Introduction
Malignant glioma is one of the most common primary
central nervous system tumors, which account for more than 70% of
intracranial malignant tumors (1).
The character of malignant glioma is diffuse growth and highly
invasive (1). Active angiogenesis is
the basic pathological feature of most malignant tumors (including
glioma), and plays an important role in the occurrence, development
and treatment of tumors (2). Tumor
angiogenesis is not only associated with vascular endothelial cell
division and proliferation. Extracellular matrix remodeling is also
associated with vascular endothelium cell migration to the tumor
tissue and tube-like structure formation (3). Recently, many studies have launched to
understand the mechanism of glioma angiogenesis, to search for more
effective treatment targets and therapeutics to extend the
asymptomatic survival time and improve the prognosis of glioma
patients.
Angiogenesis of glioma is influenced by various
factors in the tumor microenvironment, in which vascular
endothelial growth factor A (VEGF-A) is considered as the key
factor and is highly expressed in glioma (4). The VEGF-A secreted by glioma is an
important source of tumor angiogenesis microenvironment. It binds
to the VEGF-A receptor (VEGFR), then activates its downstream
signaling pathway, stimulates tumor vascular endothelial cell
proliferation, migration and tube-like structure formation, finally
promotes tumor neovascularization (5). Therefore, inhibition of tumor
angiogenesis targeting VEGF-A is considered to be an effective
treatment for glioma.
The secretion VEGF-A is regulated by various
factors. One of the critical regulatory pathways is the epidermal
growth factor receptor (EGFR) signaling pathway (6). The main downstream pathway to regulate
VEGF expression of EGFR signaling pathway is PI3K-AKT, RAS-MAPK and
JAK-signal transducer and activator of transcription (STAT)
signaling pathway (7). EGFR
downstream signaling pathway affects transcription factors such as
STAT, transcription factor second component 1 (SP1) and
hypoxia-inducible factor (HIF) (8).
These three transcription factors bind to the promoter of VEGF-A
mRNA, then promote the expression of VEGF-A mRNA and increase the
secretion of VEGF-A (8). The
overexpression, mutation or gene amplification of EGFR is found in
most malignant glioma, which abnormal activates EGFR signaling
pathway, results in increased secretion level of VEGF-A (8). Hence, studying the mechanism of VEGF
secretion through EGFR signaling pathway is expected to achieve the
purpose of inhibiting angiogenesis of glioma.
Previous studies show that EGFR signaling pathway
was regulated by the leucine-rich repeats and immunoglobulin-like
domains (LRIG) family which contains LRIG1, 2 and 3 (7). The LRIG family expresses in various
normal tissues and tumors (9–12). LRIG1 and 3 which are considered as
tumor suppressor genes, inhibit a variety of tyrosine kinase
receptors such as EGFR, Met and Ret, thus affect tumor biological
characteristics (13–16).
However, LRIG2 is used as a prognostic indicator of
cervical cancer and glioma patients, whose survival time is
negatively correlated with the cytoplasm staining of LRIG2
(11,17,18). A
preliminary study in our laboratory suggests that downregulation of
LRIG2 leads to decreasing phosphorylation of EGFR, inhibit cell
growth and promote cell apoptosis in a human glioma cell line
(GL15) (19). However, the effect of
LRIG2 on glioma angiogenesis is not well known.
In the present study, we tested the expression of
LRIG2, EGFR, VEGF-A and CD31 in glioma tissues and normal brain
tissues, then analyzed the correlation between LRIG2 in glioma
tissues and the MVD. In vitro, we studied the possible
effects of downregulation of LRIG2 on HUVEC migration and tube
formation induced by coculture with glioma cells. These findings
clearly demonstrate that LRIG2 is a potential target in glioma
angiogenesis, which is possibly mediated via the EGFR/VEGF-A
pathway.
Materials and methods
Regents
Human glioma cell lines U87 and U251 cells, human
umbilical vein endothelial cell (HUVEC) was obtained from Cell
Bank, Chinese Academy of Sciences, (Shanghai, China). Dulbecco's
Modified Eagle's Medium (DMEM) and fetal bovine serum (FBS) were
obtained from Thermo Fisher Scientific (Waltham, MA, USA).
Anti-LRIG2 antibody (ab157492), anti-EGFR antibody (ab52894),
anti-VEGF-A antibody (ab46154), anti-CD31 antibody (ab28364),
anti-p-EGFR antibody (ab40815) and biotinylated anti-rabbit
immunoglobulin G were obtained from Abcam (Cambridge, MA, USA).
Matrigel was obtained from BD Bioscience (San Diego, CA, USA). HTS
Transwell-24 Well Permeable Supports were obtained from Corning
Life Sciences (Corning, CA, USA). TRIzol reagent, reverse
transcription kit and SYBR-Green qPCR SuperMix were obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA). BCA protein assay
kit was obtained from Beyotime Institute of Biotechnology (Nantong,
China). PVDF membranes were obtained from Pall Corporation (New
York, NY, USA). HRP substrate was obtained from Millipore
(Billerica, MA, USA). Streptavidin-biotinylated horseradish
peroxidase complex was obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). DAB was obtained from DakoCytomation
(Carpinteria, CA, USA). Cresyl violet counterstaining was obtained
from Sigma-Aldrich (St. Louis, MO, USA).
Ethical statement
This study was performed with the approval of
research ethic committee of Huazhong University of Science and
Technology. All subjects provided written informed consent.
All the procedures involving subjects were performed
in the study after obtaining ethical approval of the Medical Ethics
Committee of Huazhong University of Science and Technology.
Tissue samples
Glioma tissues (n=50) and normal brain tissues
(n=20) were collected from Department of Neurosurgery, Tongji
Hospital, Tongji Medical College, Huazhong University of Science
and Technology, from 2012 to 2015, as study samples with complete
clinicopathological data.
Immunohistochemistry
The expression of LRIG2, EGFR, VEGF-A and CD31 in
the normal brain tissues and glioma tissues was detected using
immunohistochemistry. The tissues were transferred to 4%
paraformaldehyde, and embedded in paraffin. The tissue was cut into
3-µm serial sections using a Leica microtome (Leica Microsystems
GmbH, Wetzlar, Germany). IHC was performed according to a
previously published protocol. Briefly, sections were incubated in
3% H2O2 in PBS for 7 min, washed with PBS
three times, treated with BSA 0.3% Triton X-100 for 2 h. Further,
the sections were incubated overnight at 4°C with rabbit anti-LRIG2
antibodies (1:750), rabbit anti-EGFR antibodies (1:500), rabbit
anti-VEGF-A antibodies (1:500) or rabbit anti-CD31 antibodies
(1:500). After washing twice with PBS, sections were treated for 40
min with biotinylated anti-rabbit immunoglobulin G, washed three
times, and processed using streptavidin-biotinylated horseradish
peroxidase complex. The reaction was visualized using DAB and
cresyl violet counterstaining. Images were obtained using a
microscope (Olympus Corporation, Tokyo, Japan).
Evaluation of immunohistochemical
staining
The scoring standards were defined as follows
(20). Briefly, the immunoreactive
score (IRS) was derived by multiplying the positive cell staining
intensity (SI) with the percentage of positive cells (PP). SI was
judged from 0 to 3 points: 0, no staining; 1, weakly positive
staining; 2, moderately positive staining; and 3, strongly positive
staining; PP was judged from 0 to 4 points: 0, no staining; 1,
1–10%; 2, 11–50%; 3, 51–80%; and 4, 80–100%.
Assessment of microvessel density
(MVD)
The vessels of tissues were assessed by
immunohistochemical staining for CD31 in three areas of highest
vascular density per section of tumor specimen (×400 magnification)
as described before (21). Vessels
were counted in 10 high power fields the average was considered as
MVD. All clinical data were blinded to the pathologist.
Culture and transfection of the cell
lines
Human glioma cell lines U87 and U251 were cultured
in DMEM medium supplemented with 10% FBS and
penicillin/streptomycin under 5% CO2 in air at 37°C. The
cells were seeded in 24-well plates and cultured for 24 h.
Subsequently, cells were transfected with LRIG2 siRNA (si-LRIG2) or
negative control siRNA (si-NC) with Lipofectamine® 2000
reagent according to the manufacturer's instruction. The sequences
for si-LRIG2 and si-NC are as follows: si-LRIG2-sense:
5′-UCGGUUGUCUAACUGGAACTT-3′, si-LRIG2-antisense:
5′-GUUCCAGUUAGACAACCGATT-3′, si-NC-sense:
5′-UUCUCCGAACGUGUCACGUTT-3′, and si-NC-antisense:
5′-ACGUGACACGUUCGGAGAATT-3′.
Tube formation assay under co-culture
system
To investigate tube formation ability of HUVEC
induced by coculture with glioma cells, the tube formation assay
was detected by using co-culture system (0.4 µm pore size) as
described before. Briefly, 50 µl diluted matrigel was prepared and
added to the upper chamber of each transwell chamber, then
incubated at 37°C for 30 min, UV irradiation overnight, jelling for
30 min before the formal experiment. U87 and U251 cells
(1×105/well) were seeded on the lower chamber for 24 h,
then cocultivated with HUVECs (1×105/well) in the upper
chamber. After 24 h, the number of tubular structures was counted
and photographed using an inverted microscope. All the experiments
were repeated in triplicate.
Cell migration assay under co-culture
system
To investigate the migration ability of HUVEC
induced by coculture with glioma cells, the cell migration assay
was detected by using co-culture system (8 µm pore size) as
described before. Briefly, U87 and U251 cells
(1×105/well) were seeded on the lower chamber for 24 h,
then cocultivation with HUVECs (1×105/well) in the upper
chamber. After 24 h, HUVECs on the upper surface of the membrane
were then removed by cotton swabs. The migrated cells were fixed
with 10% methanol and stained with 0.1% crystal violet. Then the
migrated cells were counted under a microscope. All the experiments
were repeated in triplicate.
Quantitative PCR (qPCR)
U87 and U251 cells were seeded in 6-well plates and
received the indicated treatment. After treatment for 24 h, total
RNA was extracted using Trizol reagent according to the
manufacturer's instructions. The primer sequences for LRIG2 and
β-actin are as follows: LRIG2, forward: 5′-CATGTGCCCTCACTACCA-3′,
and reverse: 5′-CTCCAGGACCCGAGAATA-3′; β-actin, forward:
5′-TGGCACACCTTCTACAA-3′, and reverse: 5′-AGCCTGGATAGCAACGTACA-3′.
Reverse transcription and qPCR were performed in accordance with
the protocol recommended by the manufacturers of SYBR-Green qPCR
SuperMix. The relative expression of mRNA was assessed by the
comparative 2−ΔΔCq method. β-actin was used as an
internal standard.
Western blotting
Each sample was lysed using RIPA buffer. Total
protein concentration was determined with a BCA protein assay kit,
according to the manufacturer's instruction. Equal amounts of total
protein were separated in 10% SDS-polyacrylamide gels and
transferred to PVDF membranes. After blocking with 5% milk in TBS
containing 0.05% Tween-20 (TBST) for 1 h at 37°C, membranes were
incubated for 40 min with anti-LRIG2 antibody (1:1,000), anti-EGFR
antibody (1:1,000), anti-VEGF-A antibody (1:1,000), anti-p-EGFR
antibody (1:750), washed with TBST and incubated with secondary
antibody, and visualized using immobilon western chemiluminescent
HRP substrate.
Statistical analysis
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA)was employed
for statistical analysis. All data were expressed as the mean ±
standard deviation. Differences between two groups were assessed
using Student's t-test. The differences between multiple groups
were assessed using one-way ANOVA or Kruskal-Wallis H tests.
Correlation analysis of two variables was assessed using Pearson
correlation coefficient. P<0.05 was considered to indicate a
statistically significant difference.
Results
LRIG2 was upregulated in glioma
tissues and was directly correlated with MVD
We used immunochemistry assay to analyze the protein
expression (evaluated by IRS) of LRIG2, EGFR, VEGF-A and CD31 in
glioma tissues and normal brain tissues. As shown in Table I, with the increase of tumor
malignancy, the expression of LRIG2, EGFR, VEGF-A and CD31 was
significantly enhanced. The expression of LRIG2, EGFR, VEGF-A and
CD31 was significantly higher in malignant gliomas than that in low
grade. As shown in Fig. 1A, the
expression level of these four protein was highly expressed in
glioma tissues. However, the level of these four protein was not
expressed or low expressed in normal tissues (Fig. 1B).
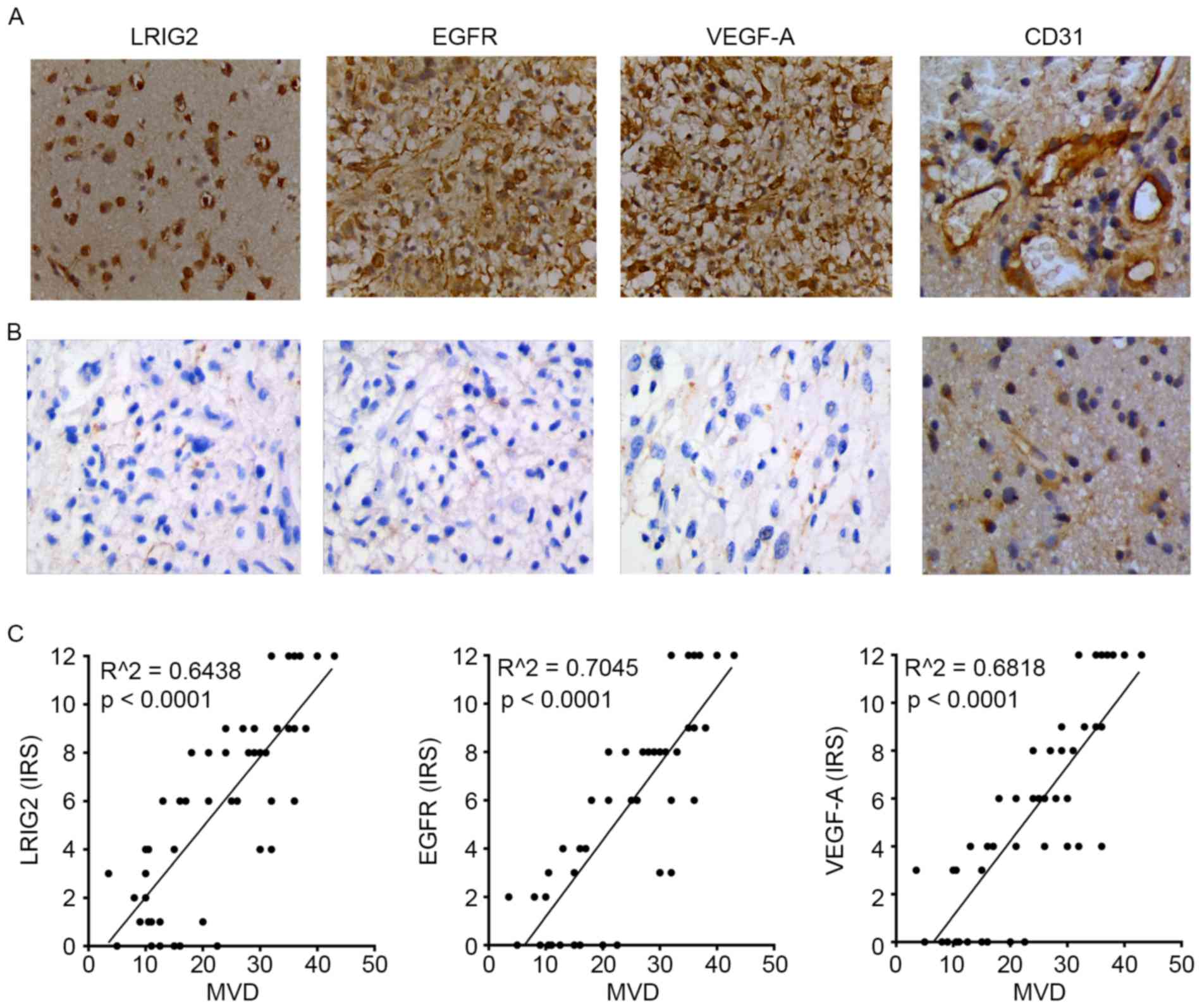 | Figure 1.We used immunochemistry assay to
analyze the protein expression (evaluated by immunoreactive score)
of LRIG2, EGFR, VEGF-A and CD31 in glioma tissues and normal brain
tissues. The vessels of tissues were expressed by
immunohistochemical staining for CD31 highlight for calculation of
MVD. The expression level of LRIG2, EGFR, VEGF-A and CD31 was
highly expressed in glioma tissues (A), but was not expressed or
lowly expressed in normal tissues (B). The expression of LRIG2,
EGFR, VEGF-A was directly correlated with MVD. (C) Results are
representative of three different experiments. Original
magnification, ×400. LRIG2, leucine-rich repeats and
immunoglobulin-like domains 2; EGFR, epidermal growth factor
receptor; VEGF-A, vascular endothelial growth factor A; CD31,
cluster of differentiation 31; MVD, microvessel density. |
 | Tabel I.Expression of LRIG2, EGFR, VEGF-A and
CD31 in normal brains and gliomas. |
Tabel I.
Expression of LRIG2, EGFR, VEGF-A and
CD31 in normal brains and gliomas.
| Group | No. | LRIG2 protein
(IRS) | EGFR protein
(IRS) | VEGF-A protein
(IRS) | CD31 protein
(MVD) |
|---|
| Normal brain | 20 | 0.90±0.64 | 0.15±0.37 | 0.55±0.51 | 5.35±2.62 |
| Grade I-II | 24 | 3.25±2.80 | 3.46±3.44 | 4.08±3.65 | 13.25±5.00 |
| Grade III-IV | 26 |
8.12±3.15a |
6.96±3.82a |
6.12±4.20a |
31.88±5.05a |
Then the correlation between the expression of
LRIG2, EGFR, VEGF-A in 50 glioma tissues and the MVD were further
analyzed. The results showed that LRIG2, EGFR, VEGF-A expression in
glioma tissues remained significantly correlated to the MVD,
respectively (Fig. 1C).
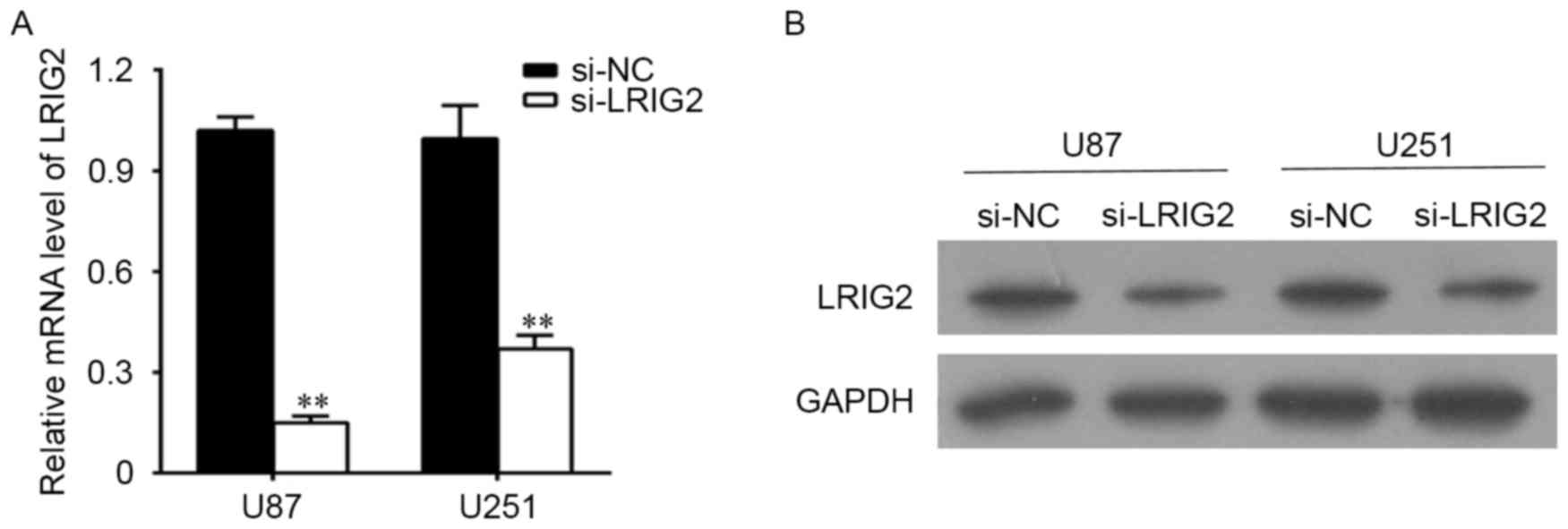
The effects of RNA Interference
U87 and U251 cells were transfected with si-LRIG2 or
si-NC, then the mRNA level and the protein level of LRIG2 were
determined. As shown in Fig. 2A, when
compared with the NC group, the LRIG2 mRNA expression was
significantly decreased when transfected with si-LRIG2 both in U87
and U251 cell lines. Consistent with the results of qPCR detection,
the protein level of LRIG2 analyzing by western blotting was
significantly decreased in si-LRIG2 transfected group, when
compared with the NC group (Fig.
2B).
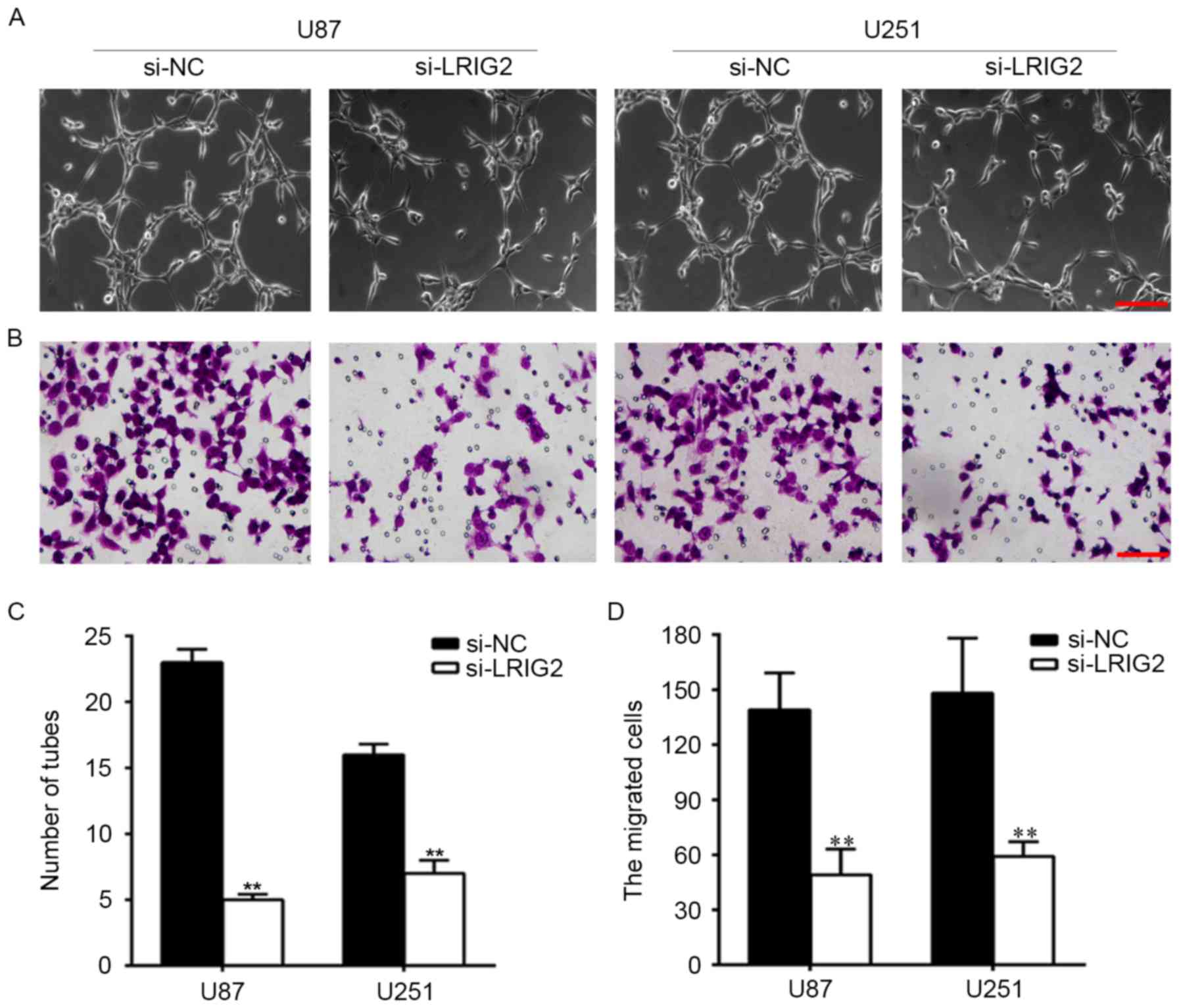
Downregulation of LRIG2 in glioma cell
lines inhibited tube formation and cell migration of HUVECs
To observe the effect of downregulation of LRIG2 on
HUVECs angiogenesis. We knockdown the expression of LRIG2 in glioma
cells, then tube formation was assessed by using transwell
co-cultured model. The number of formed tubular structures were was
significantly decreased when transfected with si-LRIG2 both in U87
and U251 cell lines, when compared with the NC group (Fig. 3A and C).
Then we analyzed the effect of downregulation of
LRIG2 on HUVECs migration. As shown in Fig. 3B and D, when compared with the NC
group, the migrated cells were was significantly decreased when
transfected with si-LRIG2 both in U87 and U251 cells.
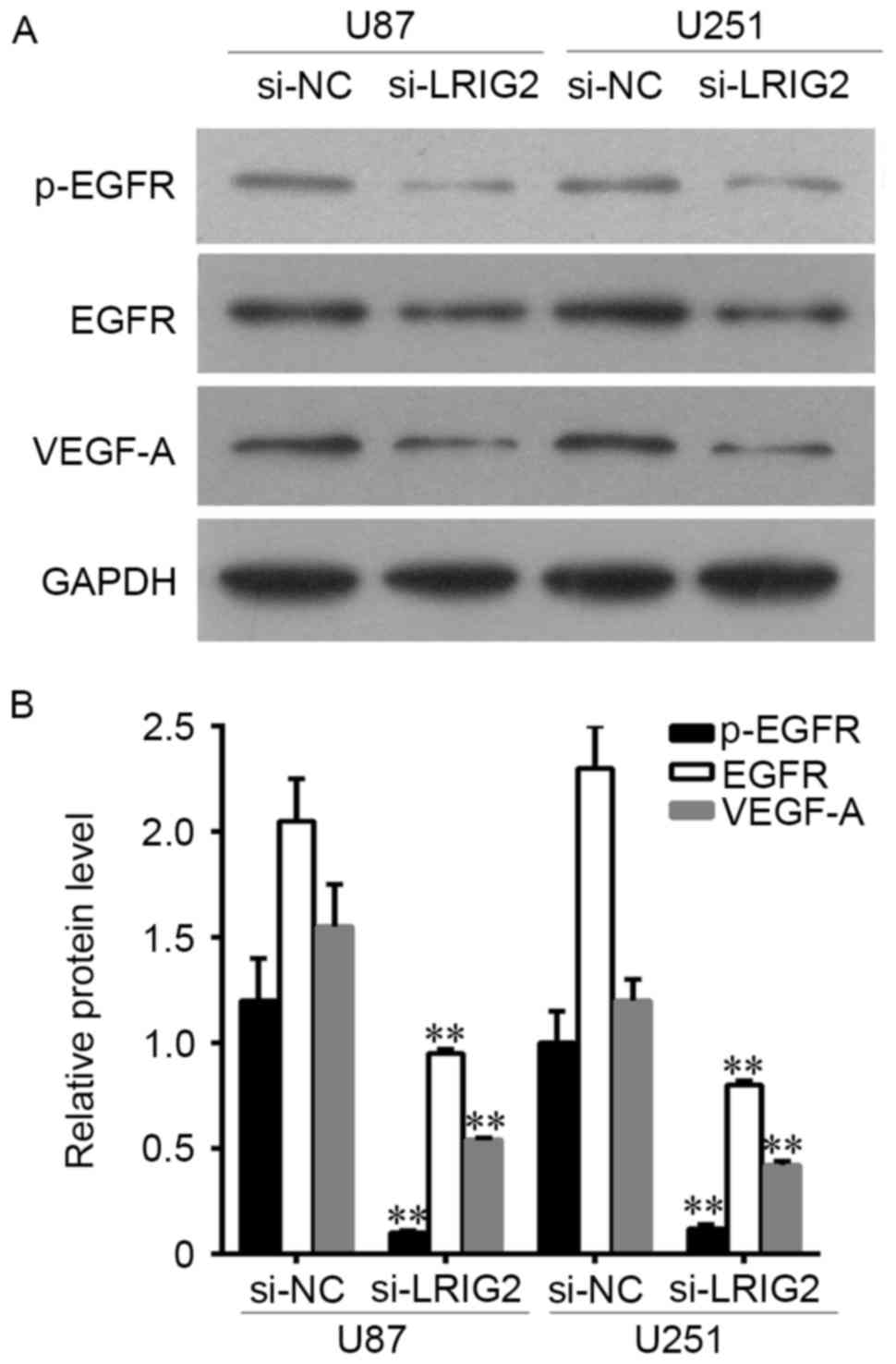
Downregulation of LRIG2 decreased the
expression of EGFR and VEGF-A
The protein expression level of EGFR, p-EGFR and
VEGF-A was assessed by Western blot assay. The results showed that
EGFR, p-EGFR and VEGF-A expression was significantly decreased when
transfected with si-LRIG2 both in U87 and U251 cells, when compared
with the NC group (Fig. 4).
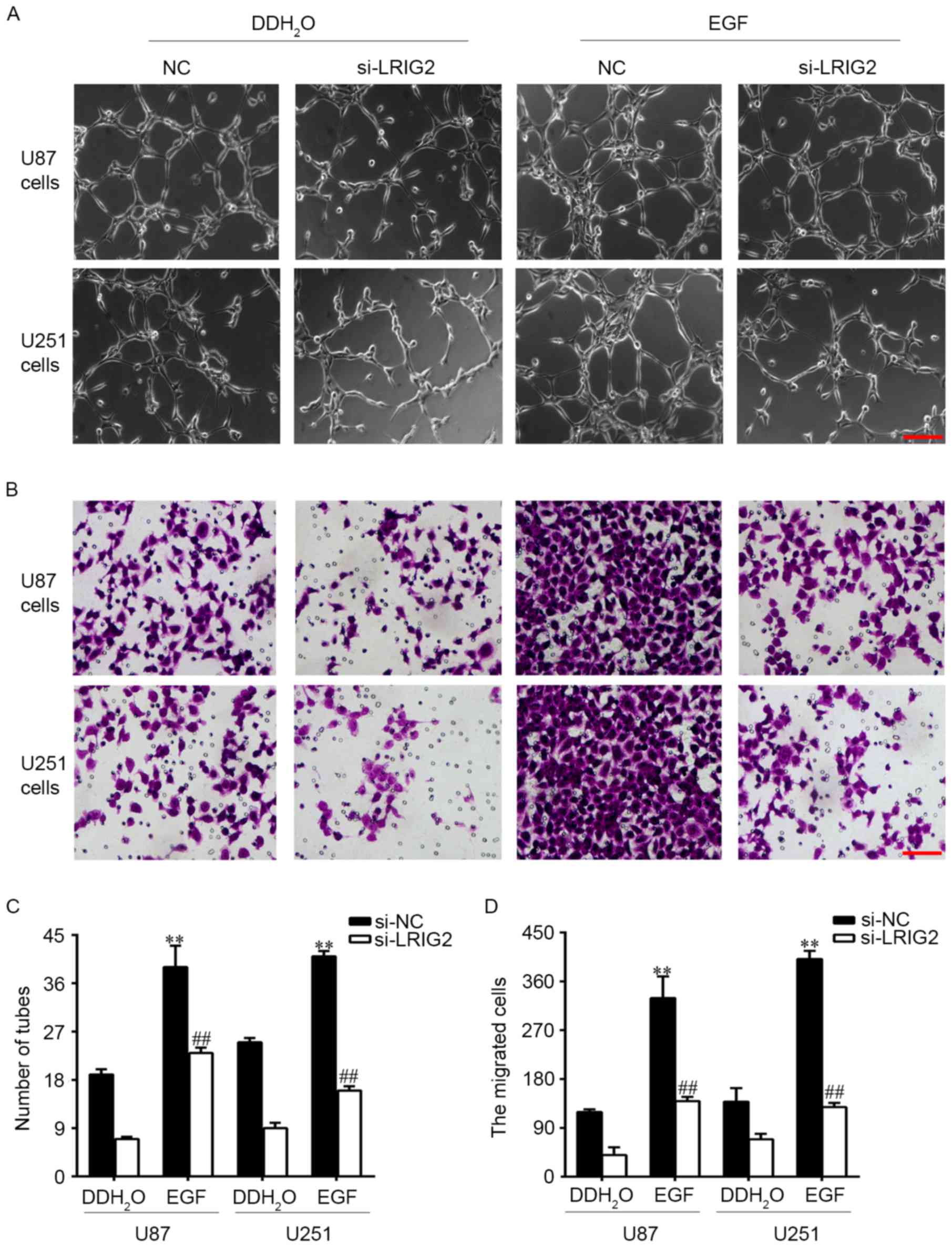
Downregulation of LRIG2 in glioma cell
lines inhibited tube formation and cell migration of HUVECs via
inhibition of EGFR/VEGF-A pathway
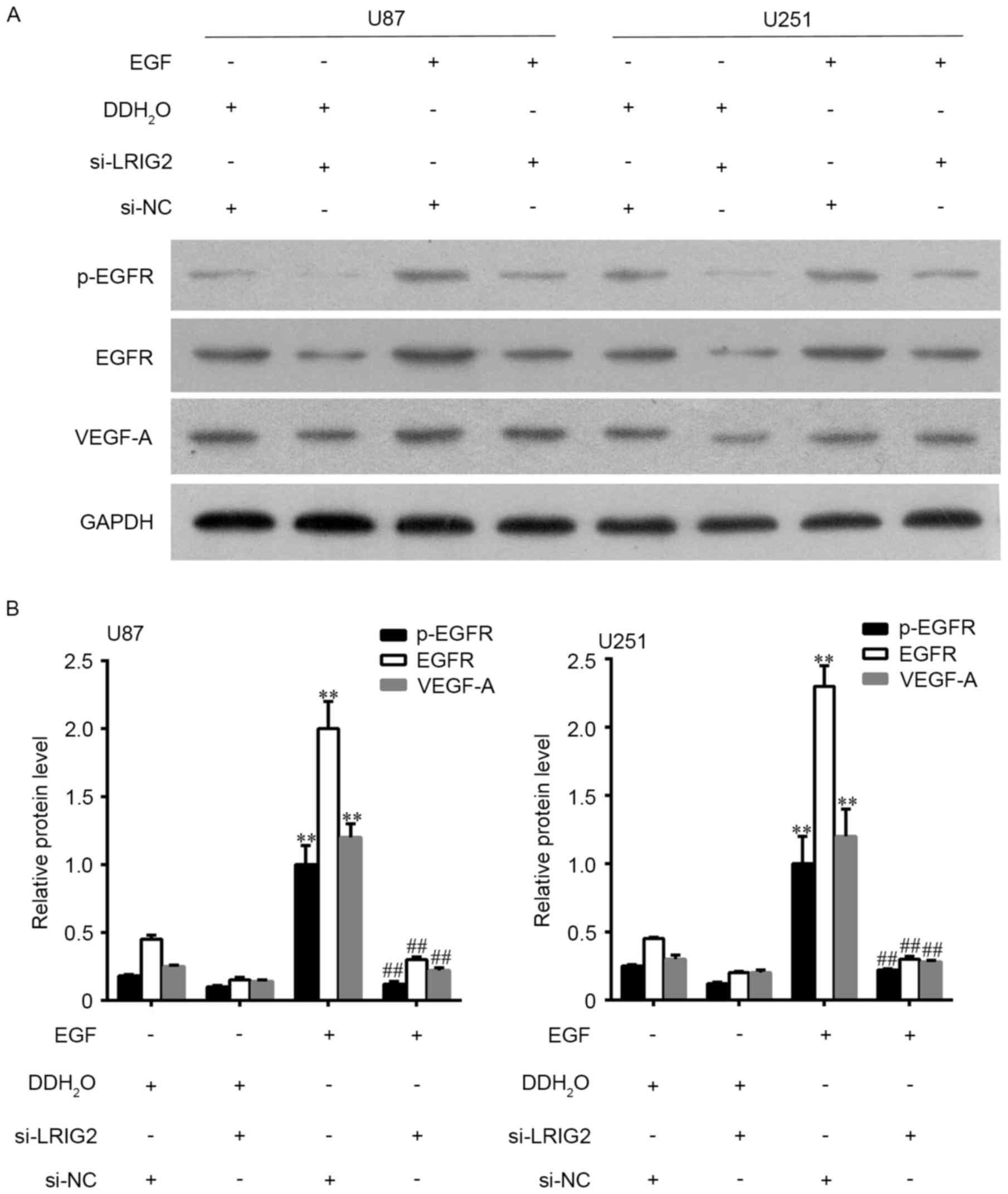
EGFR could be activated by EGF, hence we added EGF
into the glioma cells to observe the potential involvement of
EGFR/VEGF-A pathway. U87 and U251 cell lines were transfected with
si-LRIG2 or NC siRNA, then treated with 100 ng/ml EGF or
DDH2O. The results showed that EGF treatment in si-NC
transfected glioma cells significantly enhanced the number of
formed tubular structures (Fig. 5A and
C) and cell migration (Fig. 5B and
D) in HUVECs, when compared to the DDH2O treatment
group. The expression of EGFR, p-EGFR and VEGF-A was further
increased (Fig. 6). However, the
enhanced effect of EGF was diminished in si-LRIG2-transfected
glioma cells (Figs. 5 and 6).
Discussion
Tumor angiogenesis is not only associated with
vascular endothelial cell division proliferation, extracellular
matrix remodeling, but is also related to vascular endothelium cell
migration to the tumor tissue and tube-like structure formation. In
this study, we revealed the role of LRIG2 in glioma angiogenesis.
The results showed that the expression level of LRIG2, EGFR, VEGF-A
and CD31 was highly expressed in glioma tissues. The LRIG2
expression in glioma tissues was positively correlation with the
MVD. Downregulation of LRIG2 inhibited HUVEC migration and tube
formation induced by coculture with glioma cells. Mechanistically,
downregulation of LRIG2 decreased the expression of EGFR, p-EGFR
and VEGF-A. The decreased effect was diminished by EGF
treatment.
LRIG2 is one of the members of the LRIG gene family,
which include three members: LRIG1 (gene no. AF381545), LRIG2 (gene
no. AY505340), LRIG3 (gene no. AY505341). The gene products of LRIG
gene family have a unique protein structure: a signal peptide, 15
consecutive leucine-rich repeats (LRRs), three immunoglobulin-like
regions (Ig-like), a transmembrane segment and an intracellular
fraction. Previous studies demonstrate that LRIG1 negatively
regulates tumor growth and development and is viewed as a tumor
suppressor (15). However, LRIG2
might have a function distinct from that of LRIG1, and possibly
contributing to the etiology of oligodendroglioma (18). LRIG2 acts as a prognostic indicator of
various cancers such as cervical cancer, (12) non-small cell lung cancer (22) and glioma (18). Consistent with the previous study, our
study found that the expression level of LRIG2 was highly expressed
in glioma tissues, but was not expressed or low expressed in normal
brain tissues.
Research confirmed that glioma is a highly
vascularize human tumor, and its proliferation and invasion are
dependent on tumor angiogenesis (2).
CD31 is a vascular endothelial cell marker, which is used primarily
to study tumor angiogenesis. MVD is an important index for the
detection of tumor angiogenesis which is assessed by the
immunochemistry staining of CD31. Consistent with Cui et al
(23), our study revealed that the
expression of CD31 was highly expressed in glioma tissues, which
indicated that angiogenesis in glioma was higher than in normal
brain tissues. LRIG2 expression in glioma tissues was positivity
correlated with the MVD. It is suggested that the expression of
LRIG2 in human glioma might be related to tumor angiogenesis, which
might be related to the regulation of LRIG2.
Our previous study demonstrated that knockdown of
LRIG2 by RNA interference inhibited glioma cell (GL15) growth,
caused cell cycle redistribution and increased cell apoptosis in
vitro, suggested that LRIG2 was an attractive target in glioma
therapy (19). In this study, we
further revealed that downregulation of LRIG2 inhibited the number
of formed tubular structures and cell migration of HUVECs induced
by glioma cells, indicated that knockdown of LRIG2 inhibited glioma
angiogenesis. However, the results showed that the presence of
ectodomain of LRIG2 in the culture medium of si-LRIG2 treated
cells. Studies using conditioned culture medium by si-LRIG2 treated
cell still need studying in the future.
EGFR promotes the expression and secretion of VEGF-A
through its downstream transcription factors including STAT, SP1
and HIF. (8) VEGF-A binds to VEGFR,
then stimulates vascular endothelial cell proliferation, migration
and tube-like structure formation, finally promotes tumor
neovascularization (8). Previous
study reported that anti-EGFR and VEGF/VEGFR therapy significantly
prolonged survival of patients with cancers (24,25).
Therefore, inhibition of tumor angiogenesis targeting EGFR/VEGF-A
is considered to be an effective treatment for glioma. A
preliminary study in our laboratory suggested that downregulation
of LRIG2 decreased phosphorylation of EGFR, then result in
inhibition of glioma cell proliferation. Our study revealed that
downregulation of LRIG2 decreased the expression of EGFR, p-EGFR
and VEGF-A, then result in anti-angiogenesis of glioma cells. The
decreased effect was diminished by EGF (EGFR agonist) treatment. In
conclusion, these findings clearly demonstrate that downregulation
of LRIG2 is a potential target to inhibit glioma angiogenesis,
which is possibly involved in the EGFR/VEGF-A pathway.
Acknowledgements
This study was supported by the Nature Science
Foundation of Hubei Province (no. 2014CFB151).
References
|
1
|
Wen PY and Reardon DA: Neuro-oncology in
2015: Progress in glioma diagnosis, classification and treatment.
Nat Rev Neurol. 12:69–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malla RR, Gopinath S, Gondi CS, Alapati K,
Dinh DH, Gujrati M and Rao JS: Cathepsin B and uPAR knockdown
inhibits tumor-induced angiogenesis by modulating VEGF expression
in glioma. Cancer Gene Ther. 18:419–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babykutty S SPPJNR, Kumar MA, Nair MS,
Srinivas P and Gopala S: Nimbolide retards tumor cell migration,
invasion, and angiogenesis by downregulating MMP-2/9 expression via
inhibiting ERK1/2 and reducing DNA-binding activity of NF-κB in
colon cancer cells. Mol Carcinog. 51:475–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrillo M, Borriello M, Fuoco G, Legge F,
Iannone V and Ferrandina G: Novel VEGF-independent strategies
targeting tumor vasculature: Clinical aspects. Curr Pharm Des.
18:2702–2712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reardon DA, Turner S, Peters KB,
Desjardins A, Gururangan S, Sampson JH, McLendon RE, Herndon JE II,
Jones LW, Kirkpatrick JP, et al: A review of VEGF/VEGFR-targeted
therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw.
9:414–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cea V, Sala C and Verpelli C:
Antiangiogenic therapy for glioma. J Signal Transduct.
2012:4830402012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Casaletto JB and McClatchey AI: Spatial
regulation of receptor tyrosine kinases in development and cancer.
Nat Rev Cancer. 12:387–400. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Larsen AK, Ouaret D, El Ouadrani K and
Petitprez A: Targeting EGFR and VEGF(R) pathway cross-talk in tumor
survival and angiogenesis. Pharmacol Ther. 131:80–90. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malik U and Javed A: LRIGs: A
prognostically significant family with emerging therapeutic
competence against cancers. Curr Cancer Drug Targets. 17:3–16.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Erp S, van den Heuvel DM, Fujita Y,
Robinson RA, Hellemons AJ, Adolfs Y, Van Battum EY, Blokhuis AM,
Kuijpers M, Demmers JA, et al: Lrig2 negatively regulates
ectodomain shedding of Axon guidance receptors by ADAM proteases.
Dev Cell. 35:537–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo D, Nilsson J, Haapasalo H, Raheem O,
Bergenheim T, Hedman H and Henriksson R: Perinuclear leucine-rich
repeats and immunoglobulin-like domain proteins (LRIG1-3) as
prognostic indicators in astrocytic tumors. Acta Neuropathol.
111:238–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lindström AK, Asplund A and Hellberg D:
Correlation between LRIG1 and LRIG2 expressions and expression of
11 tumor markers, with special reference to tumor suppressors, in
CIN and normal cervical epithelium. Gynecol Oncol. 122:372–376.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Powell AE, Wang Y, Li Y, Poulin EJ, Means
AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE,
et al: The pan-ErbB negative regulator Lrig1 is an intestinal stem
cell marker that functions as a tumor suppressor. Cell.
149:146–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong VW, Stange DE, Page ME, Buczacki S,
Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter
MW, et al: Lrig1 controls intestinal stem-cell homeostasis by
negative regulation of ErbB signalling. Nat Cell Biol. 14:401–408.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu L, Teixeira VH, Yuan Z, Graham TA,
Endesfelder D, Kolluri K, Al-Juffali N, Hamilton N, Nicholson AG,
Falzon M, et al: LRIG1 regulates cadherin-dependent contact
inhibition directing epithelial homeostasis and pre-invasive
squamous cell carcinoma development. J Pathol. 229:608–620. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai L, McEachern D, Yang CY, Lu J, Sun H
and Wang S: LRIG1 modulates cancer cell sensitivity to Smac
mimetics by regulating TNFα expression and receptor tyrosine kinase
signaling. Cancer Res. 72:1229–1238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hedman H, Lindström AK, Tot T, Stendahl U,
Henriksson R and Hellberg D: LRIG2 in contrast to LRIG1 predicts
poor survival in early-stage squamous cell carcinoma of the uterine
cervix. Acta Oncol. 49:812–815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holmlund C, Haapasalo H, Yi W, Raheem O,
Brännström T, Bragge H, Henriksson R and Hedman H: Cytoplasmic
LRIG2 expression is associated with poor oligodendroglioma patient
survival. Neuropathology. 29:242–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Han L, Chen R, Cai M, Han F, Lei T
and Guo D: Downregulation of LRIG2 expression by RNA interference
inhibits glioblastoma cell (GL15) growth, causes cell cycle
redistribution, increases cell apoptosis and enhances cell adhesion
and invasion in vitro. Cancer Biol Ther. 8:1018–1023. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
21
|
Tai YT, Acharya C, An G, Moschetta M,
Zhong MY, Feng X, Cea M, Cagnetta A, Wen K, van Eenennaam H, et al:
APRIL and BCMA promote human multiple myeloma growth and
immunosuppression in the bone marrow microenvironment. Blood.
127:3225–3236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang G, Wu J and Song H: LRIG2 expression
and prognosis in non-small cell lung cancer. Oncol Lett. 8:667–672.
2014.PubMed/NCBI
|
|
23
|
Cui L, Xu S, Song Z, Zhao G, Liu X and
Song Y: Pituitary tumor transforming gene: A novel therapeutic
target for glioma treatment. Acta Biochim Biophys Sin (Shanghai).
47:414–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang T, Qiao M, Zhou F, Ren S, Su C and
Zhou C: Effect of combined therapy inhibiting EGFR and VEGFR
pathways in non-small-cell lung cancer on progression-free and
overall survival. Clin Lung Cancer. Dec 29–2016.(Epub ahead of
print).
|
|
25
|
Yu X, Li W, Deng Q, You S, Liu H, Peng S,
Liu X, Lu J, Luo X, Yang L, et al: Neoalbaconol inhibits
angiogenesis and tumor growth by suppressing EGFR-mediated VEGF
production. Mol Carcinog. 56:1414–1426. 2017. View Article : Google Scholar : PubMed/NCBI
|