Introduction
The underlying molecular mechanism of multiple
myeloma (MM) is associated with oncogene activation caused by the
translocation of the immunoglobulin heavy chain gene (1,2). Mutations
or deletions of certain genes are also involved (3,4). Although
MM is incurable, the median survival time is currently 5–7 years
compared with 3 years before 2000 due to the development of novel
therapies (5). The prognosis of
patients with MM is associated with genetic abnormalities (3), and the identification of molecular
genetic markers for predicting prognosis is therefore essential.
The application of high-throughput next-generation sequencing
techniques has contributed to efforts to understanding the
pathogenesis of MM (3,4); however, full coverage of the genomic
alterations involved remains unclear.
Chromosomal instability is a feature of certain
cancer types and is associated with the presence of chromosomal
fragile sites (CFSs) (6). Common
fragile sites are present in normal chromosomes, and are prone to
forming chromosomal gaps and breaks under conditions that partially
inhibit DNA synthesis (7).
Furthermore, CFSs are regions of profound genomic instability and
are frequent sites for deletions and other alterations in cancer
cells (8,9). Fragile site aphidicolin type common
fragile site (16) (q23.2) (FRA16D)
on chromosome 16q23.2 is the second most frequently expressed CFS
region (10) and has been identified
as deleted in multiple types of cancer (11–13).
The WW domain containing oxidoreductase (WWOX) gene
was identified as a putative tumor suppressor gene (14–17) and
spans FRA16D (16,18,19).
Genomic alterations, including homozygous and hemizygous deletions
that affect the WWOX locus, occur in certain types of cancer
(20–22). Deletion of all or part of chromosome
16q and loss of heterozygosity occurs in patients with MM (23–25). In
addition, relatively low expression levels of WWOX and the
cylindromatosis gene, which reside on chromosome 16q, have been
associated with worse prognosis (24).
WWOX is the target of recurrent deletions of
chromosome 16q (15–17,19) that
disrupt one WWOX allele by removing exons 6–8 that encode the
oxidoreductase domain, leading to the production of variant
transcripts. WWOX variant transcripts are frequently identified in
breast, lung, esophageal and hematological malignancies (17,26–30).
There is evidence to indicate that the loss of full
length WWOX expression is due to the localization of WWOX at one of
the most active human CFSs (15–17,19);
however, other studies have demonstrated that the methylation of
the WWOX promoter leads to decreased expression (31–33).
Furthermore, epigenetic processes, particularly DNA methylation,
are involved in carcinogenesis, and multiple studies have reported
an association between the methylation of tumor suppressor genes
and poor prognosis of patients with MM (34–40).
Genes with CFSs are often methylated in various
types of malignancy (41,42), but little information is available
regarding hematologic malignancy types (43,44). In
addition, the methylation of the WWOX promoter has been associated
with worse prognosis of patients with different types of cancer
(31,45–47). To
the best of our knowledge, no previous studies have reported an
association between alterations of WWOX and progression of MM. The
present study aimed to elucidate the function of WWOX in the
pathogenesis of MM.
Materials and methods
Cell lines
The human myeloma cell line RPMI8226 was obtained
from the American Type Culture Collection (Manassas, VA, USA), and
KMS11, KMS12PE, KMM1, KMS18, and KMS26 human myeloma cell lines
were provided by Dr Takemi Otsuki (Kawasaki Medical School,
Okayama, Japan). The cell lines were cultured in 10 ml RPMI-1640
medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented
with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in an atmosphere containing 5%
CO2.
Patients
Subjects included 165 patients with MM (82 male and
83 female) and an average age of 67 (range 34–87), 33 patients (16
male and 17 female) with monoclonal gammopathy of undetermined
significance (MGUS) with an average age of 67 (range 44–81),
diagnosed according to International Myeloma Working Group (IMWG)
Criteria for the Diagnosis of Multiple Myeloma (48) and 25 patients with lymphoma lacking
infiltration of the bone marrow as a control. All patients were
treated at Gunma University Hospital between January 2004 and
September 2011. The present study was approved by the Institutional
Review Board of Gunma University Hospital (IRB no. 810). Written
informed consent was obtained from all patients prior to 3 ml of
bone marrow aspirate collection.
Plasma cell purification
The plasma cells were purified from BM mononuclear
cells from 30 MM patients using anti-CD138 antibody conjugated with
PE (Beckman-Coulter, Brea, CA) and Easy Sep PE positive selection
containing anti-PE antibody conjugated with micro-magnetic beads
kit (STEMCELL Technologies, Vancouver, BC, Canada). The purity of
the CD138 positive plasma cells was analyzed using a flow cytometer
(FACSCanto II, Becton Dickinson, San Jose, CA, USA).
Isolation of nucleic acids
DNA was extracted from 88 patients with MM using
QIAamp DNA Blood Midi kit (Qiagen, Inc., Valencia, CA, USA),
according to the manufacturer's protocol. DNA and RNA were
extracted from 77 patients with MM and 33 patients with MGUS and 25
patients with lymphoma lacking infiltration of the bone marrow,
respectively, using an All-Prep mini-kit (Qiagen, Inc.) according
to the manufacturer's protocol.
Nested reverse
transcription-polymerase chain reaction (RT-PCR) analysis of WWOX
transcripts
cDNA was synthesized from 10 ng total RNA obtained
from 77 patients with MM, 33 patients with MGUS and 25 control
patients, and cell lines KMM1, KMS11, KMS12PE, KMS18, KMS26 and
RPMI8226 using a PrimeScript RT-PCR kit with gDNA Eraser (Takara
Bio, Inc., Otsu, Japan). The first and second PCR amplifications
were performed using the nested primers as follows: First forward,
5′-AGTTCCTGAGCGAGTGGACC-3′ and reverse,
5′-TTACTTTCAAACAGGCCACCAC-3′ and second forward,
5′-AGGTGCCTCCACAGTC-3′ and reverse, 5′-GTGTGTGCCCATCCGCTCT-3′
(29,30). Each reaction (50 µl each) contained
0.2 µmol of each primer, 2.0 mM MgCl2, 0.2 mM dNTP mix,
1X PCR buffer and 1.25 units of Takara ExTaq Hot Start Version
(Takara Bio, Inc.). The thermocycling conditions maintained were as
follows: 95°C for 8 min; 35 cycles at 94°C for 30 sec, 57°C for 30
sec, and 72°C for 1 min; and an extension step at 72°C for 5 min. A
total of 1 µl amplification product from the first reaction was
used for the second reaction. The amplicons were electrophoresed
through a 2% agarose gel and visualized using ethidium bromide.
Methylation-specific PCR (MSP)
The CpG island of the WWOX gene is located 406 bp
upstream of the transcription start site and is considered the
promoter region. MSP was used to detect the methylation levels of
this region. DNA obtained from 165 patients with MM, 33 patients
with MGUS and 25 control patients, and cell lines KMM1, KMS11,
KMS12PE, KMS18, KMS26 and RPMI8226 were used for the MSP analysis.
Each 0.5 µg sample of genomic DNA was treated with sodium bisulfite
using the MethylEasy Xceed Rapid DNA Bisulfite Modification kit
(Takara Bio, Inc.) following manufacturer's protocol, and the
converted DNA was subjected to PCR. MSP was performed using
specific primers designed to amplify methylated or unmethylated
sequences of the WWOX promoter. The primer sequences for methylated
or unmethylated DNA are as follows: Methylated forward,
5′-TATGGGCGTCGTTTTTTTAGTT-3′ and reverse,
5′-CAATCTCCGCAATATCGCGACA-3′; unmethylated forward,
5′-TATGGGTGTTGTTTTTTTAGTT-3′ and reverse,
5′-CAATCTCCACAATATCACAACA-3′ (31).
Each reaction (20 µl each) contained 0.2 µmol of each primer, 2.0
mM MgCl2, 0.2 mM dNTP mix, 1X PCR buffer and 1.25 units
of Takara ExTaq Hot Start Version (Takara Bio, Inc.). The
thermocycling conditions maintained were as follows: 95°C for 8
min; 35 cycles at 94°C for 30 sec, 58°C for 30 sec, and 72°C for 1
min; and an extension step of 72°C for 5 min. The amplicons were
electrophoresed through a 2% agarose gel and visualized using
ethidium bromide.
Statistical analysis
IBM SPSS software (version 22.0; IBM SPSS, Armonk,
NY, USA) was used for statistical analysis. Frequencies were
compared using the χ2 test, and mean values were
compared using the Student's t-test or the Mann-Whitney U test.
Overall survival (OS) and statistical significance were calculated
using the Kaplan-Meier estimator method, log-rank test, and
generalized Wilcoxon test.
Results
Analysis of WWOX mRNAs
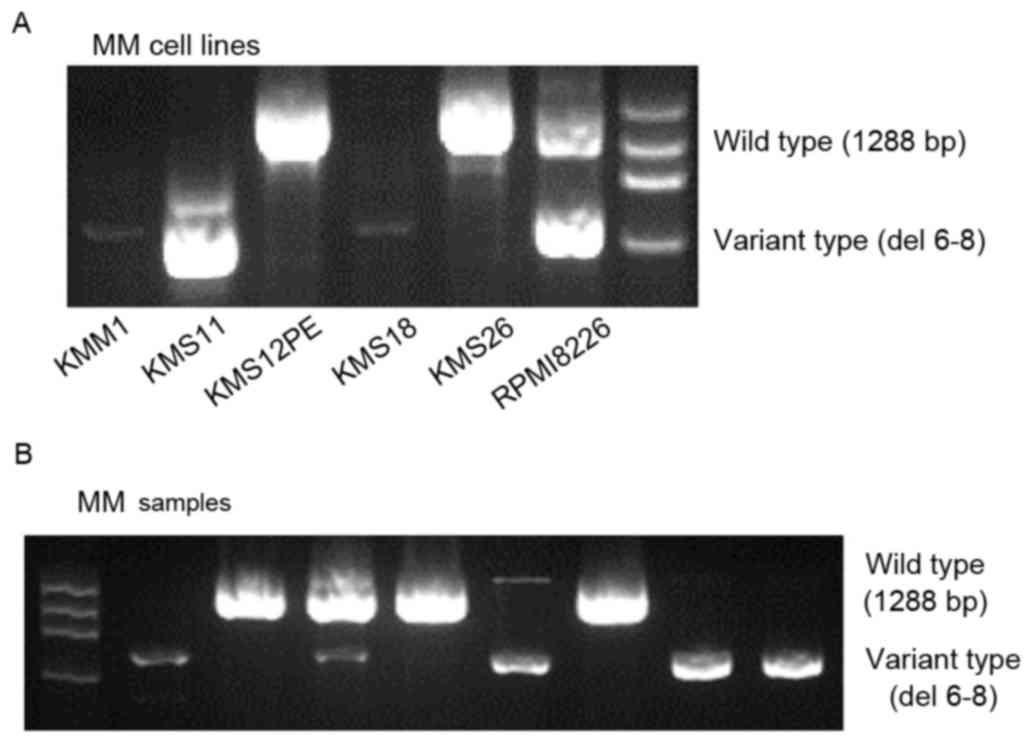
Nested PCR assays (Fig.
1A) detected the full-length wild-type WWOX mRNA and a short
variant WWOX mRNA lacking exons 6–8. The variant type was expressed
at low levels by KMM1 and KMS18 cells, and at higher levels by
KMS11 and RPMI8226 cells. Full-length wild-type WWOX was detected
in KMS12PE and KMS26 cells, and RPMI8226 cells expressed the
wild-type and variant transcripts.
WWOX transcripts were undetectable in 9 patients
with MM, 7 patients with MGUS and 4 patients with lymphoma (data
not shown), and those patients were excluded from the following
analysis. The variant WWOX mRNA was detected in 44/68 (65%)
patients with MM (Fig. 1B), 13/26
(50%) cases of patients with MGUS and in 2/21 (10%) patients with
lymphoma (Table I). This indicated
that MM and MGUS bone marrow cells expressed the variant WWOX at a
similar frequency (P=0.16), and at a significantly higher frequency
compared with lymphoma bone marrow cells (P<0.001 and P=0.004,
respectively; Table I). The similar
high frequencies of detection of variant WWOX mRNA in patients with
MM and MGUS suggested that WWOX alteration occurred during the
premalignant stage of MM.
 | Table I.Frequencies of variant WWOX mRNA,
WWOX promoter methylation, and RASSF1A promoter methylation in
patients with MM or MGUS and control subjects. |
Table I.
Frequencies of variant WWOX mRNA,
WWOX promoter methylation, and RASSF1A promoter methylation in
patients with MM or MGUS and control subjects.
|
| No. patients
(%) |
|
|---|
|
|
|
|
|---|
| Genomic
alteration | Lymphoma | MGUS | MM | P-value |
|---|
| Variant WWOX | 2/21 (10) | 13/26 (50) | 44/68 (65) | 0.1620a |
|
|
|
|
| 0.0001b |
|
|
|
|
| 0.0030c |
| WWOX
methylation | 1/25 (4) | 2/33
(6) | 58/165 (35) | 0.0012a |
|
|
|
|
| 0.0020b |
|
|
|
|
| 0.7300c |
| RASSF1A
methylation | 3/25 (12) | 14/33 (42) | 63/165
(38) | 0.7120a |
|
|
|
|
| 0.0120b |
|
|
|
|
| 0.0190c |
CD138-positive plasma cells and CD138-negative bone
marrow cells obtained from the same patients with MM were purified
and analyzed to determine whether variant WWOX was expressed by
plasma cells. The variant WWOX mRNA was detected in the
CD138-positive plasma cells of 17/30 patients (57%), which was
equivalent to the results of the analysis of whole-marrow
mononuclear cells of patients with MM described above (P=0.45; data
not shown). This was significantly higher compared with the
detection in 6/30 (24%) of the CD138-negative cell samples
(P=0.01). These results indicated that the variant form of WWOX
mRNA was a genuine abnormality of MM cells and not a characteristic
of all hematopoietic cells.
Methylation of the WWOX promoter in MM
cell lines and tumor cells
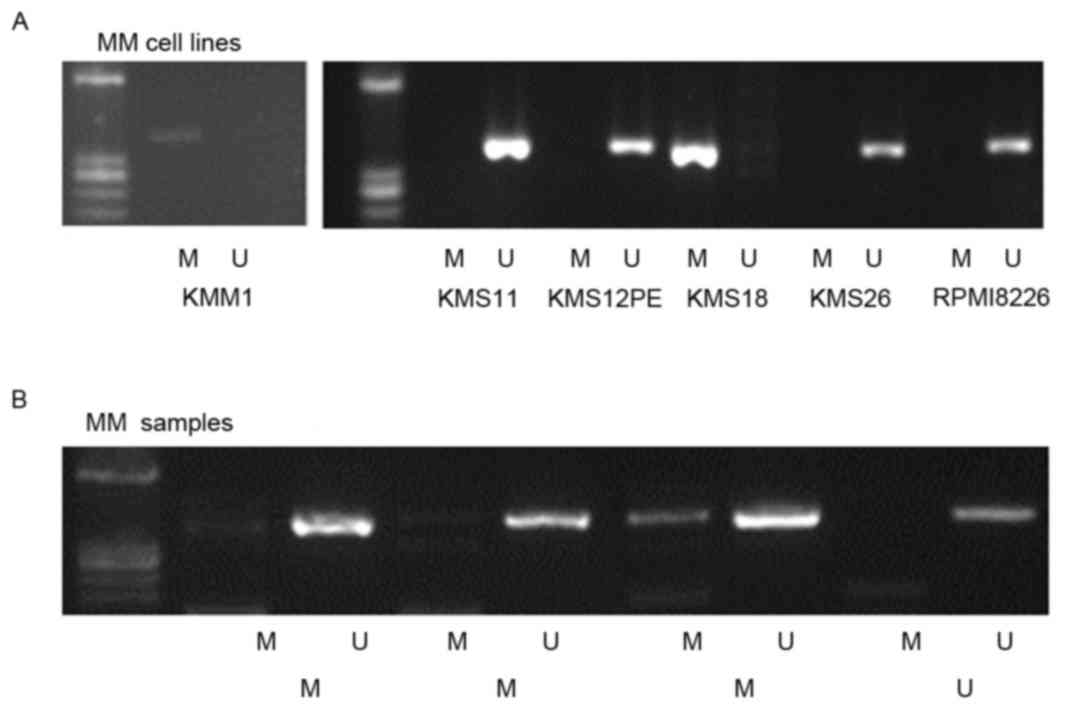
MSP analysis detected WWOX promoter methylation in
2/6 (KMM1 and KMS18) cell lines (Fig.
2A) and in 3/4 patients with MM (Fig.
2B). Methylation of the WWOX promoter was detected in samples
from 58/165 (35%) patients with MM, 2/33 (6%) patients with MGUS,
and in 1/25 patients with lymphoma (4%). The frequency of WWOX
promoter methylation in patients with MM was significantly higher
compared with those with MGUS (P=0.001) or lymphoma (P=0.002), but
the difference was not significant between patients with MGUS or
lymphoma (P=0.73; Table I). This
indicated that WWOX was preferentially methylated in MM cells.
To determine whether methylation was specific for
WWOX or reflected the methylation status of tumor suppressor genes
in MM cells, Ras association domain family member 1 isoform A
(RASSF1A) promoter methylation was analyzed. RASSF1A promoter
methylation was detected in 63/165 patients with MM (38%), 14/33
patients with MGUS (42%), and in 3/25 patients with lymphoma (12%;
Table I). No significant difference
in the frequency between the number of patients with MM and MGUS
was identified (P=0.70). In patients with MM, the frequency of
RASSF1A promoter methylation was equivalent to that of WWOX
hypermethylation. However, the RASSF1A promoter was more frequently
methylated compared with WWOX in patients with MGUS (P=0.04; data
not shown), suggesting that specific methylation of WWOX was
associated with the progression of MM.
Association between the prognosis of
patients with MM and WWOX promoter methylation
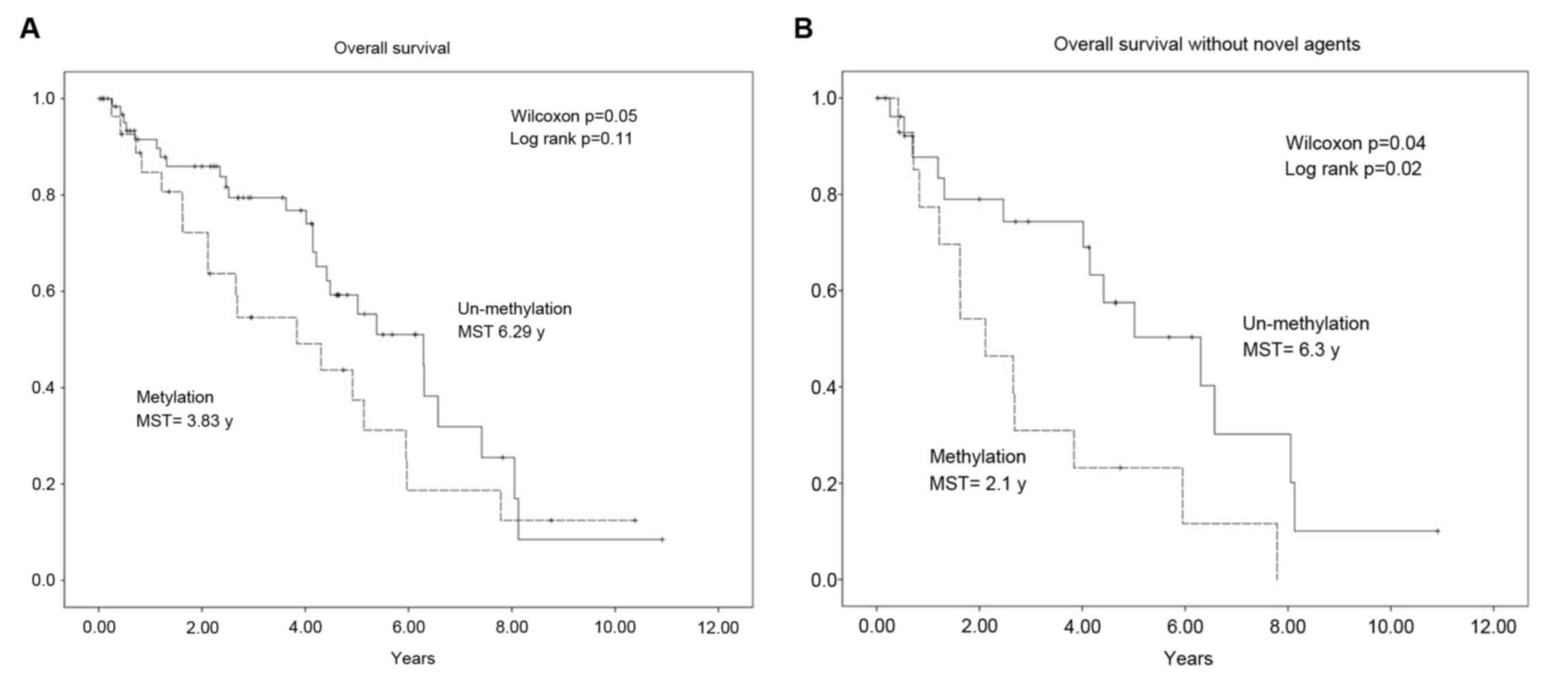
The association between WWOX promoter methylation
and the OS of patients with MM was determined using the
Kaplan-Meier estimator method, log-rank, and the generalized
Wilcoxon test. The median OS time was shorter in patients with
methylated WWOX sequences compared with those without methylation
(3.83 vs. 6.29 years; Fig. 3A). This
difference was significant according to the results of the
generalized Wilcoxon test (P=0.02) but not those of the log-rank
test (P=0.11). As novel agents, namely bortezomib, thalidomide and
lenalidomide, are effective for treating MM, the patients were
stratified according to those treated with or without novel agents
and the data were analyzed again. The median OS time of patients
with WWOX promoter methylation was shorter compared with those
without methylation (2.1 vs. 6.3 years). The difference was
significant according to the results of the generalized Wilcoxon
test (P=0.04) and the log-rank test (P=0.02; Fig. 3B). In contrast, no significant
difference between the median OS times of each class of patients
treated with novel agents were identified (methylation, 5.1 years;
undetectable methylation, 5.4 years; P=0.73; data not shown).
Analysis of WWOX promoter methylation,
β2-microglobulin levels, and International Staging System (ISS)
classification
The higher incidence of WWOX promoter methylation in
patients with MM compared with those with MGUS, and the association
between methylation and shorter OS time, indicated that WWOX
promoter methylation was associated with disease progression. To
support this hypothesis, the association between WWOX promoter
methylation, serum β2-microglobulin levels and MM stage was
analyzed according to the ISS (49).
The mean β2-microglobulin level was significantly higher in
patients with WWOX promoter methylation compared with those without
(6.80 vs. 4.68 mg/l; P=0.02; data not shown). The frequency of
patients at ISS stage 3 with WWOX promoter methylation was
significantly higher compared with those without methylation
(P=0.02; data not shown).
Discussion
In the present study, recurrent expression of a
short form of WWOX mRNA was demonstrated in patients with MM or
MGUS. Furthermore, it was revealed that the WWOX promoter was
frequently methylated in patients with MM, and that this increased
during the progression of disease from MGUS to advanced MM. In
addition, WWOX promoter methylation was identified to be associated
with a shorter median OS time.
The wild-type transcript is ubiquitously expressed
and shorter variants also occur (15,17). For
example, homozygous deletion of chromosome 16q23.2 in various
cancer cell lines includes deletions of WWOX exons (17) that generate shorter WWOX mRNA
variants. In a previous study, numerous truncated WWOX variants
lacking exons 5–8 were identified in clinical samples obtained from
patients with breast cancer (27).
Reduced expression of the full-length WWOX transcript by cancer
cells and the detection of high levels of variant WWOX transcripts
that occur specifically in tumors indicates that WWOX may be
involved in oncogenesis (27).
In the present study, variant WWOX mRNAs were
detected in myeloma cell lines and plasma cells of patients with MM
or MGUS, but at a significantly lower frequency in the cells of
control patients. These results indicated that WWOX alteration was
associated with aberrant plasma cells.
The instability of CFSs correlates with genomic
instability in precancerous lesions (50) and the early stages of oncogenesis are
associated with the DNA damage response (50,51).
Genomic instability and abnormalities are hallmarks of MM, and
aberrant DNA repair pathways are involved in disease onset and
progression (52). WWOX deficiency
reduces the levels of ATM serine/threonine kinase and impairs DNA
repair, which may drive genomic instability (51). Therefore, WWOX alterations may also
cause genomic instability. The results of the present study on
variant WWOX mRNA expression in patients with MGUS suggested that
the alteration of a CFS indicates genomic instability at an early
stage of the disease.
Studies of WWOX protein knockout and hypomorphic
mice have demonstrated that a functional defect of WWOX leads to
the induction of various types of tumor, including lymphoma and
plasmacytoma (53–55). In vitro, WWOX inhibits
β-catenin (56) and suppresses the
transcriptional activity of the nuclear factor-κB (NF-κB)-RELA
proto-oncogene, NF-κB subunit complex (57), which are involved in the pathogenesis
of MM. Variant WWOX serves as a dominant-negative factor in
vitro to inhibit the tumor suppressor function of wild-type
WWOX (26). These findings, taken
together with those of the present study, support the hypothesis
that the loss of WWOX function serves a causative role in the
pathogenesis of MM.
The frequent detection of WWOX promoter methylation
in the cells of patients with MM, in contrast to patients with MGUS
and the control group, indicates that WWOX promoter methylation is
associated with MM progression. This is consistent with findings
that WWOX methylation correlates with poor prognosis of patients
with ovarian cancer (47), head and
neck cancer (58), and
chorangiocarcinoma (59).
No significant correlation was identified between
WWOX methylation and OS. This result may be due to improved
treatment outcomes using novel agents. Therefore, patients were
stratified according to the types of therapy they received and
prognosis was identified as being worse for patients with WWOX
methylation if they had not received treatment with a novel agent,
suggesting that WWOX methylation may be associated with resistance
to conventional cytotoxic drugs.
In conclusion, the present study demonstrated that
WWOX promoter methylation is associated with β2-microglobulin
levels and ISS, indicating that WWOX methylation contributes to MM
progression. Unlike WWOX, the rate of methylated RASSF1A was
similar between patients with MGUS and MM. Therefore, the
association of WWOX methylation with a more progressive and worse
phenotype may not reflect the methylation of tumor suppressor
genes.
Genomic instability is a hallmark of the majority of
types of cancer, and is potentially involved in oncogenesis and the
response to therapy. As WWOX is a putative human tumor suppressor
gene, it appears possible that the selection for loss of function
driven by fragile site instability is involved in MM progression.
Further mechanistic studies are required to determine the role of
WWOX and other genes within other CFSs in the pathogenesis of
MM.
Acknowledgements
The present study was supported by the Ministry of
Education, Science and Culture, Japan (grant no. 20590556).
References
|
1
|
Bergsagel PL and Kuehl WM: Chromosome
translocations in multiple myeloma. Oncogene. 20:5611–5622. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chesi M and Bergsagel PL: Molecular
pathogenesis of multiple myeloma: Basic and clinical updates. Int J
Hematol. 97:313–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morgan GJ, Walker BA and Davies FE: The
genetic architecture of multiple myeloma. Nat Rev Cancer.
12:335–348. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egan JB, Shi CX, Tembe W, Christoforides
A, Kurdoglu A, Sinari S, Middha S, Asmann Y, Schmidt J, Braggio E,
et al: Whole-genome sequencing of multiple myeloma from diagnosis
to plasma cell leukemia reveals genomic initiating events,
evolution, and clonal tides. Blood. 120:1060–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vincent Rajkumar S: Multiple myeloma: 2014
Update on diagnosis, risk-stratification and management. Am J
Hematol. 89:999–1009. 2014.PubMed/NCBI
|
|
6
|
Dillon LW, Burrow AA and Wang YH: DNA
instability at chromosomal fragile sites in cancer. Curr Genomics.
11:326–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glover TW: Common fragile sites. Cancer
Lett. 232:4–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao G and Smith DI: Very large common
fragile site genes and their potential role in cancer development.
Cell Mol Life Sci. 71:4601–4615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Debatisse M, Le Tallec B, Letessier A,
Dutrillaux B and Brison O: Common fragile sites: Mechanisms of
instability revisited. Trends Genet. 28:22–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sutherland GR and Richards RI: The
molecular basis of fragile sites in human chromosomes. Curr Opin
Genet Dev. 5:323–327. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balsara BR, Pei J, De Rienzo A, Simon D,
Tosolini A, Lu YY, Shen FM, Fan X, Lin WY, Buetow KH, et al: Human
hepatocellular carcinoma is characterized by a highly consistent
pattern of genomic imbalances, including frequent loss of
16q23.1-24.1. Genes Chromosomes Cancer. 30:245–253. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paris PL, Witte JS, Kupelian PA, Levin H,
Klein EA, Catalona WJ and Casey G: Identification and fine mapping
of a region showing a high frequency of allelic imbalance on
chromosome 16q23.2 that corresponds to a prostate cancer
susceptibility locus. Cancer Res. 60:3645–3649. 2000.PubMed/NCBI
|
|
13
|
Hansen LL, Yilmaz M, Overgaard J, Andersen
J and Kruse TA: Allelic loss of 16q23.2-24.2 is an independent
marker of good prognosis in primary breast cancer. Cancer Res.
58:2166–2169. 1998.PubMed/NCBI
|
|
14
|
O'Keefe LV and Richards RI: Common
chromosomal fragile sites and cancer: Focus on FRA16D. Cancer Lett.
232:37–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ried K, Finnis M, Hobson L, Mangelsdorf M,
Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A,
Venter D, et al: Common chromosomal fragile site FRA16D sequence:
Identification of the FOR gene spanning FRA16D and homozygous
deletions and translocation breakpoints in cancer cells. Hum Mol
Genet. 9:1651–1663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bednarek AK, Laflin KJ, Daniel RL, Liao Q,
Hawkins KA and Aldaz CM: WWOX, a novel WW domain-containing protein
mapping to human chromosome 16q23.3-24.1, a region frequently
affected in breast cancer. Cancer Res. 60:2140–2145.
2000.PubMed/NCBI
|
|
17
|
Paige AJ, Taylor KJ, Taylor C, Hillier SG,
Farrington S, Scott D, Porteous DJ, Smyth JF, Gabr H and Watson JE:
WWOX: A candidate tumor suppressor gene involved in multiple tumor
types. Proc Natl Acad Sci USA. 98:11417–11422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ludes-Meyers JH, Bednarek AK, Popescu NC,
Bedford M and Aldaz CM: WWOX, the common chromosomal fragile site,
FRA16D, cancer gene. Cytogenet Genome Res. 100:101–110. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paige AJ, Taylor KJ, Stewart A, Sgouros
JG, Gabra H, Sellar GC, Smyth JF, Porteous DJ and Watson JE: A
700-kb physical map of a region of 16q23.2 homozygously deleted in
multiple cancers and spanning the common fragile site FRA16D.
Cancer Res. 60:1690–1697. 2000.PubMed/NCBI
|
|
20
|
Alsop AE, Taylor K, Zhang J, Gabra H,
Paige AJ and Edwards PA: Homozygous deletions may be markers of
nearby heterozygous mutations: The complex deletion at FRA16D in
the HCT116 colon cancer cell line removes exons of WWOX. Genes
Chromosomes Cancer. 47:437–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beroukhim R, Mermel CH, Porter D, Wei G,
Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J,
Urashima M, et al: The landscape of somatic copy-number alteration
across human cancers. Nature. 463:899–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bignell GR, Greenman CD, Davies H, Butler
AP, Edkins S, Andrews JM, Buck G, Chen L, Beare D, Latimer C, et
al: Signatures of mutation and selection in the cancer genome.
Nature. 463:893–898. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krummel KA, Roberts LR, Kawakami M, Glover
TW and Smith DI: The characterization of the common fragile site
FRA16D and its involvement in multiple myeloma translocations.
Genomics. 69:37–46. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jenner MW, Leone PE, Walker BA, Ross FM,
Johnson DC, Gonzalez D, Chiecchio L, Dachs Cabanas E, Dagrada GP,
Nightingale M, et al: Gene mapping and expression analysis of 16q
loss of heterozygosity identifies WWOX and CYLD as being important
in determining clinical outcome in multiple myeloma. Blood.
110:3291–3300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agnelli L, Mosca L, Fabris S, Lionetti M,
Andronache A, Kwee I, Todoerti K, Verdelli D, Battaglia C, Bertoni
F, et al: A SNP microarray and FISH-based procedure to detect
allelic imbalances in multiple myeloma: An integrated genomics
approach reveals a wide gene dosage effect. Genes Chromosomes
Cancer. 48:603–614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bednarek AK, Keck-Waggoner CL, Daniel RL,
Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ and Aldaz CM: WWOX,
the FRA16D gene, behaves as a suppressor of tumor growth. Cancer
Res. 61:8068–8073. 2001.PubMed/NCBI
|
|
27
|
Driouch K, Prydz H, Monese R, Johansen H,
Lidereau R and Frengen E: Alternative transcripts of the candidate
tumor suppressor gene, WWOX, are expressed at high levels in human
breast tumors. Oncogene. 21:1832–1840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gourley C, Paige AJ, Taylor KJ, Scott D,
Francis NJ, Rush R, Aldaz CM, Smyth JF and Gabra H: WWOX mRNA
expression profile in epithelial ovarian cancer supports the role
of WWOX variant 1 as a tumour suppressor, although the role of
variant 4 remains unclear. Int J Oncol. 26:1681–1689.
2005.PubMed/NCBI
|
|
29
|
Yendamuri S, Kuroki T, Trapasso F, Henry
AC, Dumon KR, Huebner K, Williams NN, Kaiser LR and Croce CM: WW
domain containing oxidoreductase gene expression is altered in
non-small cell lung cancer. Cancer Res. 63:878–881. 2003.PubMed/NCBI
|
|
30
|
Kuroki T, Trapasso F, Shiraishi T, Alder
H, Mimori K, Mori M and Croce CM: Genetic alterations of the tumor
suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer
Res. 62:2258–2260. 2002.PubMed/NCBI
|
|
31
|
Iliopoulos D, Guler G, Han SY, Johnston D,
Druck T, McCorkell KA, Palazzo J, McCue PA, Baffa R and Huebner K:
Fragile genes as biomarkers: Epigenetic control of WWOX and FHIT in
lung, breast and bladder cancer. Oncogene. 24:1625–1633. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iliopoulos D, Fabbri M, Druck T, Qin HR,
Han SY and Huebner K: Inhibition of breast cancer cell growth in
vitro and in vivo: Effect of restoration of Wwox expression. Clin
Cancer Res. 13:268–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cantor JP, Iliopoulos D, Rao AS, Druck T,
Semba S, Han SY, McCorkell KA, Lakshman TV, Collins JE, Wachsberger
P, et al: Epigenetic modulation of endogenous tumor suppressor
expression in lung cancer xenografts suppresses tumorigenicity. Int
J Cancer. 120:24–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ng MH, Chung YF, Lo KW, Wickham NW, Lee JC
and Huang DP: Frequent hypermethylation of p16 and p15 genes in
multiple myeloma. Blood. 89:2500–2506. 1997.PubMed/NCBI
|
|
35
|
Guillerm G, Gyan E, Wolowiec D, Facon T,
Avet-Loiseau H, Kuliczkowski K, Bauters F, Fenaux P and Quesnel B:
p16 (INK4a) and p15 (INK4b) gene methylations in plasma cells from
monoclonal gammopathy of undetermined significance. Blood.
98:244–246. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stanganelli C, Arbelbide J, Fantl DB,
Corrado C and Slavutsky I: DNA methylation analysis of tumor
suppressor genes in monoclonal gammopathy of undetermined
significance. Ann Hematol. 89:191–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Braggio E, Maiolino A, Gouveia ME,
Magalhães R, Souto Filho JT, Garnica M, Nucci M and Renault IZ:
Methylation status of nine tumor suppressor genes in multiple
myeloma. Int J Hematol. 91:87–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gonzalez M, Mateos MV, García-Sanz R,
Balanzategui A, López-Pérez R, Chillón MC, González D, Alaejos I
and San Miguel JF: De novo methylation of tumor suppressor gene
p16/INK4a is a frequent finding in multiple myeloma patients at
diagnosis. Leukemia. 14:183–187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heuck CJ, Mehta J, Bhagat T, Gundabolu K,
Yu Y, Khan S, Chrysofakis G, Schinke C, Tariman J, Vickrey E, et
al: Myeloma is characterized by stage-specific alterations in DNA
methylation that occur early during myelomagenesis. J Immunol.
190:2966–2975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kaiser MF, Johnson DC, Wu P, Walker BA,
Brioli A, Mirabella F, Wardell CP, Melchor L, Davies FE and Morgan
GJ: Global methylation analysis identifies prognostically important
epigenetically inactivated tumor suppressor genes in multiple
myeloma. Blood. 122:219–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tanaka H, Shimada Y, Harada H, Shinoda M,
Hatooka S, Imamura M and Ishizaki K: Methylation of the 5′ CpG
island of the FHIT gene is closely associated with transcriptional
inactivation in esophageal squamous cell carcinomas. Cancer Res.
58:3429–3434. 1998.PubMed/NCBI
|
|
42
|
Zöchbauer-Müller S, Fong KM, Maitra A, Lam
S, Geradts J, Ashfaq R, Virmani AK, Milchgrub S, Gazdar AF and
Minna JD: 5′ CpG island methylation of the FHIT gene is correlated
with loss of gene expression in lung and breast cancer. Cancer Res.
61:3581–3585. 2001.PubMed/NCBI
|
|
43
|
Ishii H, Vecchione A, Furukawa Y,
Sutheesophon K, Han SY, Druck T, Kuroki T, Trapasso F, Nishimura M,
Saito Y, et al: Expression of FRA16D/WWOX and FRA3B/FHIT genes in
hematopoietic malignancies. Mol Cancer Res. 1:940–947.
2003.PubMed/NCBI
|
|
44
|
Uehara E, Takeuchi S, Tasaka T, Matsuhashi
Y, Yang Y, Fujita M, Tamura T, Nagai M and Koeffler HP: Aberrant
methylation in promoter-associated CpG islands of multiple genes in
therapy-related leukemia. Int J Oncol. 23:693–696. 2003.PubMed/NCBI
|
|
45
|
Nakayama S, Semba S, Maeda N, Matsushita
M, Kuroda Y and Yokozaki H: Hypermethylation-mediated reduction of
WWOX expression in intraductal papillary mucinous neoplasms of the
pancreas. Br J Cancer. 100:1438–1443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang X, Chao L, Jin G, Ma G, Zang Y and
Sun J: Association between CpG island methylation of the WWOX gene
and its expression in breast cancers. Tumour Biol. 30:8–142. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan H and Sun J: Methylation status of
WWOX gene promoter CpG islands in epithelial ovarian cancer and its
clinical significance. Biomed Rep. 1:375–378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
International Myeloma Working Group:
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: A report of the
International Myeloma Working Group. Br J Haematol. 121:749–757.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Le Tallec B, Koundrioukoff S, Wilhelm T,
Letessier A, Brison O and Debatisse M: Updating the mechanisms of
common fragile site instability: How to reconcile the different
views? Cell Mol Life Sci. 71:4489–4494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Abu-Odeh M, Salah Z, Herbel C, Hofmann TG
and Aqeilan RI: WWOX, the common fragile site FRA16D gene product,
regulates ATM activation and the DNA damage response. Proc Natl
Acad Sci USA. 111:E4716–E4725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gourzones-Dmitriev C, Kassambara A, Sahota
S, Rème T, Moreaux J, Bourquard P, Hose D, Pasero P, Constantinou A
and Klein B: DNA repair pathways in human multiple myeloma: Role in
oncogenesis and potential targets for treatment. Cell Cycle.
12:2760–2773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aqeilan RI, Trapasso F, Hussain S,
Costinean S, Marshall D, Pekarsky Y, Hagan JP, Zanesi N, Kaou M,
Stein GS, et al: Targeted deletion of Wwox reveals a tumor
suppressor function. Proc Natl Acad Sci USA. 104:3949–3954. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ludes-Meyers JH, Kil H, Nuñez MI, Conti
CJ, Parker-Thornburg J, Bedford MT and Aldaz CM: WWOX hypomorphic
mice display a higher incidence of B-cell lymphomas and develop
testicular atrophy. Genes Chromosomes Cancer. 46:1129–1136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ludes-Meyers JH, Kil H, Parker-Thornburg
J, Kusewitt DF, Bedford MT and Aldaz CM: Generation and
characterization of mice carrying a conditional allele of the Wwox
tumor suppressor gene. PloS One. 4:e77752009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bouteille N, Driouch K, Hage PE, Sin S,
Formstecher E, Camonis J, Lidereau R and Lallemand F: Inhibition of
the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein.
Oncogene. 28:2569–2580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fu J, Qu Z, Yan P, Ishikawa C, Aqeilan RI,
Rabson AB and Xiao G: The tumor suppressor gene WWOX links the
canonical and noncanonical NF-κB pathways in HTLV-I Tax-mediated
tumorigenesis. Blood. 117:1652–1661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ekizoglu S, Bulut P, Karaman E, Kilic E
and Buyru N: Epigenetic and genetic alterations affect the WWOX
gene in head and neck squamous cell carcinoma. PloS One.
10:e01153532015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang C, Tian Y, Peng R, Zhang C, Wang D,
Han S, Jiao C, Wang X, Zhang H, Wang Y and Li X: Association of
downregulation of WWOX with poor prognosis in patients with
intrahepatic cholangiocarcinoma after curative resection. J
Gastroenterol Hepatol. 30:421–433. 2015. View Article : Google Scholar : PubMed/NCBI
|