Introduction
Epithelial-mesenchymal transition (EMT) is a
cornerstone phenomenon in which epithelial cells lose cell polarity
and their cell-to-cell adhesion and acquire increased motility and
invasive hallmarks to become mesenchymal-like cells (1). Cells undergoing EMT degrade the
neighboring microenvironment, and migrate from the primary site to
new frontier organs. Several families of transcriptional
repressors, including zinc finger E-box binding homeobox 1 (ZEB1),
Twist, SNAIL, and basic helix-loop-helix factors, have been
identified as direct downregulators of E-cadherin transcription and
representative inducers of EMT (2,3). Among
these molecules, ZEB1 is known as a member of the zinc-finger E-box
binding homeobox (ZFH) family, and this molecule suppresses the
expression of certain microRNAs, such as miR-183, miR-203, and
miR-200 family members, which function as inhibitors of stem-like
hallmarks as well as positive inducers of epithelial
differentiation (4). Also, it has
been reported to be a major transcriptional factor in cancer
progression/metastasis (5). In fact,
earlier studies reported that ZEB1 promotes tumor invasiveness and
metastasis and is correlated with a poorer clinical prognosis in
patients with several solid cancers (6–8). According
to the prior report from Siebzehnrubl et al (9), ZEB1 is an important marker of
glioblastoma recurrence, including the capability of evading
chemotherapy, suggesting that this molecule acts in both
glioblastoma invasion and chemoresistance. In addition, silencing
ZEB1 expression could significantly restore the chemosensitivity of
docetaxel-resistant human lung adenocarcinoma cells as well as
inhibit their migratory ability through reversing the mesenchymal
phenotype (10).
Epithelial ovarian cancer (EOC) is the one of the
most lethal cancers among the gynecologic malignancies worldwide,
with more than 238,700 newly diagnosed cases and 151,900 reported
deaths per year (11). In general,
epithelial ovarian carcinoma (EOC) is a neoplasm originating from
surface epithelial cells of the ovary, and it is called a silent
killer because most patients with this disease are less symptomatic
until the tumor has widely formed metastases in the peritoneal
cavity, systematic lymph nodes, and distant parenchymal organs.
Therefore, a number of EOC patients are frequently diagnosed when
they enter an advanced stage (12).
The majority of patients with EOC are categorized into four
histological subtypes: High/low-grade serous, clear-cell,
endometrioid, and mucinous carcinoma (13). In this context, EOC is biologically
heterogeneous in nature, with different epidemiological and genetic
backgrounds, molecular profiles, and behavioral responses toward
chemotherapy and other treatments (13,14),
resulting in the difficulties in establishing unified, satisfactory
treatments. Moreover, approximately three in four EOC patients show
a favorable initial response to cytotoxic chemotherapy; however
they gradually become chemoresistant, leading to recurrence and
death. Correctively, intrinsic and/or acquired resistance to
chemotherapeutic agents is the primary obstacle in the actual
treatment of patients with EOC. The lack of strategies to cope with
the biological complexity and change to being treatment-refractory
is one of the main causes preventing improvement of the patient
prognosis. Thus, it is crucial to develop more precise and
effective therapies from a biological point of view.
These clinical and molecular backgrounds led us to
hypothesize that ZEB1 plays a central role in cancer progression,
and that positive ZEB1 expression may be a helpful indicator to
predict an unfavorable clinical outcome in patients with EOC. In
the present study, the need for a novel investigation of the
possible correlation between immunostaining expression of ZEB1 and
other clinicopathologic indicators and the oncologic outcome of EOC
patients was proposed.
Patients and methods
Patients and immunohistochemical
staining
The total of 40 ovarian carcinomas were categorized
into the following pathological types: 11 serous, 18 clear cell, 8
endometrioid, and 3 mucinous carcinomas. As the histological types,
we adopted the World Health Organization (WHO) classification
criteria. The clinical stage was assigned according to the
International Federation of Gynecology and Obstetrics (FIGO)
staging system (15,16).
Tissue samples of EOC were collected after obtaining
informed consent from EOC patients who had been surgically treated
at Nagoya University Hospital between 2001 and 2006. The present
study was approved by the Ethics committee of Nagoya University
(Approval no. 1234). Formalin-fixed, paraffin-embedded tissue
sections were cut at a thickness of 4 µm. For heat-induced epitope
retrieval, deparaffinized sections in 0.01 M citrate buffer (Target
Retrieval Solution pH 6.1; Dako Japan Co., Ltd., Tokyo, Japan) were
heated three times at 90°C for 5 min using a microwave oven.
Immunohistochemical staining was performed using the avidin-biotin
immunoperoxidase technique with the Histofine SAB-PO kit (Nichirei,
Tokyo, Japan) according to the manufacturer's protocol, and the
experimental procedure was comprehensively described previously
(3). Sections were incubated at 4°C
for 12 h with primary antibody (anti-rabbit-ZEB1 polyclonal, at a
1:1,000 dilution; Cell Signaling Technology, Inc., Danvers, MA,
USA). The sections were rinsed and incubated for 30 min with
biotinylated anti-rabbit IgG antibody (second antibody). As a
negative control, the primary antibody was replaced with normal
rabbit IgG at an appropriate dilution.
Evaluation of immunohistochemical
staining
For the evaluation of the results of
immunohistochemical staining, 10 fields of each specimen were
selected, and evaluated with both low- (×100) and high-power (×400)
microscopy. Two investigators assessed the slides without knowledge
of the clinicopathologic features and were blinded to each other's
evaluation. The two investigators were in agreement on all the
slides examined. Based on the ZEB1 immunostaining activity, a
four-tiered semiquantitative score was assigned according to the
intensity and area of stained cells as follows: For the evaluation
of ZEB1 expression, the staining intensity was scored as 0
(negative), 1 (weak), 2 (medium), or 3 (strong). Percentage of
staining the area was scored as 0 (0%), 1 (1–10%), 2 (10–50%), and
3 (51% <) relative to the total tumor area. The sum of the
staining intensity and area scores was calculated as the final
score (0–6) for ZEB1. Tumors with a final score of 0, 1–2, 3–4, or
5–6 were classified as showing negative, weakly, moderately, and
strongly positive expression, respectively.
Statistics
The distributions of clinicopathologic factors were
statistically assessed using the Chi-square test or Fisher's exact
test. The Cochran-Armitage test for trend was used to examine
whether the frequency of recurrence was significantly different
with each staining intensity. The recurrence/progression-free
survival (RFS) was defined as the time interval between the date of
surgery and date of the last follow-up or recurrence/progression.
The survival curves were compared employing the Log-rank test.
Survival analysis was conducted using the Kaplan-Meier method. The
prognostic significance of ZEB1 expression concerning other
clinicopathologic variables was assessed using the multivariate
Cox's proportional hazard's analysis. All statistical analyses were
performed with JMP Pro Ver.10.0 (SAS Institute Japan). P<0.05
was considered to indicate a statistically significant
difference.
Results
Patients' characteristics
Patients' characteristics are detailed in Table I. The median (range) age was 52
(22–72) years. The distributions of the FIGO stage were 37.5%
(15/40) stage I, 25.0% (10/40) stage II, 32.5% (13/40) stage III,
and 5.0% (2/40) stage IV, respectively. Of all patients, 38 (95%)
were postoperatively administered more than 3 cycles of
chemotherapy. Two patients (5.0%) did not undergo postoperative
chemotherapy owing to their strong wishes or severe complications.
A total of 6 patients had residual tumor at the initial surgery.
The ZEB1 immunoreactivity was classified into the four scoring
types as described in ‘Patients and methods’ (negative, weakly,
moderately, and strongly positive expressions). Representative
images of each histological feature are shown in Fig. 1.
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
| Characteristics | No. | % |
|---|
| Total | 40 |
|
| Age |
|
|
| Median (range) | 54 (22–72) |
|
| ≤50 | 13 | 32.5 |
|
>50 | 27 | 32.5 |
| FIGO stage |
|
|
| I | 15 | 37.5 |
| II | 10 | 25.0 |
| III | 13 | 32.5 |
| IV | 2 | 5.0 |
| Histological
type |
|
|
|
Serous | 11 | 27.5 |
|
Mucinous | 3 | 7.5 |
|
Endometrioid | 8 | 20.0 |
|
Clear-cell | 18 | 45.0 |
| Surgery |
|
|
| Standard
surgery + RPN | 19 | 47.5 |
| Standard
surgery + intestine resection | 4 | 10.0 |
| Standard
surgerya | 13 | 32.5 |
|
Exploratory laparotomy | 4 | 10.0 |
| Chemotherapy |
|
|
| Taxane
plus platinum | 37 | 92.5 |
|
Conventinal
platinum-based | 1 | 2.5 |
| None | 2 | 5.0 |
|
Recurrence/progression |
|
|
| Yes | 19 | 47.5 |
| No | 21 | 52.5 |
| ZEB1 immunostaining
classification |
|
|
|
Negative | 7 | 17.5 |
| Weakly
positive | 14 | 35.0 |
|
Moderately positive | 11 | 27.5 |
|
Strongly positive | 8 | 20.0 |
In several cases, the immunoexpression of ZEB1 was
found in the stroma as well as carcinoma tissues. Of the 40
carcinomas, negative, weakly, moderately, and strongly positive
ZEB1 immunoexpressions were observed in 7 (17.5%), 14 (35.0%), 11
(27.5%), and 8 (20.0%) patients, respectively.
Table II shows the
association between ZEB1 expression and clinicopathologic
parameters of primary EOC. Two categorized ZEB1 expressions
(negative + weakly, moderately + strongly positive) were not
correlated with any of the clinicopathologic parameters examined:
Age, histological type, FIGO stage, and surgical procedure.
 | Table II.Relationship between the expression
of ZEB1 and clinicopathologic parameters of primary EOC. |
Table II.
Relationship between the expression
of ZEB1 and clinicopathologic parameters of primary EOC.
|
| ZEB1 |
|
|---|
|
|
|
|
|---|
|
| Negative-Weak |
Moderate-Strong |
|
|---|
|
|
|
|
|
|---|
| Parameters | N | % | N | % | P-value |
|---|
| Total | 21 | 52.5 | 19 | 47.5 |
|
| Age |
|
|
|
|
|
|
≤50 | 6 | 15.0 | 7 | 17.5 | 0.737 |
|
>50 | 15 | 37.5 | 12 | 30 |
|
| FIGO |
|
|
|
|
|
|
I+II | 15 | 37.5 | 10 | 25.0 | 0.328 |
|
III+IV | 6 | 15.0 | 9 | 22.5 |
|
| Histological
type |
|
|
|
|
|
|
Serous | 4 | 10.0 | 7 | 17.5 | 0.163 |
|
Mucinous | 3 | 7.5 | 0 | 0 |
|
|
Endometrioid | 3 | 7.5 | 5 | 12.5 |
|
|
Clear-cell | 11 | 27.5 | 7 | 17.5 |
|
| Surgery |
|
|
|
|
|
|
Standard surgery + RPN | 11 | 27.5 | 8 | 20.0 | 0.692 |
|
Standard surgery + intestine
resection | 2 | 5 | 2 |
5.0 |
|
|
Standard surgerya | 7 | 17.5 | 6 | 15.0 |
|
|
Exploratory laparotomy | 1 | 2.5 | 3 |
7.5 |
Oncologic outcome and the extent of
ZEB1 positivity
The median follow-up duration was 94.8, ranging from
3.8–202.0 months in the surviving patients. During this period, 19
patients (47.5%) developed recurrence. The median time to
recurrence was 10.8 months. The five-year RFS rate of all EOC
patients was 52.5%.
Regarding the frequency of recurrence according to
the extent of ZEB1 positivity, there was no patient with negative
expression of ZEB1 who experienced recurrence. Patients with higher
ZEB1 positivity showed a higher rate of recurrence
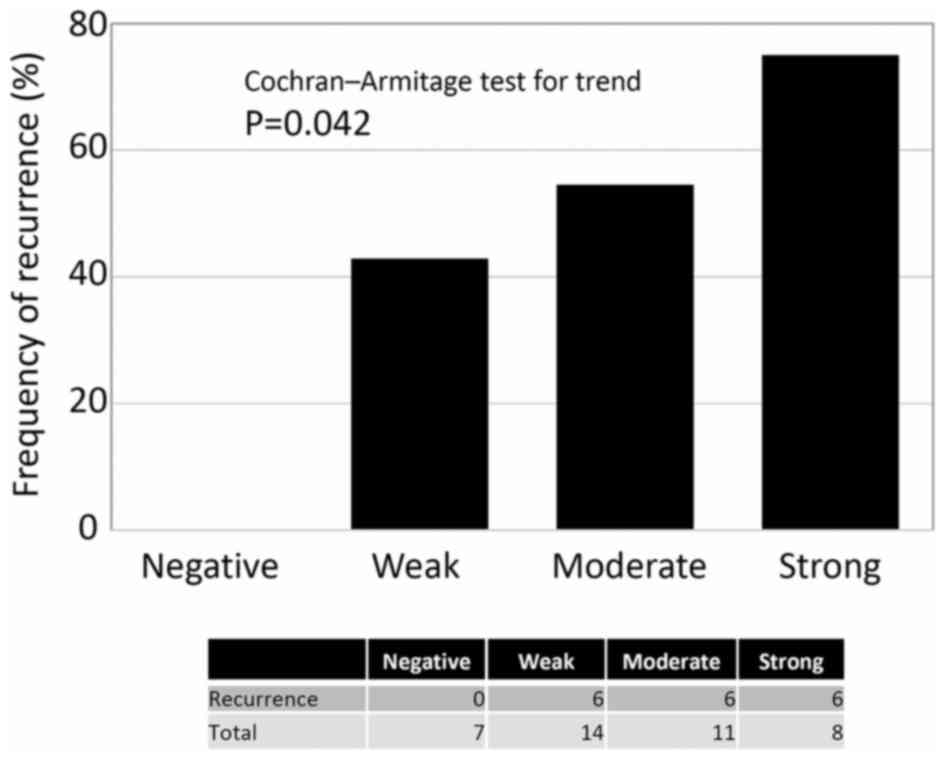
(Cochran-Armitage test for trend, P=0.042) (Fig. 2). Confining analysis to patients with
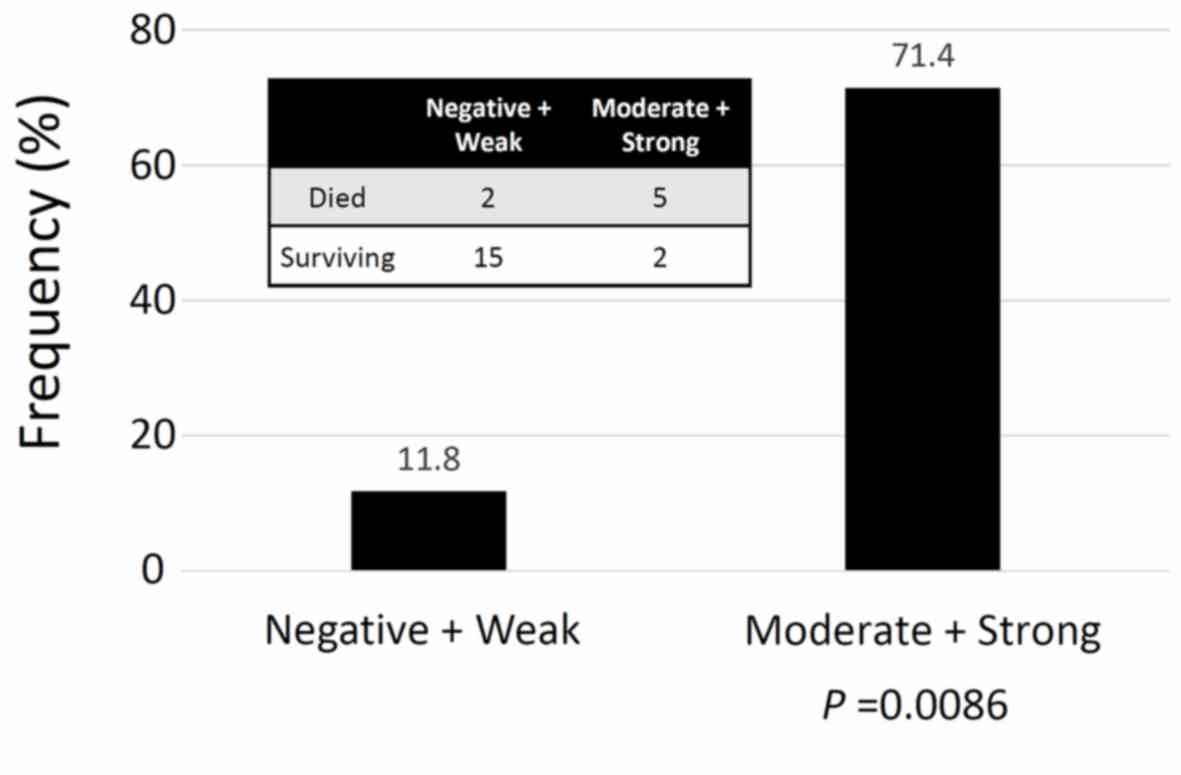
the mucinous/clear-cell histological type, the frequency of an
unfavorable oncologic outcome (death) was higher in patients with
higher ZEB1 positivity (Moderate + Strong expression) (P=0.0086)
(Fig. 3). Furthermore, compared with
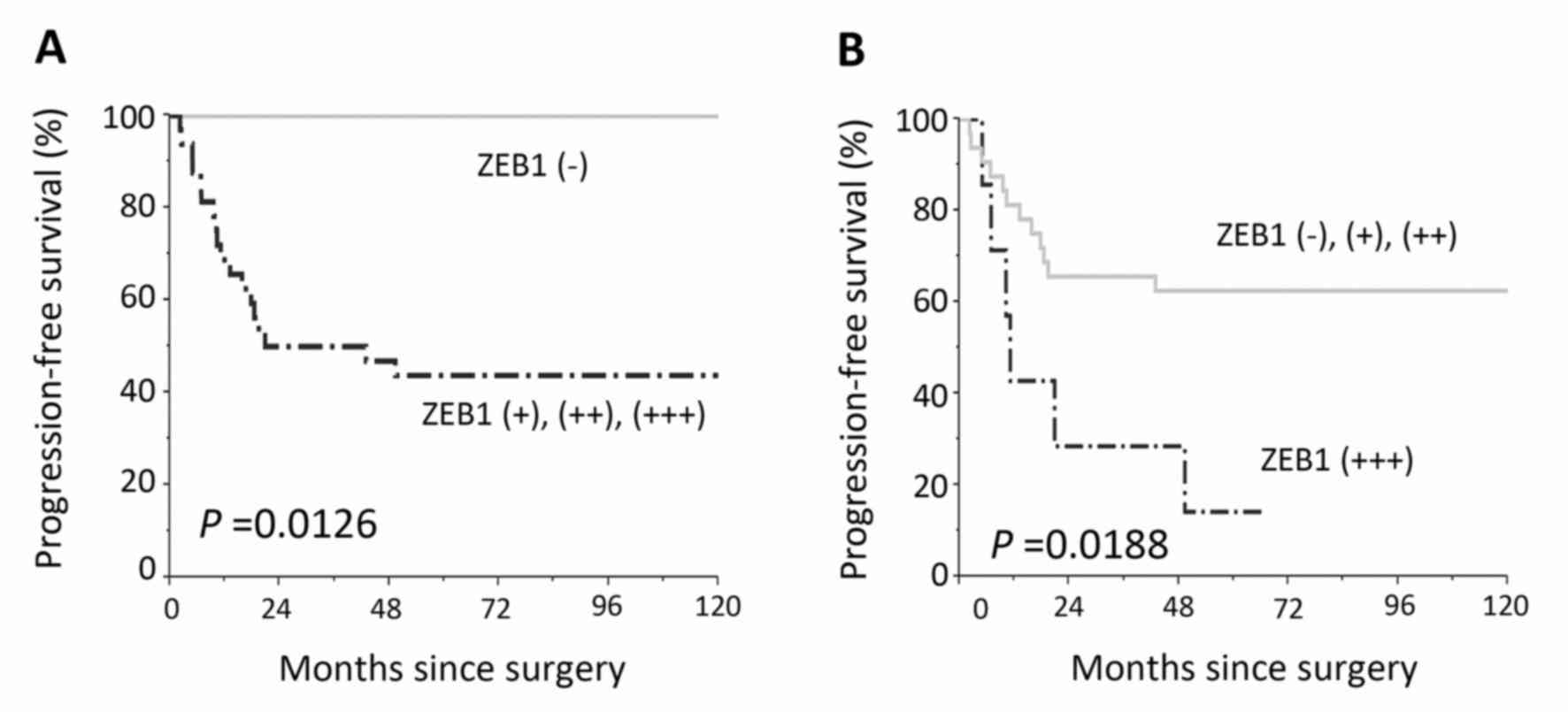
negative expression, two-scale positive ZEB1 expression predicted a
significantly poorer RFS {Negative vs. weak, moderate, and strong
(P=0.002), Negative plus weak vs. moderate plus strong (P=0.001)}
(Fig. 4). Confining analysis to
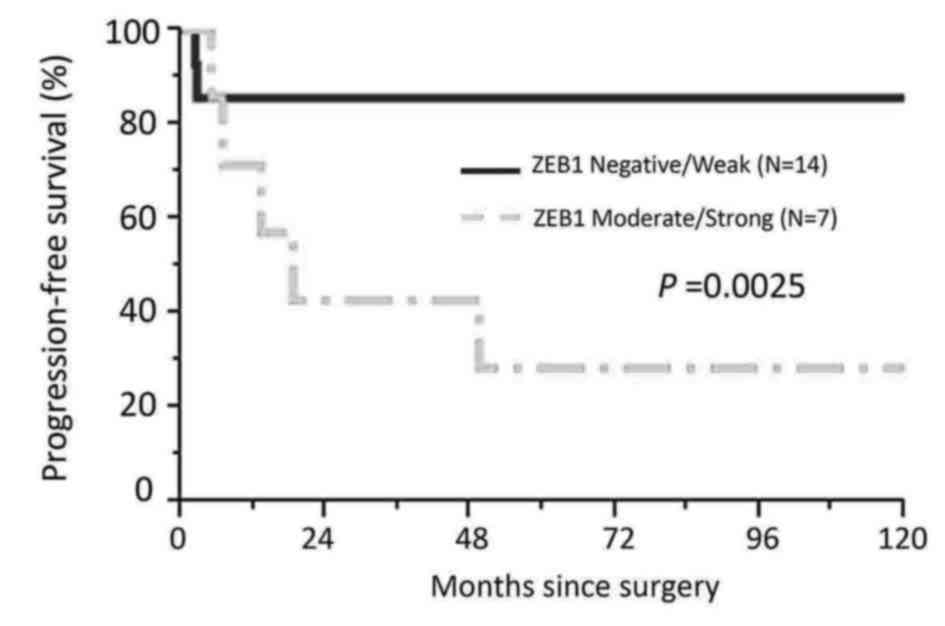
patients with the mucinous/clear-cell histological type, patients
with lower ZEB1 expression (negative/weak) showed poorer RFS than
those with higher ZEB1 expression (moderate/strong) (P=0.0025)
(Fig. 5). In the current survival
analyses, the post-hoc powers we calculated were ranging from 0.565
to 0.786.
Multivariate analysis
In multivariate RFS analyses, age (≤50 vs. >50),
FIGO stage (I+II vs. III+IV), histological type
(serous/endometrioid vs. mucinous/clear-cell), and ZEB1
immunoreactivity (negative/weak vs. moderate/strong) were included
in the Cox proportional hazard analysis. The age, histological
type, and ZEB1 expression were significant independent prognostic
indicators of a poor RFS. The hazard ratio (HR) for
moderately/strongly positive ZEB1 expression was as follows: HR:
2.2265, 95% CI: 1.102–8.021; P=0.0349) (Table III).
 | Table III.Uni- and multivariable analyses of
clinicopathologic parameters in relation to
recurrence/progression-free survival of patients |
Table III.
Uni- and multivariable analyses of
clinicopathologic parameters in relation to
recurrence/progression-free survival of patients
|
|
Recurrence/progression-free survival |
|---|
|
|
|
|---|
|
| Univariable
analysis | Multivariable
analysis |
|---|
|
|
|
|
|---|
| Parameters | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age |
|
|
|
|
| ≤50 | 1 |
| 1 |
|
| >50 | 0.469
(0.188–1.183) | 0.106 | 0.323
(0.114–0.908) | 0.0325 |
| FIGO |
|
|
|
|
| I–II | 1 |
| 1 |
|
| III–IV | 3.450
(1.373–9.057) | 0.0087 | 5.013
(1.679–15.952) | 0.0038 |
| Histological
type |
|
|
|
|
| S/E | 1 |
| 1 |
|
| M/C | 0.529
(0.202–1.320) | 0.172 | 0.738
(0.422–3.211) | 0.2966 |
| ZEB1
expression |
|
|
|
|
| Negative/weak | 1 |
| 1 |
|
|
Moderate/strong | 3.415
(1.329–9.865) | 0.010 | 2.265
(1.072–8.021) | 0.0349 |
Recurrence site in relapsed
patients
Table IV shows the
clinical features in the 18 relapsed patients. ZEB1 was expressed
in 17 of the 18 (94.4%) patients. In the majority of relapsed
patients, the most frequent site of recurrence was the peritoneal
cavity (16/18:88.9%).
 | Table IV.Clinical features and the ZEB1
immunostaining intensity in the relapsed patients. |
Table IV.
Clinical features and the ZEB1
immunostaining intensity in the relapsed patients.
| Case | Age | Histological
type | FIGO stage | Rec. site | Time to Rec.
(Mo) | Follow-up (Mo) | ZEB1 expression
score |
|---|
| 1 | 70 | S |
IIIC | PC | 15.9 | 34.0 | 2 |
| 2 | 50 | S |
IIC | PC, Distant | 43.4 | 107.3 | 3 |
| 3 | 57 | S |
IIIC | PC | 21.0 | 55.0 | 4 |
| 4 | 61 | S |
IVB | PC | 10.2 | 52.5 | 4 |
| 5 | 69 | S |
IIIC | PC | 10.4 | 38.8 | 3 |
| 6 | 47 | S |
IVB | PC | 17.9 | 32.4 | 2 |
| 7 | 48 | S |
IIIC | PC |
9.6 | 31.4 | 2 |
| 8 | 22 | M |
IIB | PC |
2.2 |
3.8 | 2 |
| 9 | 49 | E |
IIIC | PC | 11.2 | 31.3 | 4 |
| 10 | 53 | E |
IIIC | RPM, Distant | 19.6 | 38.6 | 2 |
| 11 | 53 | E |
IIC | RPM, Distant |
4.9 | 84.2 | 4 |
| 12 | 58 | E |
IIIC | PC |
6.9 | 14.4 | 4 |
| 13 | 53 | C | IC | PC |
6.8 | 10.8 | 3 |
| 14 | 38 | C |
IIB | PC | 13.3 | 24.1 | 3 |
| 15 | 39 | C | IA | PC | 18.6 | 49.7 | 3 |
| 16 | 57 | C |
IIB | PC, Distant |
2.4 |
4.3 | 2 |
| 17 | 57 | C |
IIIC | PC | 49.9 | 74.8 | 4 |
| 18 | 48 | C |
IIIC | PC |
4.9 | 88.2 | 3 |
Discussion
In general, patients with EOC show an unfavorable
prognosis, principally owing to its asymptomatic hallmark until the
late stage, and it is frequently linked with disseminated
intraperitoneal and/or distant metastases (17–19).
Especially, peritoneal dissemination is the most common
presentation, consisting of multi steps: First, tumor cells are
released from the original tumor, and then they migrate in the
abdominal cavity. When the tumor cells attach to the peritoneum,
they start to invade tissues through the mesothelium (20,21). In
the current investigation of 40 EOC patients, various levels of
ZEB1 expression were identified in 82.5% (33 of 40), and patients
with higher ZEB1 expressions showed a significantly poorer
prognosis. Furthermore, multivariate analyses showed that a higher
expression of ZEB1 was an independent prognostic indicator of a
poorer RFS of EOC patients. Currently, studies demonstrated the
important association between ZEB1 expression and aggressive
phenotypes in several solid malignancies. Spoelstra et al
(22) revealed that ZEB1 was
aberrantly expressed in carcinoma cells of aggressive
poorly-differentiated endometrioid carcinomas and other kinds of
aggressive endometrial cancers, including uterine serous
carcinomas. In addition, Hashiguchi et al (8) reported the clinical effect of ZEB1 and
E-cadherin expression in 108 patients with primary hepatocellular
carcinoma. They demonstrated that positive immunohistochemical
activity of ZEB1 was significantly correlated with a reduced
expression of E-cadherin, and those with positive ZEB1/reduced
E-cadherin expression particularly showed a poorer overall
survival. These previous findings are consistent with our current
results. If ZEB1 is directly linked with EMT of EOC, a
ZEB1-positive clone may easily spread into the peritoneal cavity
and have a greater opportunity to adhere to the mesothelium,
resulting in the increasing formation of micro-and/or macroscopic
peritoneal disseminations. According to earlier study from Chen
et al (23), silencing of ZEB1
expression induced the colony-forming, wound-healing, and cellular
migration abilities were downregulated with enhanced the expression
of miR-200c to inhibit the epithelial-mesenchymal transition in
ovarian cancer cells. In our next study, we aim to perform
functional analysis of ZEB1 in EOC. At least, the current findings
indicate that the immunoreactive identification of ZEB1 expression
might be a crucial predictor of patients who will show a poor
oncologic outcome and its identification may lead to the selection
of better treatment strategies.
As well as metastasis to the peritoneal cavity and
distant parenchymal organs, intrinsic or acquired chemoresistance
remains a major challenge to improve the prognosis of patients with
EOC. Recently, several studies suggested that the EMT shows therapy
resistance, resulting in tumor recurrence (24–26). Also,
the EMT-inducer ZEB1 was revealed to be involved in tumor stemness
and treatment resistance. ZEB1 represses miR-200 as well as
miR-203, which can also suppress stemness hallmarks (27). The level of ZEB1 is also upregulated
in melanoma cells with acquired resistance and in biopsies from
relapsed patients during treatment (28). Additionally, patients with a mucinous
and clear-cell histology generally showed a very low response rate
to platinum-based chemotherapy, leading to intrinsic
chemoresistance (29,30). Indeed, in patients with the
mucinous/clear-cell histological type, the frequency of an
unfavorable oncologic outcome was higher in those with higher ZEB1
positivity. ZEB1 expression may be involved in inheriting or the
acquisition of EOC chemoresistance. The mechanism of patients with
ZEB1 expression showing an unfavorable clinical outcome may be due
to both the chemoresistant hallmark and metastasis-promoting effect
of ZEB1 in the peritoneum via EMT. Indeed, in our study, the
majority of patients with positive ZEB1 expression experienced
recurrence {17/18 (94.4%)}. The remaining chemoresistant clone,
which is linked with ZEB1 expression, may be a cause of the high
rate of recurrence. However, a functional analysis of
ZEB1-expressing EOC cells was not carried out. Therefore, we can
only hypothesize regarding the possibility of close linkage between
chemoresistance and ZEB1 expression in EOC at present. We hope to
test this hypothesis in the next study in order to clarify the
EOC-specific biological hallmarks.
In the present study, there were several
limitations, including a non-prospective, exploratory study,
limited patient number, heterogeneous treatment modalities, and
different follow-up periods. Especially, reflecting the small-scale
patient number, our study did not have sufficient power.
Nevertheless, we observed statistically significant difference at
least in the two group comparison regarding ZEB1 expression
(negative/weak vs. moderate/strong). The result indicates our
patients were not necessary insufficient to withdraw the conclusion
that the ZEB1 expression was significant prognostic indicator of
EOC. In addition, the heterogeneity of EOC is now the biggest
challenge in all relevant studies. To investigate the effect of the
ZEB1 expression in each histological type, we had categorized
patients into the mucinous/clear-cell and other histological type.
Nevertheless, we sincerely felt that the number of patients was so
limited. Therefore, our finding that there was an association
between ZEB1 expression and the unfavorable oncologic outcome of
EOC patients is only weakly supported. We need to reanalyze and
confirm the expression of ZEB1 in EOC samples in a larger patient
population.
In conclusion, to our knowledge, this is the first
study showing that the expression of ZEB1 was closely associated
with a poor oncologic outcome of patients with EOC. The current
findings may be based on the metastasis- and/or
chemoresistant-promoting effects of ZEB1, although further
investigation is needed to clarify the molecular mechanisms of
ZEB1. In addition, at present, there are a number of problems,
including the histological heterogeneity and lack of power.
Nevertheless, our evidence provided sheds some light on the
clinical and biological behavior of this malignancy. Although the
current findings must be confirmed by other future studies, the
expression of ZEB1 can be a helpful predictor factor for metastasis
and/or relapse of EOC. We believe that this will help improve EOC
treatment by adding criteria for the administration of systematic
therapy in the future.
Acknowledgements
We sincerely thank Dr K. Tamakoshi (Nagoya
University, School of Health Science), for his advice on
statistical analyses as an expert statistician.
References
|
1
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hosono S, Kajiyama H, Terauchi M, Shibata
K, Ino K, Nawa A and Kikkawa F: Expression of Twist increases the
risk for recurrence and for poor survival in epithelial ovarian
carcinoma patients. Br J Cancer. 96:314–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Ahn YH, Chen Y, Tan X, Guo L,
Gibbons DL, Ungewiss C, Peng DH, Liu X, Lin SH, et al: ZEB1
sensitizes lung adenocarcinoma to metastasis suppression by PI3K
antagonism. J Clin Invest. 124:2696–2708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu WS, Heinrichs S, Xu D, Garrison SP,
Zambetti GP, Adams JM and Look AT: Slug antagonizes p53-mediated
apoptosis of hematopoietic progenitors by repressing puma. Cell.
123:641–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spaderna S, Schmalhofer O, Wahlbuhl M,
Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A,
et al: The transcriptional repressor ZEB1 promotes metastasis and
loss of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanchez-Tilló E, Liu Y, de Barrios O,
Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A
and Postigo A: EMT-activating transcription factors in cancer:
Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 69:3429–3456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hashiguchi M, Ueno S, Sakoda M, Iino S,
Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, et
al: Clinical implication of ZEB-1 and E-cadherin expression in
hepatocellular carcinoma (HCC). BMC Cancer. 13:5722013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siebzehnrubl FA, Silver DJ, Tugertimur B,
Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT,
Kupper MD, Neal D, et al: The ZEB1 pathway links glioblastoma
initiation, invasion and chemoresistance. EMBO Mol Med.
5:1196–1212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren J, Chen Y, Song H, Chen L and Wang R:
Inhibition of ZEB1 reverses EMT and chemoresistance in
docetaxel-resistant human lung adenocarcinoma cell line. J Cell
Biochem. 114:1395–1403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prat J: New insights into ovarian cancer
pathology. Ann Oncol. 23:(Suppl 10). x111–x117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Groen RS, Gershenson DM and Fader AN:
Updates and emerging therapies for rare epithelial ovarian cancers:
One size no longer fits all. Gynecol Oncol. 136:373–383. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeppernick F and Meinhold-Heerlein I: The
new FIGO staging system for ovarian, fallopian tube, and primary
peritoneal cancer. Arch Gynecol Obstet. 290:839–842. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen VW, Ruiz B, Killeen JL, Coté TR, Wu
XC and Correa CN: Pathology and classification of ovarian tumors.
Cancer. 97:(10 Suppl). S2631–S2642. 2003. View Article : Google Scholar
|
|
17
|
Kajiyama H, Shibata K, Mizuno M, Umezu T,
Suzuki S, Yamamoto E, Fujiwara S, Kawai M, Nagasaka T and Kikkawa
F: Long-term clinical outcome of patients with recurrent epithelial
ovarian carcinoma: Is it the same for each histological type? Int J
Gynecol Cancer. 22:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kikkawa F, Nawa A, Ino K, Shibata K,
Kajiyama H and Nomura S: Advances in treatment of epithelial
ovarian cancer. Nagoya J Med Sci. 68:19–26. 2006.PubMed/NCBI
|
|
19
|
Yoshikawa N, Kajiyama H, Mizuno M, Shibata
K, Kawai M, Nagasaka T and Kikkawa F: Clinicopathologic features of
epithelial ovarian carcinoma in younger vs. older patients:
Analysis in Japanese women. J Gynecol Oncol. 25:118–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kajiyama H, Shibata K, Terauchi M, Ino K,
Nawa A and Kikkawa F: Involvement of SDF-1alpha/CXCR4 axis in the
enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int
J Cancer. 122:91–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Terauchi M, Kajiyama H, Yamashita M, Kato
M, Tsukamoto H, Umezu T, Hosono S, Yamamoto E, Shibata K, Ino K, et
al: Possible involvement of TWIST in enhanced peritoneal metastasis
of epithelial ovarian carcinoma. Clin Exp Metastasis. 24:329–339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spoelstra NS, Manning NG, Higashi Y,
Darling D, Singh M, Shroyer KR, Broaddus RR, Horwitz KB and Richer
JK: The transcription factor ZEB1 is aberrantly expressed in
aggressive uterine cancers. Cancer Res. 66:3893–3902. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen D, Wang J, Zhang Y, Chen J, Yang C,
Cao W, Zhang H, Liu Y and Dou J: Effect of down-regulated
transcriptional repressor ZEB1 on the epithelial-mesenchymal
transition of ovarian cancer cells. Int J Gynecol Cancer.
23:1357–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sahai E and Marshall CJ: Differing modes
of tumour cell invasion have distinct requirements for Rho/ROCK
signalling and extracellular proteolysis. Nat Cell Biol. 5:711–719.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Wever O, Demetter P, Mareel M and
Bracke M: Stromal myofibroblasts are drivers of invasive cancer
growth. Int J Cancer. 123:2229–2238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richard G, Dalle S, Monet MA, Ligier M,
Boespflug A, Pommier RM, de la Fouchardière A, Perier-Muzet M,
Depaepe L, Barnault R, et al: ZEB1-mediated melanoma cell
plasticity enhances resistance to MAPK inhibitors. EMBO Mol Med.
8:1143–1161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sugiyama T, Kamura T, Kigawa J, Terakawa
N, Kikuchi Y, Kita T, Suzuki M, Sato I and Taguchi K: Clinical
characteristics of clear cell carcinoma of the ovary: A distinct
histologic type with poor prognosis and resistance to
platinum-based chemotherapy. Cancer. 88:2584–2589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimada M, Kigawa J, Ohishi Y, Yasuda M,
Suzuki M, Hiura M, Nishimura R, Tabata T, Sugiyama T and Kaku T:
Clinicopathological characteristics of mucinous adenocarcinoma of
the ovary. Gynecol Oncol. 113:331–334. 2009. View Article : Google Scholar : PubMed/NCBI
|