Introduction
Colorectal cancer (CRC) is the second-leading
death-related malignant tumor in Poland in both sexes (1). The prognosis of patients is performed by
staging of primary tumor and the involvement of metastases in the
lymph nodes and distant organs by standard classification of the
Union for International Cancer Control/American Joint Committee on
Cancer (UICC/AJCC) classification based upon the
tumor-node-metastasis (TNM) (2).
However, recent reports in the literature indicated that a
considerable heterogeneity of the primary tumor of colon cancer
requires a more detailed qualitative analysis, all of its
components such as inflammatory response and connective tissue
stroma (3,4).
Vascular connective tissue is an important part of
the tumor which forms its framework. It transfers the nutrients
into proliferating, neoplastic cells. Due to certain elements, the
stromal tumor retains its integrity and has the ability to increase
as a destructive parasite on the host organism. The production of
biologically active compounds can be done during the interaction
between the structural elements of the stroma such as inflammatory
cell infiltration and cancer cells (5). It has been shown that the tumor stroma
including fibroblasts, endothelial cells, and inflammatory cells
play an important role in promoting the progression of the disease
(6,7).
Desmoplasic stromal reaction (DR) is a poor prognostic factor for
patients with CRC. Moreover, the appearance of liver metastases is
accompanied by a large DR with SMA-positive myofibroblasts
(8,9).
Furthermore, Conti et al (10)
found that DR stimulates the growth of primary tumor and decreases
the chemosensitivity of CRC metastasis in the liver.
Tumor stroma, as myofibroblasts, may affect the
organization of the inflammatory response. Dysregulation of the
immune response is already visible in the early stages of
precancerous adenoma-colorectal carcinoma sequence in which
decrease in the activity of Th1 cells is observed (11). By contrast, the development of CRC on
the basis of inflammatory bowel disease is characterized by the
stimulation of immune responses by CD3+ T-cells
(12). Recent reports showed that
both the inflammatory cell infiltration and tumor stroma affect the
development and progression of CRC (13). Therefore, the aim of our study was to
assess inflammatory cell infiltrate in the invasive front and in
the center of primary tumor mass, and tumor stroma percentage (TSP)
in correlation with anatomoclinical features of CRC patients.
Materials and methods
Patients
The study group consisted of 160 patients diagnosed
with colorectal carcinoma (female, 56, male, 88) and operated on at
the Department of Oncological Surgery, in the Comprehensive Cancer
Center of Bialystok, during years 2014–2016. The data collection
procedures and statistical analysis were designed before the
collection of study material had started. The mean age was 67.5
years, including 40 patients under 60 years of age and 120 patients
over 6 decades of life. Mostly, patients were complained about
abdominal ache, anemia and bleeding from rectum. Family's medical
history of malignant neoplasms was noted in 16 out of 160 cases.
Patients were taking medicine against hypertension, type II
diabetes, osteoarthritis and coronary heart disease in most of
cases. We excluded patients with clinical evidence of active
infection and/or chronic inflammatory condition. Colonoscopy
examination was performed in 62 cases that confirmed the presence
of cancerous infiltrate in the intestinal wall. Macroscopically,
cancerous infiltrate was limited to the gut wall in 69 cases,
exceeded the wall focally in 17 cases and continuously in 74
cases.
All patients, during routine diagnostics, underwent
a basic diagnostic laboratory examination, ECG, spirometry,
arterial blood gasometric study and X-ray and computerized
tomography of the chest. The clinical efficiency was performed by
5-point scale of Zubroda (WHO) (14).
The clinical staging of CRC was evaluated according to TNM
classification (2). Patients
diagnosed with neoplasms in rectum received preoperative therapy
(N=53). Patients received radiotherapy (N=39), chemotherapy (N=7)
and radio-chemotherapy (N=7). They took a dose of 25 Gy in
fractions of 5 Gy during one week in the pelvic area. Patients with
tumors situated on other localization had received neither
inflammatory nor immunosuppressive therapy. The response to
preoperative therapy was estimated according to the Response
Evaluation Criteria in Solid Tumors (RECIST) criteria (15).
The study was performed in conformity with the
Declaration of Helsinki for Human Experimentation and the protocol
was approved by the Bioethics Committee of the Medical University
of Bialystok (no. R-I-002/353/2016). Written informed consent was
obtained from all participants.
Histopathological examination of CRC
tumor
Sections, 4 µm-thick, were cut from paraffin blocks
and stained with hematoxylin and eosin (H&E) (cat no.
468802128; POCH S.A., Poland). The routine histopathological
assessment of the sections referred to type of tumor growth, tumor
size, histological type and percentage of the mucinous component,
grade of malignancy, pTNM and Duke stages. We also analyzed venous,
lymphatic and perineural invasions, characteristic features of
lymph node invasion such as number of resected and invaded lymph
nodes, the presence of micro- and macrometastases, invasion of the
pouch lymph node; presence of the distant metastases and their size
in millimetre. We also assessed the presence of deposits, their
number and size in millimetre (16).
We analyzed tumor budding according to Morodomi et al
(17). The extent of necrosis and
fibrosis in the central tumor was evaluated according to Richards
et al (18) and graded as
‘absent’ (none), ‘focal’ (<10% of tumor area), ‘moderate’
(10–30%) or ‘extensive’ (>30%). Crohn's-like aggregates of
lymphocyte (CRL) were performed in the basis of Väyrynen's et
al criteria (19). Histological
categorization of fibrotic cancer stroma was performed based on
classification described by the Ueno et al (20).
Examination of inflammatory cell
infiltration, TSP and Glasgow microenvironment score (GMS)
The inflammatory cell infiltrates were assessed
according to Klintrup-Makinen (K-M) (21) criteria and performed by two
independent pathologists who have been blinded to the clinical
information. Briefly, inflammatory reaction in the invasive margin
and centre of tumor were scored on 4-point scale where score ‘0’
defined no increase in inflammatory cell infiltrate; score ‘1’
defined a mild or patchy increase; score ‘2’ denoted a prominent
inflammatory reaction with some evidence of cancer cell destruction
and score ‘3’ denoted florid ‘cup-like’ inflammatory infiltrate.
The inflammatory cell infiltrate were classified into low-group
(score 0–1) and high-group (score 2–3). Invasive front of tumor was
defined as the most progressed few cancer cells localized on the
advanced edge of tumor. We assessed the TSP according to criteria
described by Huijbers et al (22). TSP ratio was dived into two groups:
‘high TSP group’ (>50%) and ‘low TSP group’ (≤50%). We also
analyzed the GMS (4) based on the K-M
grade and TSP. Patients were characterized as having, i) good
prognosis (high score of K-M and any TSP score); ii) intermediate
prognosis (low K-M score and low TSP score) and iii) poor prognosis
(low K-M score and high TSP score).
Follow-up
Patients were followed-up during last 2–2.5 years.
They were monitored by the measurement of carcinoembryonic antigen
(CEA) and CA19-9 levels, physical examination, colonoscopy or/and
radiological imaging including computerized tomography of the
chest, abdomen, and pelvis, bone scan, and positron emission
tomography scans. Local and distant recurrences were defined as
pathologic evident of the spread of tumors in the region of the
anastomosis (local recurrence) or/and present outside of the
primary tumor at other sites such as liver, lungs, bones, brain
(distant recurrence) and confirmed by mentioned above
techniques.
Statistical analysis
Statistical analysis was conducted using the
STATISTICA 10.0 program (StatSoft, Kracow, Poland). Mann-Whitney
U-test was use to compare the groups. Correlations between the
parameters were calculated by the Spearman's correlation
coefficient tests. Disease-free survival (DFS) was calculated from
the date of diagnosis to the date of disease progression (local or
distant relapse). DFS were estimated using Kaplan Meier method and
the survival curves were compared using log-rank tests.
Multivariate Cox proportional hazards models were used to estimate
hazard ratios. A P-value of <0.05 was considered statistically
significant.
Results
Distribution of inflammatory cell
infiltrates and characteristics of TSP and GMS in CRC
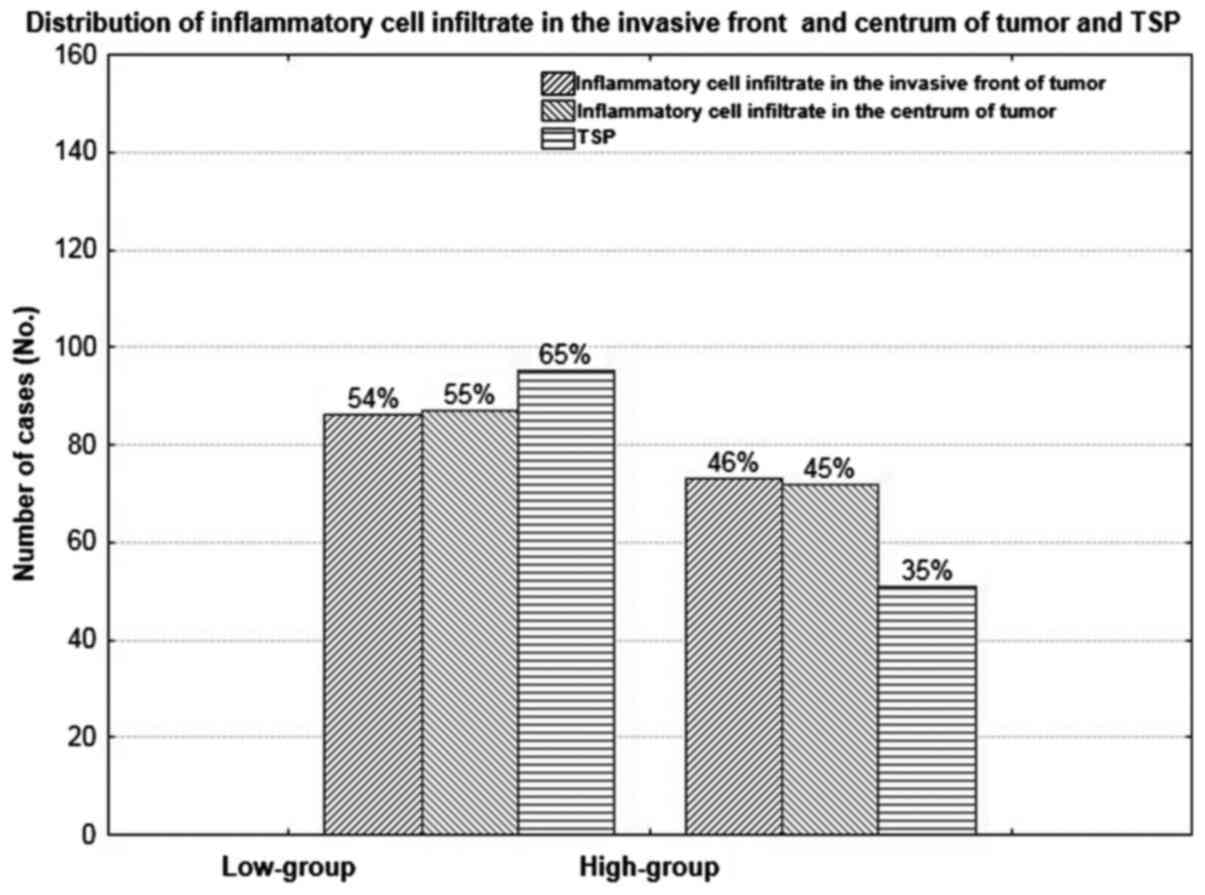
The inflammatory cell infiltrate in the invasive
front of tumor was low in 86 (54%) and high in 73 (46%) of cases,
and was similar to those observed in the centrum of tumor mass.
Low-group of TSP was present in 95 (65%) cases in comparison with
51 (35%) cases observed in high TSP group (Figs. 1–3).
Examined group of parameters did not differ significantly (P=0.059;
P=0.065; P=0.910). Patient prognosis, based on GMS was: good in 55,
intermediate in 49 and poor in 56 cases.
Inflammatory cell infiltrates in the
invasive front of CRC and its correlation with anatomoclinical
variables
Inflammatory cell infiltrate in the invasive front
was found to correlate negatively with female (P=0.018, R=−0.197),
venous and lymphatic invasion (P=0.020, R=−0.193; P=0.038,
R=−0.173, respectively), invasion of lymph node pouch (P=0.020,
R=−0.212), TSP (P=0.015, R=−0.212) and the stage of fibrosis
(P<0.000, R=−0.293). The increase of the inflammatory cell
infiltrate in the invasive front of tumor was associated with
increase of stromal maturation (P=0.004, R=0.238) (Tables I and II).
 | Table I.Correlation between inflammatory cell
infiltration in the invasive front and main mass of primary tumor
and anatomoclinical variables of colorectal cancer. |
Table I.
Correlation between inflammatory cell
infiltration in the invasive front and main mass of primary tumor
and anatomoclinical variables of colorectal cancer.
|
|
| Inflammatory cell
infiltration in the invasive front of tumor | Inflammatory cell
infiltration in the center of tumor mass |
|---|
|
|
|
|
|
|---|
| Variables | N 160 | R | P-value | R | P-value |
|---|
| Age |
|
|
|
|
|
|
<60 | 40 | NS | NS | NS | NS |
|
>60 | 120 |
|
|
|
|
| Gender |
|
|
|
|
|
|
Female | 64 | -0.197 | 0.018 | NS | NS |
| Male | 96 |
|
|
|
|
| Localization |
|
|
|
|
|
|
Right-side | 20 | NS | NS | NS | NS |
|
Transverse | 14 |
|
|
|
|
|
Left-side | 15 |
|
|
|
|
|
Sigmoid | 29 |
|
|
|
|
|
Rectum | 82 |
|
|
|
|
| Tumor growth |
|
|
|
|
|
|
Expanding | 133 | NS | NS | NS | NS |
|
Infiltrate | 27 |
|
|
|
|
| Tumor size, cm |
|
|
<2.5 | 27 | NS | NS | NS | NS |
|
2.5–5.0 | 106 |
|
|
|
|
|
>5.0 | 27 |
|
|
|
|
| TNM stage |
|
|
|
|
|
| 1 | 42 | NS | NS | -0.200 | 0.012 |
| 2 | 31 |
|
|
|
|
| 3 | 69 |
|
|
|
|
| 4 | 18 |
|
|
|
|
| Duke stage |
|
|
|
|
|
| A | 39 | NS | NS | -0.218 | 0.009 |
| B | 35 |
|
|
|
|
| C | 69 |
|
|
|
|
| D | 17 |
|
|
|
|
| Adenocarcinoma
type |
|
|
|
|
|
| Partim
muc | 30 | NS | NS | NS | NS |
|
Nonmuc | 130 |
|
|
|
|
| Percentage of
mucinous component |
|
|
|
|
|
|
10–30% | 15 | NS | NS | NS | NS |
|
30–50% | 15 |
|
|
|
|
| Grade of
malignancies |
|
|
|
|
|
| 2 | 148 | NS | NS | NS | NS |
| 3 | 12 |
|
|
|
|
| Preoperative
treatment |
|
|
|
|
|
|
Yes | 53 | NS | NS | NS | NS |
| No | 107 |
|
|
|
|
| Treatment
response |
|
|
|
|
|
| SD | 26 | NS | NS | NS | NS |
| PR | 27 |
|
|
|
|
| pT stage |
|
|
|
|
|
| 1 | 3 | NS | NS | -0.175 | 0.036 |
| 2 | 62 |
|
|
|
|
| 3 | 91 |
|
|
|
|
| 4 | 4 |
|
|
|
|
 | Table II.Correlation between inflammatory cell
infiltration in the invasive front and main mass of primary tumor
and morphological variables of colorectal cancer. |
Table II.
Correlation between inflammatory cell
infiltration in the invasive front and main mass of primary tumor
and morphological variables of colorectal cancer.
|
|
| Inflammatory cell
infiltration in the invasive front of tumor | Inflammatory cell
infiltration in the center of tumor mass |
|---|
|
|
|
|
|
|---|
| Variables | N 160 | R | P-value | R | P-value |
|---|
| Venous
invasion |
|
|
|
|
|
|
Absent | 113 | -0.193 | 0.020 | NS | NS |
|
Present | 46 |
|
|
|
|
| Lymphatic
invasion |
|
|
|
|
|
|
Absent | 121 | -0.173 | 0.038 | NS | NS |
|
Present | 38 |
|
|
|
|
| Perineural
invasion |
|
|
|
|
|
|
Absent | 143 | NS | NS | -0.191 | 0.022 |
|
Present | 17 |
|
|
|
|
| No. of removed
lymph nodes |
|
|
|
|
|
|
<5 | 13 | NS | NS | NS | NS |
|
5–10 | 29 |
|
|
|
|
|
>10 | 116 |
|
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
Absent | 81 | NS | NS | -0.230 | 0.005 |
|
Present | 79 |
|
|
|
|
| Type of lymph node
metastasis |
|
|
|
|
|
|
Micro | 27 | NS | NS | -0.198 | 0.017 |
|
Macro | 52 |
|
|
|
|
| Number of
metastatic lymph nodes |
|
|
|
|
|
|
<5 | 49 | NS | NS | -0.323 | 0.012 |
|
>5 | 26 |
|
|
|
|
| Lymph node pouch
invasion |
|
|
|
|
|
|
Absent | 11 | -0.212 | 0.010 | -0.191 | 0.021 |
|
Present | 68 |
|
|
|
|
| Distant
metastasis |
|
|
|
|
|
|
Absent | 143 | NS | NS | NS | NS |
|
Present | 17 |
|
|
|
|
| Distant metastasis
size (mm) |
|
|
|
|
|
|
<10 | 11 | NS | NS | NS | NS |
|
>10 | 6 |
|
|
|
|
| Tumor
deposits |
|
|
|
|
|
|
Absent | 133 | NS | NS | NS | NS |
|
Present | 27 |
|
|
|
|
| Size of tumor
deposits (mm) |
|
|
|
|
|
|
<2.5 | 10 | NS | NS | NS | NS |
|
>2.5 | 17 |
|
|
|
|
| TSP (%) |
|
|
|
|
|
|
<50 | 94 | -0.212 | 0.015 | NS | NS |
|
>50 | 66 |
|
|
|
|
| Tumor budding |
|
|
|
|
|
|
Absent | 94 | NS | NS | NS | NS |
|
Present | 66 |
|
|
|
|
| Crohn's-like
aggregates of lymphocyte |
|
|
|
|
|
|
Absent | 113 | NS | NS | 0.195 | 0.019 |
|
Present | 42 |
|
|
|
|
| Necrosis |
|
|
|
|
|
|
Absent | 45 | NS | NS | NS | NS |
|
Focal | 61 |
|
|
|
|
|
Moderate | 36 |
|
|
|
|
|
Extensive | 18 |
|
|
|
|
| Fibrosis |
|
|
|
|
|
|
Absent | 11 | -0.293 | 0.000 | NS | NS |
|
Focal | 72 |
|
|
|
|
|
Moderate | 43 |
|
|
|
|
|
Extensive | 34 |
|
|
|
|
| Maturation of
fibrotic stroma |
|
|
|
|
|
|
Immature | 12 | 0.238 | 0.004 | 0.256 | 0.002 |
|
Intermediate | 91 |
|
|
|
|
|
Mature | 57 |
|
|
|
|
Inflammatory cell infiltrates in the
centrum of the mass of CRC in correlation with anatomoclinical
variables
Inflammatory cell infiltrate in the centrum of the
tumor mass was associated with parameter response for disease
progression. Inflammatory cell infiltrate in this localization in
tumor was negatively correlated with TNM and Duke stage (P=0.012,
R=−0.200; P=0.009, R=−0.218), pT stage (P=0.036, R=−0.175),
invasion of perineural structures (P=0.022, R=−0.191), lymph node
status (P=0.005, R=−0.230), type of lymph nodes (P=0.017,
R=−0.198), number of metastatic lymph nodes (P=0.012, R=−0.323) and
the invasion of lymph node pouches (P=0.021, R=−0.151).
Cronh's-like aggregates of lymphocyte and maturation of fibrotic
stroma were positively associated with the increase of inflammatory
cell infiltrate in the centre of tumor mass (P=0.019, R=0195;
P=0.002, R=0.256, respectively). Results of correlation are showed
in Tables I and II.
Inflammatory cell infiltrates TSP and
GMS in CRC DFS
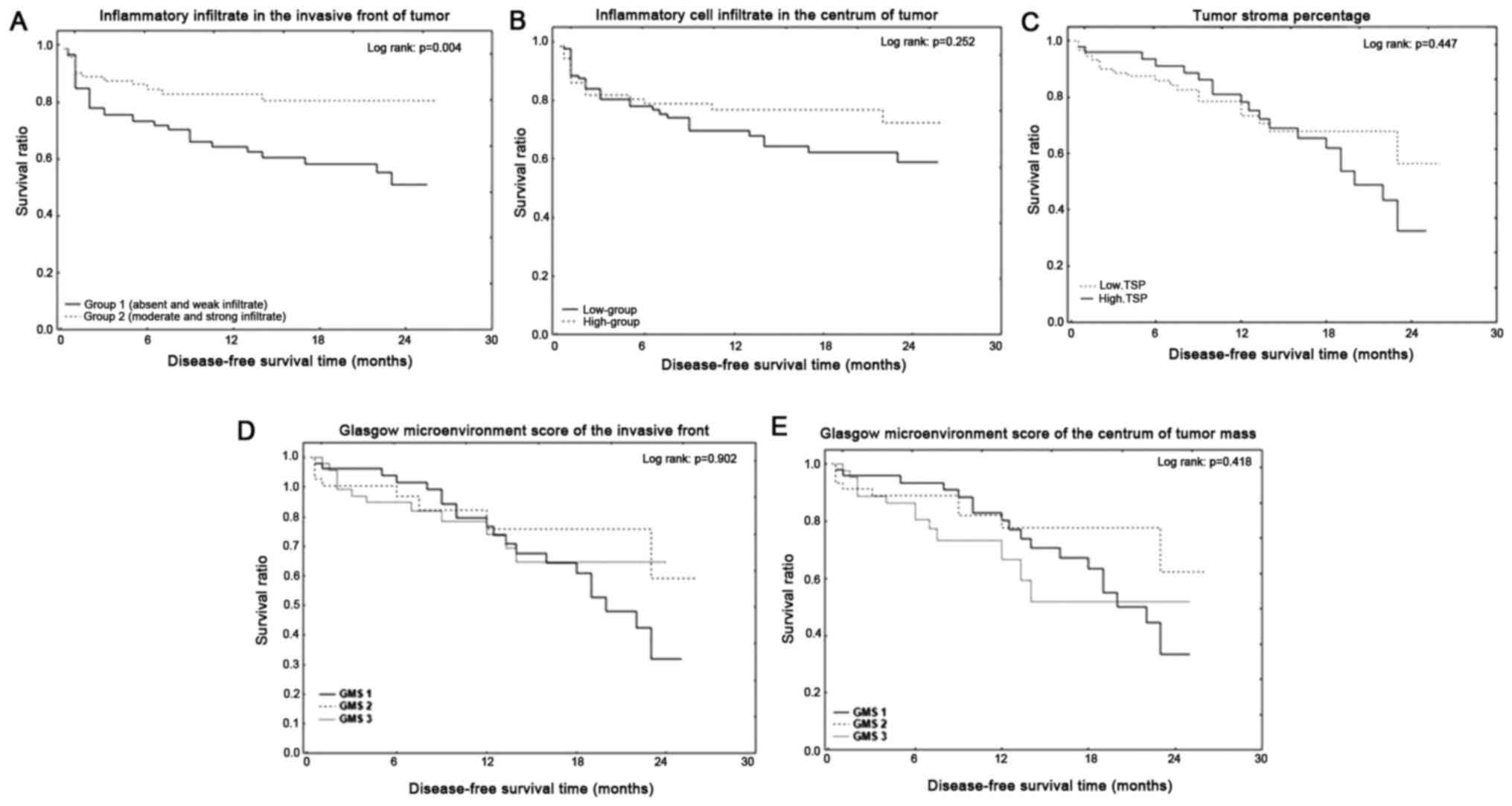
Low-group who showed an absent or weak inflammatory
cell infiltrate in the invasive front of the tumor had
statistically significant shorter DFS (P=0.004). The 1-year and
2-year DFS of the low group with inflammatory cell infiltrate in
the invasive front of the tumor were 64 and 51%, whereas patients
with high inflammatory cell infiltrate had 1- and 2-year DFS of 83
and 80%. DFS did not differ in the inflammatory cell infiltrate in
the centrum of the tumor mass, TSP and GMS (P=0.252, P=0.447,
P=0.902, P=0.418) (Fig. 4). Low TSP
group had 1-year DFS of 76% and 2-year DFS of 56%, whereas patients
with high TSP group had 1-year DFS of 78% and 2-year DFS of 35%.
Univariate analysis showed that preoperative treatment (P=0.048),
type of lymph nodes (P=0.039), number of tumor deposits (P=0.025)
have prognostic values. Moreover, the multivariate Cox-analysis
proved that inflammatory cell infiltrate in the invasive front was
an independent predictive factors in CRC (P=0.041) (Table III).
 | Table III.Prognostic factors in patients with
CRC. |
Table III.
Prognostic factors in patients with
CRC.
| Variables | Univariate
p-value | Multivariate
p-value | HR (95% CI) |
|---|
| Age (≤60 vs.
≥60) | 0.059 | – | 1.21
(0.36–1.53) |
| Gender (female vs.
male) | 0.597 | – | 2.19
(1.88–3.53) |
| Tumor growth
(expanding vs. infiltrate) | 0.288 | – | 1.68
(1.06–1.90) |
| Tumor size (<2.5
vs. 2.5–5 vs. >5 cm) | 0.349 | – | 0.77
(0.27-.87) |
| TNM stage
(I–IV) | 0.258 | – | 0.85
(0.13–1.27) |
| Duke stage
(A-D) | 0.628 | – | 1.22
(0.42–1.45) |
| Adenocarcinoma type
(nonmuc. vs. partim mucin) | 0.359 | – | 0.62
(0.51–0.84) |
| Grade of
malignancies (2 vs. 3) | 0.220 | – | 0.39
(0.23–1.49) |
| Preoperative
treatment (yes vs. no) | 0.048 | 0.784 | 1.05
(0.75–1.52) |
| pT stage (1–4) | 0.674 | – | 1.00
(0.17-.1.2) |
| Venous invasion
(yes vs. no) | 0.109 | – | 2.72
(1.62–2.93) |
| Lymphatic invasion
(yes vs. no) | 0.149 | – | 0.36
(0.23–2.08) |
| Perineural invasion
(yes vs. no) | 0.121 | – | 2.30
(1.54–2.40) |
| No. of removed
lymph nodes (<5 vs. 5–10 vs. <10) | 0.816 | – | 0.94
(0.05–1.42) |
| Lymph node
metastasis (yes vs. no) | 0.079 | – | 1.47
(0.92–3.07) |
| Type of lymph node
metastasis (micro vs. macro) | 0.039 | 0.103 | 1.21
(0.19–2.65) |
| Number of
metastatic lymph nodes (<5 vs. >5) | 0.951 | – | 0.96
(0.61–1.22) |
| Lymph node pouch
invasion (yes vs. no) | 0.374 | – | 0.55
(0.45–0.78) |
| Distant metastasis
(yes vs. no) | 0.702 | – | 0.96
(0.14–1.23) |
| Distant metastasis
size <10 vs. >10 mm | 0.637 | – | 1.05
(0.22–1.24) |
| Tumor deposits (yes
vs. no) | 0.099 | – | 0.53
(0.37–2.72) |
| Tumor budding (yes
vs. no) | 0.267 | – | 0.65
(0.47–0.77) |
| Number of tumor
budding | 0.025 | 0.059 | 1.05
(0.57–3.53) |
| Fibrosis (low vs.
high) | 0.524 | – | 1.25
(0.40–1.48) |
| Necrosis (low vs.
high) | 0.615 | – | 0.84
(0.25–1.12) |
| Maturation of tumor
stroma (low vs. high) | 0.471 | – | 0.83
(0.51–1.26) |
| Inflammatory cell
infiltrate in the invasive front of tumor (present vs. absent) | 0.037 | 0.041 | 0.50
(0.33–4.14) |
| Inflammatory cell
infiltrate in center of tumor (present vs. absent) | 0.733 | – | 0.92
(0.23–1.56) |
| TSP (low vs.
high) | 0.054 | – | 0.46
(0.35–4.66 |
Discussion
Inflammatory infiltration located in both the
invasive front and in the center of the primary tumor may play a
significant role in the development of malignant tumors. In our
study, we noted the lack of weak inflammatory infiltration in the
invasive front and in center of the tumor in 50% of cases
comparable to a medium or large infiltration in both locations
(45–46%). Richards et al (18)
reported a low-grade inflammatory cell infiltrate in 48% and
high-grade inflammatory cell infiltrate in 52% cases in peritumoral
stroma. We also noted low-TSP in 65% of cases and high-TSP in 35%
of cases. Also, Park et al (23) observed that the TSP was low in 75% of
cases and high in 25% of cases. These observations confirmed that
the presence of inflammatory infiltrate may be different in the
cases of CRC patients. Probably, it is determined by the activity
of the immune system, the speed of its reorganization during
detection of tumor-associated antigen (TAA), the preoperative
treatment modulating pathway of inflammatory response or the
ability of tumor cells to produce specific antigens directly
blocking immunocompetitive cells.
Inhibition or impartation of the inflammatory
response allows malignant tumor cells to invade into the tissue. We
confirmed such observation by correlations, in which together with
the decrease in the inflammatory response increased tumor stage,
TNM, and Duke's stage, including the primary tumor stage (pT), the
presence of tumor cell emboli in blood and the lymphatic vessels,
in perineutral spaces. Moreover, the degree of inflammatory
infiltration was negatively correlated with the the presence of
lymph node metastasis, its size, exceeding the lymph pouches and
infiltration structures near to metastatic lymph nodes. Our results
are consistent with the observations of Galon et al
(12), Menon et al (24) and Väyrynen et al (25). They confirmed that patients with high
TNM stage linked with presence of distant metastases were
correlated with lower immune response. Moreover, authors showed
that peritumoral inflammatory cell infiltrate was higher in
advanced stage than in intratumoral densities. Also, Richards et
al (18,26) demonstrated the presence of the
relationship between the inflammatory cell infiltrate and pT
status, positive lymph node status, TNM stage, venous invasion,
necrosis and character of tumor growth.
Several studies confirmed an association between
tumor inflammatory infiltrates and survival of patients with
malignant neoplasms (27–29). In our study, we showed that patients
with low inflammatory cell infiltrate located in the invasive front
had a shorter DFS), which was 64% after 12 months and 51% after 24
months after the surgery. Mei et al (13) showed that a high level of
CD3+ cells in the invasive front was associated with
good overall survival (OS) and DFS. Moreover, a high level of
CD8+ cells, but not CD3+ or FOXP3+
was correlated with better prognosis and longer OS. Also, Väyrynen
et al (25) confirmed the
relationship between the degree of inflammatory infiltration
assessed in the basis of M-K criteria and the occurrence of relapse
in patients with CRC. We also analyzed the relationship between
TSP, GMS and DFS of patients with CRC. Patients with low-TSP had
1-year DFS of 76% whereas the 2-year DFS of patients with high TSP
was 56%. Unfortunately, the differences were not statistically
significant, in contrast to the results of Park et al
(23) who reported a shorter
cancer-specific survival (CSS) in CRC patients in stage I–III of
the high-TSP compared to those in which low-TSP was found. In
subsequent studies, the author confirmed that the 5-year survival
of low TSP was 80% and in the high TSP group in 90% (4). We also assessed the overall parameters
of inflammatory cell infiltrate and TSP by GMS, which did not
confirmed statistically significant differences in DFS. Our
observations are contrary to the results of Park et al
(4). These differences may be due to
sample size, nationality of the selected population and the scope
of the TNM staging of patients enrolled in the study.
Multivariate analysis showed that the inflammatory
cell infiltrate in the invasive front of the primary tumor is an
independent prognostic factor in patients with CRC. Richards et
al (26) presented that a low
grade of local immune response, TNM, venous invasion were
associated indecently with reduced CSS. On the other hand, Park
et al (23) demonstrated that
low TSP in stage I to III of patients with CRC is associated with
N0 status and those who received adjuvant chemotherapy had reduced
CSS. It seems that the inflammatory cell infiltration is a very
important part of the tumor, which, along with routinely assessed
morphological, may provide additional prognostic factor in patients
with CRC.
In conclusion, the degree of inflammatory cell
infiltration in the invasive front of the primary tumor and
especially TSP of patients with CRC affects significantly the
variables that determine disease progression and DFS. Moreover, the
routine, histopathological assessment of both parameters in the
basis of tissue material stained with H&E may have potential
diagnostic and prognostic values.
References
|
1
|
Kubiak A, Kycler W and Trojanowski M:
Epidemiology and prevention of colorectal cancer in Poland. Probl
Hig Epidemiol. 95:636–642. 2014.
|
|
2
|
Hamilton SR and Aaltonen LA: Tumours of
the colon and rectum. In: World Health Organization Classification
of TumoursPathology and Genetics of Tumours of the Digestive
System. IARC Press; Lyon: pp. 103–104. 2000
|
|
3
|
Park JH, McMillan DC, Edwards J, Horgan PG
and Roxburgh CS: Comparison of the prognostic value of measures of
the tumor inflammatory cell infiltrate and tumor-associated stroma
in patients with primary operable colorectal cancer.
Oncoimmunology. 21:e10988012016. View Article : Google Scholar
|
|
4
|
Park JH, McMillan DC, Powell AG, Richards
CH, Horgan PG, Edwards J and Roxburgh CS: Evaluation of a tumor
microenvironment-based prognostic score in primary operable
colorectal cancer. Clin Cancer Res. 21:882–888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dvorak HF: Tumor stroma, tumor blood
vessels, and antiangiogenesis therapy. Cancer J. 21:237–243. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conti J and Thomas G: The role of tumour
stroma in colorectal cancer invasion and metastasis. Cancers
(Basel). 3:2160–2168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Wever O, Demetter P, Mareel M and
Bracke M: Stromal myofibroblasts are drivers of invasive cancer
growth. Int J Cancer. 123:2229–2238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crispino P, De Toma G, Ciardi A, Bella A,
Rivera M, Cavallaro G, Polistena A, Fornari F, Unim H, Pica R, et
al: Role of desmoplasia in recurrence of stage II colorectal cancer
within 5 years after surgery and therapeutic implication. Cancer
Invest. 26:419–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoong KF, Afford SC, Randhawa S, Hubscher
SG and Adams DH: Fas/Fas ligand interaction in human colorectal
hepatic metastases: A mechanism of hepatocyte destruction to
facilitate local tumor invasion. Am J Pathol. 154:693–703. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conti JA, Kendall TJ, Bateman A, Armstrong
TA, Papa-Adams A, Xu Q, Packham G, Primrose JN, Benyon RC and
Iredale JP: The desmoplastic reaction surrounding hepatic
colorectal adenocarcinoma metastases aids tumor growth and survival
via alphav integrin ligation. Clin Cancer Res. 14:6405–6413. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui G, Goll R, Olsen T, Steigen SE,
Husebekk A, Vonen B and Florholmen J: Reduced expression of
microenvironment Th1 cytokines accompanies adenomas-carcinomas
sequence of colorectum. Cancer Immunol Immunother. 56:985–995.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang
G, Peng H, Cui L and Li C: Tumour-infiltrating inflammation and
prognosis in colorectal cancer: Systematic review and
meta-analysis. Br J Cancer. 110:1595–1605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group'. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin Q, Wei Y, Ren L, Zhong Y, Qin C, Zheng
P, Xu P, Zhu D, Ji M and Xu J: Tumor deposit is a poor prognostic
indicator in patients who underwent simultaneous resection for
synchronous colorectal liver metastases. Onco Targets Ther.
8:233–240. 2015.PubMed/NCBI
|
|
17
|
Morodomi T, Isomoto H, Shirouzu K,
Kakegawa K, Irie K and Morimatsu M: An index for estimating the
probability of lymph node metastasis in rectal cancers. Lymph node
metastasis and the histopathology of actively invasive regions of
cancer. Cancer. 63:539–543. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Richards CH, Flegg KM, Roxburgh CS, Going
JJ, Mohammed Z, Horgan PG and McMillan DC: The relationships
between cellular components of the peritumoural inflammatory
response, clinicopathological characteristics and survival in
patients with primary operable colorectal cancer. Br J Cancer.
106:2010–2015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Väyrynen JP, Sajanti SA, Klintrup K,
Mäkelä J, Herzig KH, Karttunen TJ, Tuomisto A and Mäkinen MJ:
Characteristics and significance of colorectal cancer associated
lymphoid reaction. Int J Cancer. 134:2126–2135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ueno H, Jones AM, Wilkinson KH, Jass JR
and Talbot IC: Histological categorisation of fibrotic cancer
stroma in advanced rectal cancer. Gut. 53:581–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klintrup K, Mäkinen JM, Kauppila S, Väre
PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ and
Mäkinen MJ: Inflammation and prognosis in colorectal cancer. Eur J
Cancer. 41:2645–2654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huijbers A, Tollenaar RA, Pelt VGW,
Zeestraten EC, Dutton S, McConkey CC, Domingo E, Smit VT, Midgley
R, Warren BF, et al: The proportion of tumor-stroma as a strong
prognosticator for stage II and III colon cancer patients:
Validation in the VICTOR trial. Ann Oncol. 24:179–185. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JH, Richards CH, McMillan DC, Horgan
PG and Roxburgh CS: The relationship between tumour stroma
percentage, the tumour microenvironment and survival in patients
with primary operable colorectal cancer. Ann Oncol. 25:644–651.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Menon AG, Janssen-van Rhijn CM, Morreau H,
Putter H, Tollenaar RA, van de Velde CJ, Fleuren GJ and Kuppen PJ:
Immune system and prognosis in colorectal cancer: A detailed
immunohistochemical analysis. Lab Invest. 84:493–501. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Väyrynen JP, Tuomisto A, Klintrup K,
Mäkelä J, Karttunen TJ and Mäkinen MJ: Detailed analysis of
inflammatory cell infiltration in colorectal cancer. Br J Cancer.
109:1839–1847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Richards CH, Roxburgh CS, Anderson JH,
McKee RF, Foulis AK, Horgan PG and McMillan DC: Prognostic value of
tumour necrosis and host inflammatory responses in colorectal
cancer. Br J Surg. 99:287–294. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakano O, Sato M, Naito Y, Suzuki K,
Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H and Ohtani H:
Proliferative activity of intratumoral CD8(+) T-lymphocytes as a
prognostic factor in human renal cell carcinoma: Clinicopathologic
demonstration of antitumor immunity. Cancer Res. 61:5132–5136.
2001.PubMed/NCBI
|
|
28
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato E, Olson SH, Ahn J, Bundy B,
Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone
C, et al: Intraepithelial CD8+ tumor-infiltrating
lymphocytes and a high CD8+/regulatory T cell ratio are
associated with favorable prognosis in ovarian cancer. Proc Natl
Acad Sci USA. 102:18538–18543. 2005. View Article : Google Scholar : PubMed/NCBI
|