression patterns in BEV responders |
|
| Variable | n=16 (%) |
|
| Only clinical
progression | 2 (12.5) |
| Only radiological
progression | 5 (31.3) |
| Clinical and
radiological progression | 9 (56.2) |
|
| D. Radiological
disease progression in BEV responders |
|
| Variable | n=14 (%) |
|
| Target lesions |
|
|
Dominant enhancing
lesions | 8 (57.1) |
|
Dominant non-enhancing
lesions | 6 (42.9) |
|
Diffuse | 3 |
|
Focal | 3 |
| New
lesions | 4 |
|
Dominant enhancing
lesions | 2 |
|
Dominant
non-enhancing lesions | 2 |
|
| E. Radiological
progressive disease in BEV non-responders |
|
| Variable | n=6 (%) |
|
| Target lesions |
|
|
Dominant enhancing
lesions | 4 (66.7) |
|
Dominant non-enhancing
lesions | 2 (33.3) |
|
Diffuse | 2 |
|
Focal | 0 |
| New lesions | 2 |
|
Predominant
enhancing lesion | 1 |
|
Predominant
non-enhancing lesion | 1 |
| Subependymal
enhancement | 9/20 (45.0) |
As an initial study, the extent of tumor removal was
assessed using postoperative MRI, and removal using three subgroups
was classified as follows: Gross total resection (no obvious
residual tumor), subtotal resection (residual tumor, <50%) and
partial resection (residual tumor, ≥50%). Tumors were completely
removed from 6 patients (25.0%) and partially removed from 13
patients (54.2%). Biopsies were performed in 5 patients (20.8%). A
total of 19 patients underwent surgery followed by radiotherapy and
concomitant temozolomide chemotherapy. In total, 5 patients
received surgery followed by radiotherapy alone. The median
radiation dose was 59.4 Gy (range, 50.4–60.0). As adjuvant
treatment prior to BEV-based chemotherapy, surgical resection was
performed for recurred lesions in 6 patients, and chemotherapeutic
regimens were as follows: Treatment with temozolomide was
administered to 10 patients; procarbazine, CCNU and vincristine
(PCV) regimens were administered to 9 patients; metronomic
temozolomide was administered to 3 patients; an ifosfamide,
carboplatin and etoposide (ICE) regimen was administered to 1
patient. Gamma knife radiosurgery was performed on 3 patients and a
single patient received additional radiation.
The patients were regularly treated using BEV (10
mg/kg body weight) alone or in combination with irinotecan (125
mg/m2) every 2 weeks. In total, 6 patients received
BEV-only chemotherapy and 18 patients received BEV combined with
irinotecan.
Treatment responses following BEV-based chemotherapy
were estimated using the RANO criteria (7). The RANO criteria divides tumor responses
into four types based on alterations in MRI and clinical features:
Complete response (CR), partial response (PR), stable disease (SD)
and progressive disease (PD). A target lesion was defined as a
primary tumor site including any peritumoral edema and a new lesion
was defined as a spatially-separate tumor within the brain.
Radiological progression patterns were divided into dominant
enhancing and non-enhancing lesions, according to the alterations
in T1-enhancing and T2/FLAIR MRI images (Fig. 1). Non-enhancing lesion progression
patterns were diffuse or focal. Additionally, clinical
deterioration was considered a recurrence pattern. Ventricular
subependymal enhancement was analyzed as evidence of cerebrospinal
fluid dissemination on brain imaging studies.
Toxic effects were assessed using the National
Cancer Institute Common Terminology Criteria for Adverse Events
version 4.0 (CTCAE), with a score of 1 indicating mild adverse
effects, 2 indicating moderate adverse effects, 3 indicating severe
adverse effects, 4 indicating life-threatening adverse effects and
5 denoting mortality-associated with the adverse effects. Physical
examinations, including neurological exams, blood pressure
measurements and laboratory tests of blood and urine, were
performed biweekly. Eastern Cooperative Oncology Group (ECOG)
performance status (PS) scales were used to assess patient disease
progression and to determine the appropriate treatment prognosis
(9).
Statistical analysis
Follow-up durations were expressed as the median
average and range. The effects of single variables on
progression-free survival (PFS) and overall survival (OS) rates
were determined using univariate analyses. Single variables
included pathologic grade, chemotherapeutic regimen, radiological
progression pattern, associated subependymal enhancement and
salvage treatment following BEV-based chemotherapy. PFS was
calculated from the initiation of BEV-based chemotherapy to
clinicoradiological disease progression; OS was calculated from the
time of failure to respond to BEV-based chemotherapy to the date of
mortality (or the last follow-up visit). PFS and OS probabilities
were estimated using the Kaplan-Meier method and compared using
log-rank tests. All statistical analyses were performed using SPSS
18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Tumor response and toxicity of
BEV-based chemotherapy
Prior to BEV-based treatment, the ECOG PS scores
were 1 in 3 patients, 2 in 9 patients, 3 in 9 patients and 4 in 3
patients. In total, 2 patients discontinued treatment due to
chemotherapy-associated complications that included rectal bleeding
and severe hematologic toxicity following a single administration
of BEV combined with irinotecan. A total of 22 patients received a
median of 6 cycles (range, 2–18) of BEV, and treatment responses
were analyzed using the RANO criteria. The objective tumor response
rates were 13.6% CR, 45.5% PR, 13.6% SD and 27.3% PD. It was
considered that CR, PR and SD were BEV-responsive, but PD was
BEV-non-responsive.
According to CTCAE version 4.0, patients exhibited
chemotherapy-associated complications, including grade 4 (n=2),
grade 3 (n=1) and grade 2 (n=2) decreases in neutrophil counts,
grade 3 hypertension (n=1), grade 2 platelet loss (n=1), grade 2
hemorrhoid bleeding (n=1), grade 1 epistaxis (n=2), grade 1 general
muscle weakness (n=3) and grade 1 urticaria (n=1; Table I). Severe grade 3 and 4 toxicities
occurred in 4/24 patients (16.7%).
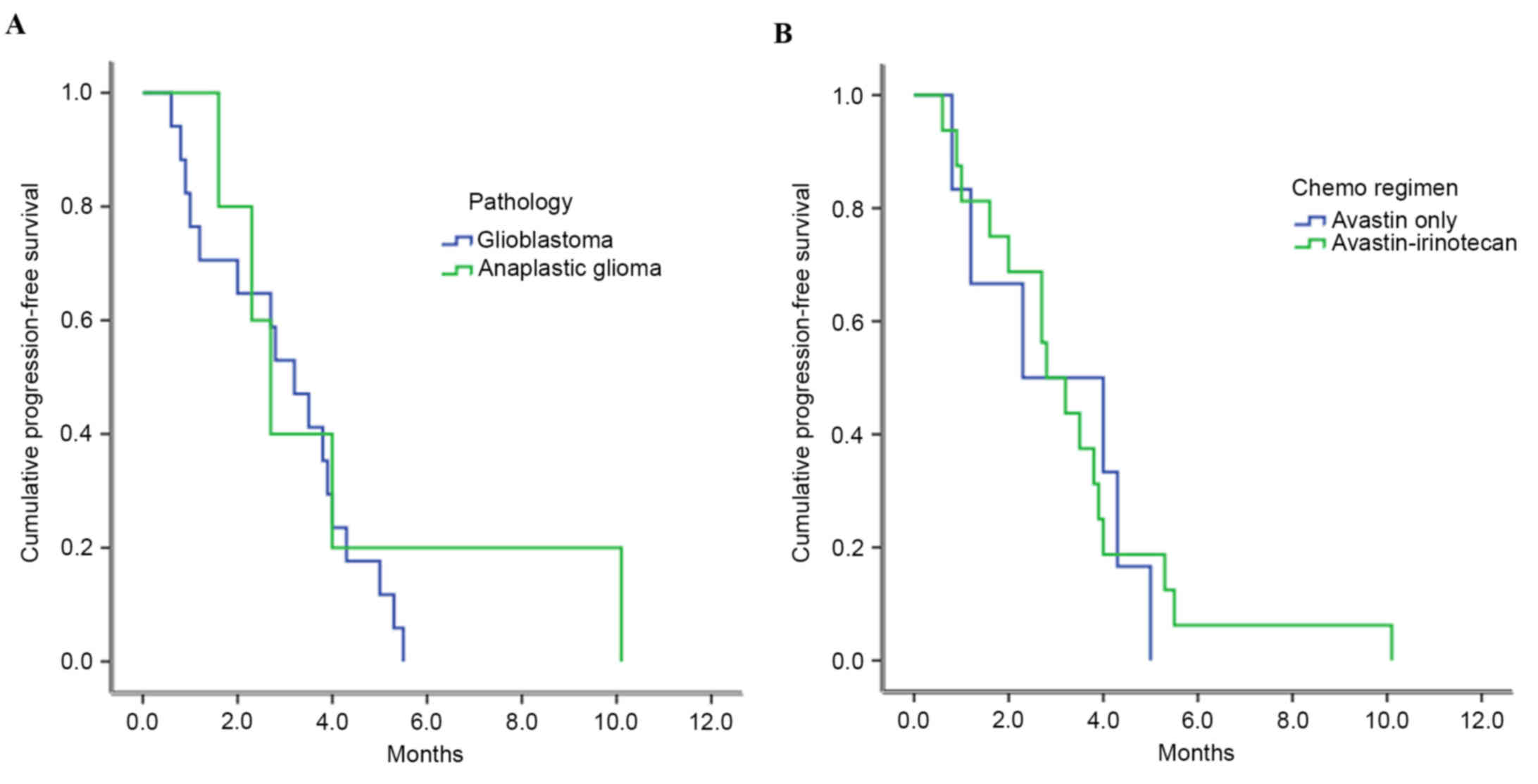
The median PFS was 2.8 months (range, 0.6–10.1)
following BEV-based chemotherapy. Depending on the pathology, the
median PFS times were 3.2±0.4 months for patients with glioblastoma
and 2.7±1.5 months for patients with anaplastic astrocytoma
(P=0.482; Fig. 2A). For
chemotherapeutic regimens, the median PFS of patients who received
BEV alone was 2.3±1.7 months, and that for patients who received
BEV combined with irinotecan was 2.8±0.5 months (P=0.823; Fig. 2B).
Patterns of disease progression
following BEV-based chemotherapy
Patterns of disease progression are summarized in
Table I and Fig. 1. In BEV responders, clinical
progression without definitive radiological progression was
observed in 2/16 patients (12.5%). In total, 5 patients (31.3%)
exhibited only radiological progression and 9 patients (56.2%)
exhibited clinical and radiological progression.
For radiological progression in BEV responders,
target lesions were dominant enhancing tumors in 8/14 patients
(57.1%), and were non-enhancing tumors in 6/14 patients (42.9%).
Out of the 6 patients with non-enhancing tumors, 3 had diffuse
lesions and 3 had focal lesions. New lesions developed in 4
patients, of which 2 were enhancing lesions and 2 were
non-enhancing lesions. For radiological disease progression in BEV
non-responders, target lesions were dominant enhancing tumors in
4/6 patients (66.7%) and were diffuse non-enhancing tumors in 2
patients (33.3%). New lesions were observed in 2 patients, of which
1 was enhancing and 1 was non-enhancing. Subependymal enhancement
was associated with 9/20 patients (45.0%), and included BEV
responders and non-responders.
The radiological progression patterns
in BEV complete responders
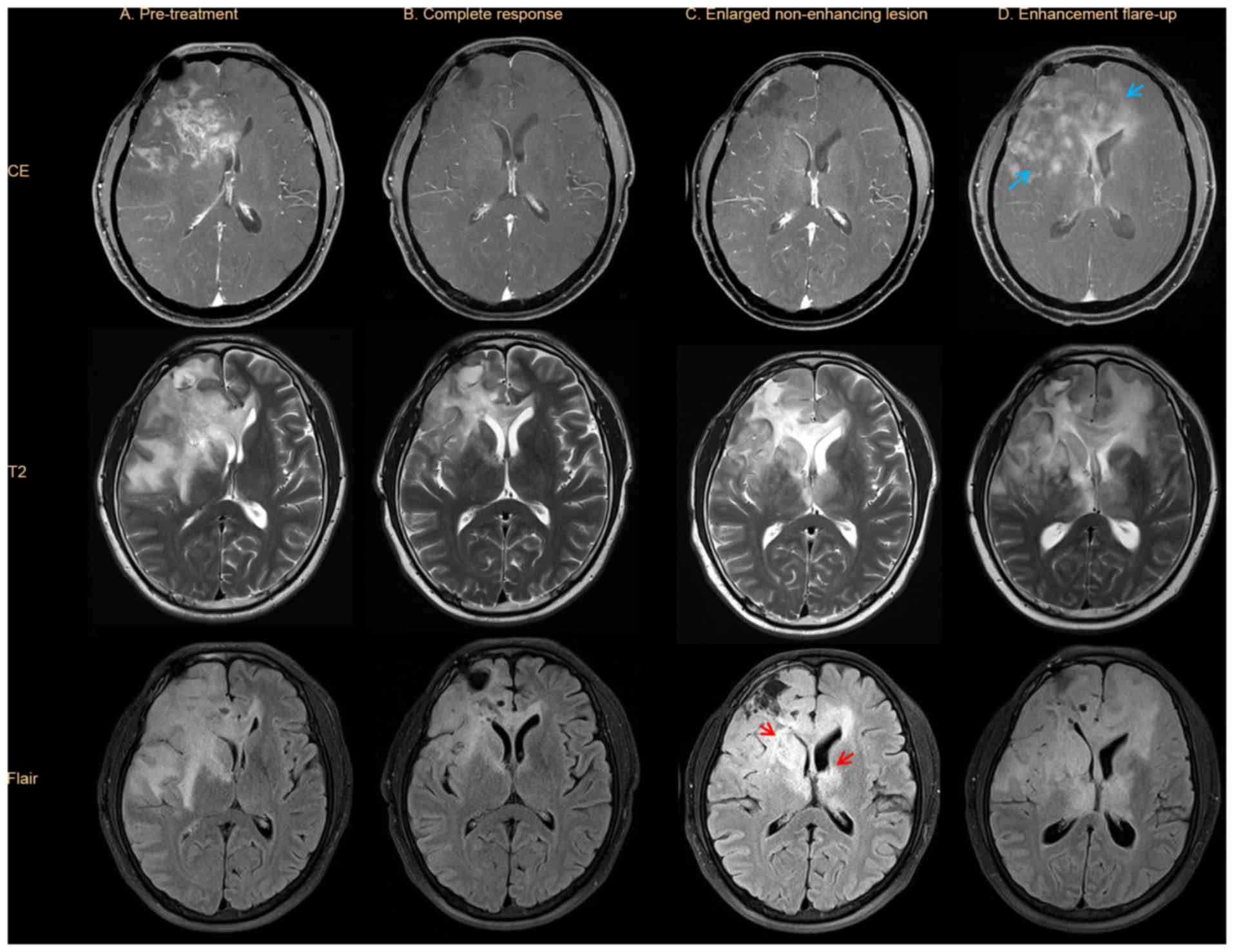
All enhancing lesions disappeared in the BEV
complete responders (n=3), and non-enhancing lesions improved
without any new lesions developing (Fig.
3). T2/FLAIR lesions enlarged in cases of radiological
progression despite stable enhancing lesions, which were associated
with clinical deterioration in 2 patients and not associated with
clinical deterioration in 1 patient. All enlarged T2/FLAIR lesions
with enhancement flare-ups were associated with clinical
deterioration.
The overall survival rate following
failure of BEV-based chemotherapy
The median OS was 4.5 months (range, 0.4–34.0)
following BEV-based chemotherapy failure. In total, 8 patients
(36.4%) received salvage therapies, including metronomic
temozolomide (n=3), ICE chemotherapy (n=2), additional radiation
therapy (n=2), PCV (n=1) and gamma knife radiosurgery (n=1). A
total of 14 patients received supportive care. The median OS for
patients who received salvage therapy was 5.1±0.7 months, as
compared with 3.0±0.7 months for patients who received supportive
care (P=0.297). According to radiological progression patterns, the
median OS was 4.5±0.4 months for patients with dominant enhancing
lesions, 4.4±2.6 months for patients with diffuse non-enhancing
lesions and 3.3±0.8 months for patients with focal non-enhancing
lesions (P=0.749; Fig. 4A). The
median OS in patients with associated subependymal enhancement was
3.0±0.6 months, as compared with 5.1±0.5 months for those without
subependymal enhancement (P=0.072; Fig.
4B).
Discussion
Malignant glial tumors are highly vascular and have
increased expression levels of VEGF, which binds to VEGF receptors
and promotes endothelial cell proliferation and tumor angiogenesis
(10). Anti-angiogenic therapy
exhibits anti-tumor effects via inducing endothelial cell
apoptosis, inhibiting novel vessel formation and decreasing tumor
perfusion, which then results in tumor starvation (11). During initial treatment, abnormal
tumor vasculature may be transiently normalized by reducing blood
vessel diameter and permeability, which subsequently increases
tumor perfusion, reduces interstitial pressure and improves tumor
oxygenation (12). At this stage, the
effects of radiotherapy and cytotoxic chemotherapeutic agents may
potentially be improved (12).
Augmentation of host immunity may also occur upon reducing
VEGF-mediated immune suppression, which in turn improves the
effects of immunotherapy (13).
Augmentation of BEV-mediated benefits has been attempted using
chemotherapeutic agents, targeted therapy and irradiation (4,12,13). A previous study identified that BEV
combined with CCNU improved the 6-month PFS rate to 41%, as
compared with 18% for BEV alone (4).
However, these combination therapies failed to improve survival
beyond that conferred by monotherapy, which is explained by the
effect of BEV decreasing drug delivery to the tumor (14). Finally, adaptive resistance promotes
mesenchymal transition and increased invasiveness in response to
BEV treatment (6). In the present
study, BEV combined with irinotecan improved survival rates, as
compared with BEV alone, although this was not to a statistically
significant extent.
The majority of retrospective studies suggest that
BEV may modestly improve patient outcomes (4,14,15). In the current study, the median PFS
was 2.8 months. Responses to anti-angiogenic therapy occur rapidly,
and tumor and edema volumes have been established to decrease
within 6 weeks of therapy initiation (16,17).
Anti-angiogenic therapy targeting the VEGF signaling pathway may
promptly reduce vessel permeability and contrast media
extravasation. This rapid and transient radiological alteration is
called a ‘pseudoresponse’ (16,17). The
decrease in edema was rapid and sustained, even following tumor
progression. Tumor progression also occurs without necrosis, which
differs from the recurrence patterns observed following treatment
with other chemotherapeutic agents. Anti-angiogenic treatments have
focused on brain tumor imaging in order to accurately define tumor
responses and progression (16,17).
The radiological findings of BEV-induced
chemotherapy responders differ from the typical radiological
results of patients who did not receive BEV-based chemotherapy, as
aforementioned. Enlargement of non-enhancing lesions with decreased
or stable enhancing lesions are a typical relapse progression
pattern following BEV-based chemotherapy, compared with typically
deteriorating enhancing lesions following non-BEV chemotherapy
(18,19). Flare-up of enhancing lesions and
clinical deterioration without significant radiological alterations
are observed in BEV chemotherapy responders. These characteristics
were introduced into the revised RANO criteria (7). Enlarged T2/FLAIR lesions, despite stable
enhancing lesions, are frequently present in BEV chemotherapy
responders. The use of RANO criteria to assess responses would
effectively estimate tumor progression, compared with other
criteria that do not consider T2/FLAIR MRI results. The RANO
criteria are reported to allow disease progression detection ~1
month earlier, as compared with criteria that do not account for
T2/FLAIR imaging (20). However,
these criteria do not state the degree of T2/FLAIR alterations
required to define tumor progression, and these results may be
treatment-associated alterations, including perioperative ischemia,
inflammation, post-radiation demyelination and leukoencephalopathy.
Furthermore, patterns of progression may differ amongst patients
with malignant glioma (21). In the
current study, clinical deterioration without definitive
radiological alterations were detected in 12.5% of patients.
Enlarged dominant enhancing lesions were noted in 57.1% of
BEV-responders and non-enhancing lesions were observed in 42.9% of
BEV responders. Among the radiological results, patients with
tumors that had associated subependymal enhancement suggesting
dissemination of tumor cells into the cerebrospinal fluid had a
poor OS, as compared with patients with tumors lacking enhancement
(P=0.072). Notably, the radiological progression of BEV complete
responders primarily demonstrated progressive enlarged T2/FLAIR
lesions, despite stable enhancing lesions with or without clinical
deterioration. Enlarged T2/FLAIR lesions with enhancement flare-ups
were associated with clinical deterioration in all patients. The
progression of non-enhancing lesions may delay detection of
treatment failure, particularly in BEV complete responders. This
delay may potentially lead to an aggressive phenotype and be a
negative prognostic factor.
Pharmacological inhibition of VEGF induces tumor
hypoxia and the growth of bone marrow-derived cells, which then
promotes the alternative angiogenesis pathway and tumor growth
(22). Considering this, tumors may
be more invasive and aggressive following anti-angiogenic treatment
and, therefore, alternative salvage treatments must be considered
following BEV-based chemotherapy failure. In one previous study,
the median OS of patients was 5.6 months with heterogeneous salvage
treatments following BEV failure, which was not reduced compared
with that of the historical salvage treatments (18). In the present study, the median OS of
patients was 4.5 months following BEV-based chemotherapy failure. A
total of 8 patients (36.4%) received salvage therapies, which
resulted in a median 5.3 month OS compared with a 2.6 month OS in
the supportive care group.
In conclusion, for patients treated with BEV-based
chemotherapy, caution is required to determine tumor progression
based on clinical and radiological alterations. In certain cases,
clinical deterioration without radiological alterations suggested
tumor progression. Additionally, tumor progression through
increased non-enhancing lesions was observed in numerous
BEV-responders. Clinical symptoms and radiological alterations of
non-enhancing lesions must be evaluated in order to assess tumor
progression in the BEV responders, particularly those patients who
experienced a complete response.
Acknowledgements
This study was supported by a grant (grant no.
HCRI14029-1) from the Chonnam National University Hwasun Hospital
Research Institute of Clinical Medicine (Gwangju, Republic of
Korea).
References
|
1
|
Levin VA, Mendelssohn ND, Chan J, Stovall
MC, Peak SJ, Yee JL, Hui RL and Chen DM: Impact of bevacizumab
administered dose on overall survival of patients with progressive
glioblastoma. J Neurooncol. 122:145–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman HS, Prados MD, Wen PY, Mikkelsen
T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen
R, et al: Bevacizumab alone and in combination with irinotecan in
recurrent glioblastoma. J Clin Oncol. 27:4733–4740. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al:
Phase II trial of single-agent bevacizumab followed by bevacizumab
plus irinotecan at tumor progression in recurrent glioblastoma. J
Clin Oncol. 27:740–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taal W, Oosterkamp HM, Walenkamp AM,
Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D,
de Vos FY, et al: Single-agent bevacizumab or lomustine versus a
combination of bevacizumab plus lomustine in patients with
recurrent glioblastoma (BELOB trial): A randomised controlled phase
2 trial. Lancet Oncol. 15:943–953. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu KV and Bergers G: Mechanisms of evasive
resistance to anti-VEGF therapy in glioblastoma. CNS Oncol.
2:49–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piao Y, Liang J, Holmes L, Henry V, Sulman
E and de Groot JF: Acquired resistance to anti-VEGF therapy in
glioblastoma is associated with a mesenchymal transition. Clin
Cancer Res. 19:4392–4403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wen PY, Macdonald DR, Reardon DA,
Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert
MR, Lassman AB, et al: Updated response assessment criteria for
high-grade gliomas: Response assessment in neuro-oncology working
group. J Clin Oncol. 28:1963–1972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lamszus K, Ulbricht U, Matschke J,
Brockmann MA, Fillbrandt R and Westphal M: Levels of soluble
vascular endothelial growth factor (VEGF) receptor 1 in astrocytic
tumors and its relation to malignancy, vascularity, and VEGF-A.
Clin Cancer Res. 9:1399–1405. 2003.PubMed/NCBI
|
|
11
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Winkler F, Kozin SV, Tong RT, Chae SS,
Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E,
et al: Kinetics of vascular normalization by VEGFR2 blockade
governs brain tumor response to radiation: Role of oxygenation,
angiopoietin-1, and matrix metalloproteinases. Cancer Cell.
6:553–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shrimali RK, Yu Z, Theoret MR, Chinnasamy
D, Restifo NP and Rosenberg SA: Antiangiogenic agents can increase
lymphocyte infiltration into tumor and enhance the effectiveness of
adoptive immunotherapy of cancer. Cancer Res. 70:6171–6180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van der Veldt AA, Lubberink M, Bahce I,
Walraven M, de Boer MP, Greuter HN, Hendrikse NH, Eriksson J,
Windhorst AD, Postmus PE, et al: Rapid decrease in delivery of
chemotherapy to tumors after anti-VEGF therapy: Implications for
scheduling of anti-angiogenic drugs. Cancer Cell. 21:82–91. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Batchelor TT, Reardon DA, de Groot JF,
Wick W and Weller M: Antiangiogenic therapy for glioblastoma:
Current status and future prospects. Clin Cancer Res. 20:5612–5619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pope WB, Lai A, Nghiemphu P, Mischel P and
Cloughesy TF: MRI in patients with high-grade gliomas treated with
bevacizumab and chemotherapy. Neurology. 66:1258–1260. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ananthnarayan S, Bahng J, Roring J,
Nghiemphu P, Lai A, Cloughesy T and Pope WB: Time course of imaging
changes of GBM during extended bevacizumab treatment. J Neurooncol.
88:339–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iwamoto FM, Abrey LE, Beal K, Gutin PH,
Rosenblum MK, Reuter VE, DeAngelis LM and Lassman AB: Patterns of
relapse and prognosis after bevacizumab failure in recurrent
glioblastoma. Neurology. 73:1200–1206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaspar LE, Fisher BJ, Macdonald DR, LeBer
DV, Halperin EC, Schold SC Jr and Cairncross JG: Supratentorial
malignant glioma: Patterns of recurrence and implications for
external beam local treatment. Int J Radiat Oncol Biol Phys.
24:55–57. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gallego Pérez-Larraya J, Lahutte M,
Petrirena G, Reyes-Botero G, González-Aguilar A, Houillier C,
Guillevin R, Sanson M, Hoang-Xuan K and Delattre JY: Response
assessment in recurrent glioblastoma treated with
irinotecan-bevacizumab: Comparative analysis of the Macdonald,
RECIST, RANO, and RECIST + F criteria. Neuro Oncol. 14:667–673.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nowosielski M, Wiestler B, Goebel G,
Hutterer M, Schlemmer HP, Stockhammer G, Wick W, Bendszus M and
Radbruch A: Progression types after antiangiogenic therapy are
related to outcome in recurrent glioblastoma. Neurology.
82:1684–1692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du R, Lu KV, Petritsch C, Liu P, Ganss R,
Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z and Bergers G:
HIF1alpha induces the recruitment of bone marrow-derived vascular
modulatory cells to regulate tumor angiogenesis and invasion.
Cancer Cell. 13:206–220. 2008. View Article : Google Scholar : PubMed/NCBI
|