Introduction
Hepatocellular adenoma (HCA) is a benign liver tumor
that occurs mainly in young females subsequent to the long-term use
of oral contraceptives (1,2). It is a type of rare tumor with low
morbidity (3). However, HCA may lead
to hemorrhage, and even malignant transformation to hepatocellular
carcinoma (HCC), which is the third leading cause of
cancer-associated mortality worldwide (4–8). A rising
incidence has been reported due to improved application of
diagnostic imaging techniques (9).
HCA is rare in children, men and post-menopausal women. The use of
androgenic steroids for Fanconi's anemia or acquired aplastic
anemia is a risk factor for the development of HCA (10). Other drugs are involved in its
development, such as clomiphene, barbiturates and recombinant human
growth hormone (11–13). Obesity and alcohol abuse have also
been reported as risk factors for developing HCA (14).
Previously, ultrasound and magnetic resonance
imaging (MRI) have been suggested as effective tools for the
diagnosis of HCA and explore its biological mechanisms (15). According to the different genetic
mutations, HCA is divided into 4 major molecular subgroups:
Hepatocellular nuclear factor-1α (HNF1α)-mutated type HCA;
β-catenin-mutated type HCA; inflammatory type HCA; and unclassified
type HCA (8,14,16). The
transcription factor 1 (TCF1) gene, which encodes HNF1α, has been
identified in the liver (16).
Overexpression of certain genes, including erb-b2 receptor tyrosine
kinase 2, mechanistic target of rapamycin, platelet-derived growth
factor α polypeptide, platelet-derived growth factor β polypeptide
and cyclin D1, has been identified in HCA, and the products of
these genes are associated with cell proliferation, cell cycle
activation and angiogenesis (17).
In the human genome, GC-rich DNA sequences, also
known as CpG islands, are frequently enriched in the first exon and
the promoter (18). DNA methylation
at CpG islands located upstream of a gene promoter is associated
with differential expression of the gene. DNA methylation regulates
gene silencing by directly inhibiting the binding of
methylation-dependent transcriptional activators or indirectly
altering the affinity of proteins, including methylated DNA binding
domain protein, involved in chromatin remodeling (19–23). At
present, DNA methylation is widely identified in human cancer,
including HCC. It was reported that long interspersed nuclear
element-1 (LINE-1) has lower DNA methylation levels in
hepatitis virus and aflatoxin-associated HCC compared with normal
liver tissue (24,25). In addition, hypomethylation of
LINE-1 was associated with advanced disease and poorer
survival in HCC (26). The DNA
methylation level of spermidine/spermine N1-acetyltransferase
family member 2 (SAT2) also has a significant role in liver
carcinogenesis. It has been suggested that decreased SAT2
methylation of white blood cell DNA was significantly associated
with increased HCC risk later in life (27).
It is challenging to diagnose HCC and HCA at an
early stage. Numerous therapies are limited when HCC enters the
advanced stage, as the advanced stage is accompanied by severe
liver dysfunction (28). Therefore,
it is necessary to identify early biomarkers of HCA. In the present
study, data of the DNA methylation profile and gene expression
profile was extracted from the Gene Expression Omnibus (GEO)
database. Certain therapeutic targets and the related pathways that
may be associated with the development of HCA were identified by
microarray analysis. This may contribute to promoting available
biomarkers for the early diagnosis, therapy and prognosis of
HCA.
Materials and methods
Microarray data
The gene expression profile and DNA methylation
profile were both downloaded from the GEO (http://www.ncbi.nlm.nih.gov/geo/) database. The gene
expression profile (GSE7473) contained 41 samples, including 8
HNF1α-mutated HCA and the corresponding non-tumor liver samples
(each sample was assessed four times using 11K_VJF-ARRAY; GPL3282),
and 5 HNF1α-mutated HCA and 4 non-related non-tumor liver samples
(the 9 samples were assessed using GPL96 Affymetrix Human Genome
U133A Array). In the present study, the 9 samples that were
assessed via GPL96 were used as the objects, and the 5
HNF1α-mutated HCA and 4 non-related non-tumor liver samples were
classified as the case and control groups, respectively. The DNA
methylation profile (GSE43091), provided by Pilati et al
(29), contained 50 HCA and 4 normal
liver tissues. These 54 samples were achieved by GPL13534 Illumina
HumanMethylation450 BeadChip (HumanMethylation450_15017482).
Data preprocessing
The raw microarray data were converted into
expression data using the affy package of R. The values of
multiple probes that correspond to the same gene were summarized.
For original DNA methylation data, the β value of every methylated
site was calculated and normalized using the IMA package of
R.
Identification of differentially
methylated sites and differentially expressed genes (DEGs)
DEGs were identified using the limma package of
R with P<0.05 and |log2 (fold-change)|>1. A paired
Student's t-test was conducted on the methylation levels between
HCA samples and normal samples, and the differentially methylated
sites with adjusted P<0.05 and |Δβ|>0.2 were selected.
Functional enrichment analysis of
DEGs
Gene Ontology (GO) and Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathway analysis of DEGs was performed using the
Database for Annotation, Visualization and Integrated Discovery
(DAVID). DAVID was used to perform functional annotation for a list
of genes, gene functional classification or gene ID conversion. All
GO terms and KEGG pathways with P<0.05 that contained at least
five genes were selected for subsequent analysis.
Comprehensive analysis of gene
expression profile and DNA methylation profile
The genes in which differentially methylated sites
were located were identified using the annotation files of the
methylation chip platform.
Results
Differentially methylated sites and
differentially expressed genes
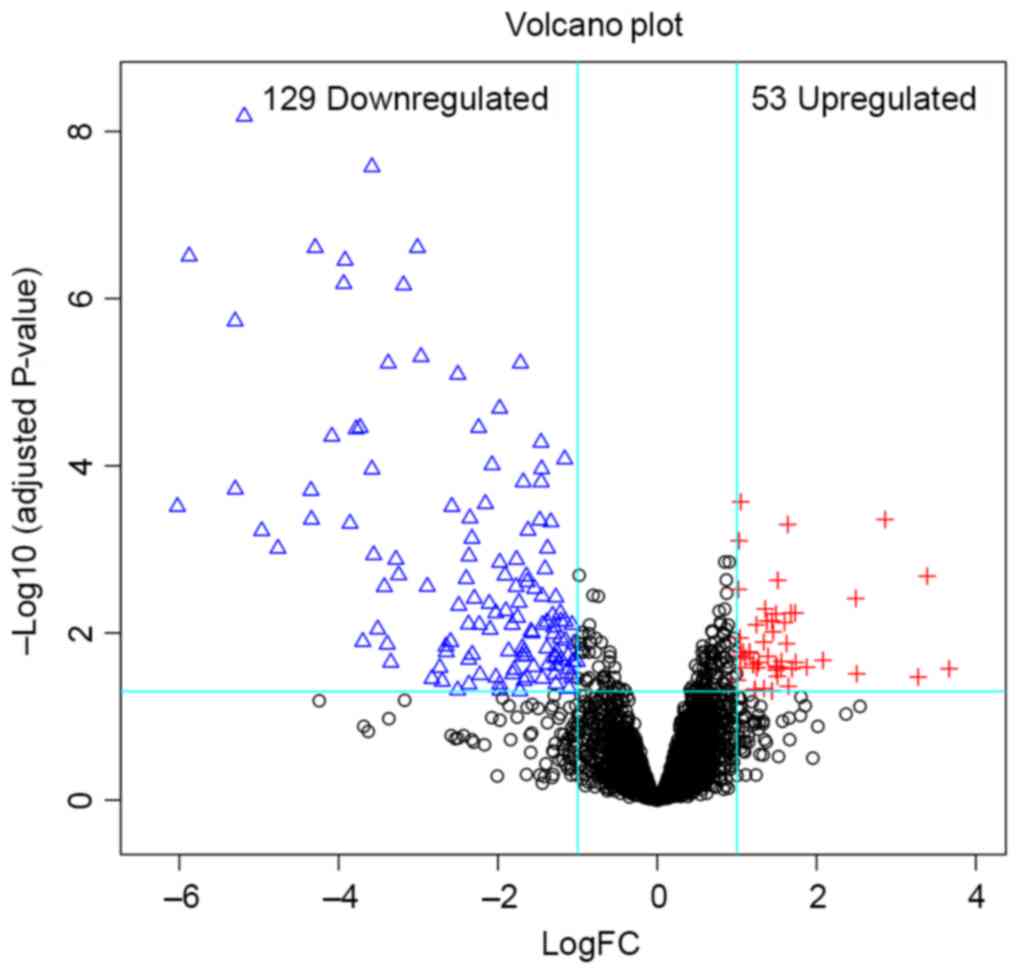
In total, 182 DEGs (53 upregulated and 129
downregulated) were identified in HCA. The volcano plot (Fig. 1) showed the distribution of DEGs. From
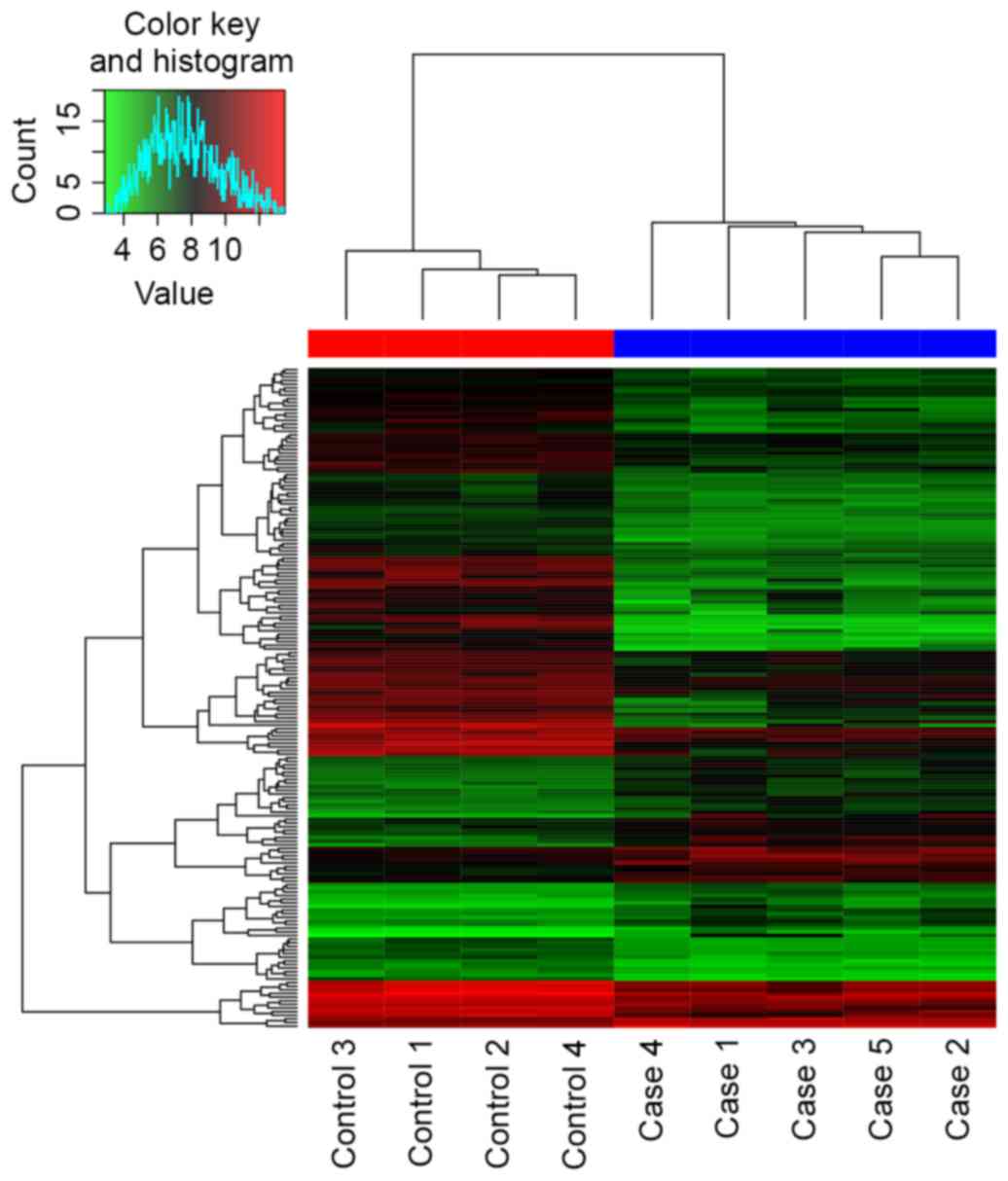
the heatmap (Fig. 2), the case
samples were found to be distinguished from the control samples.
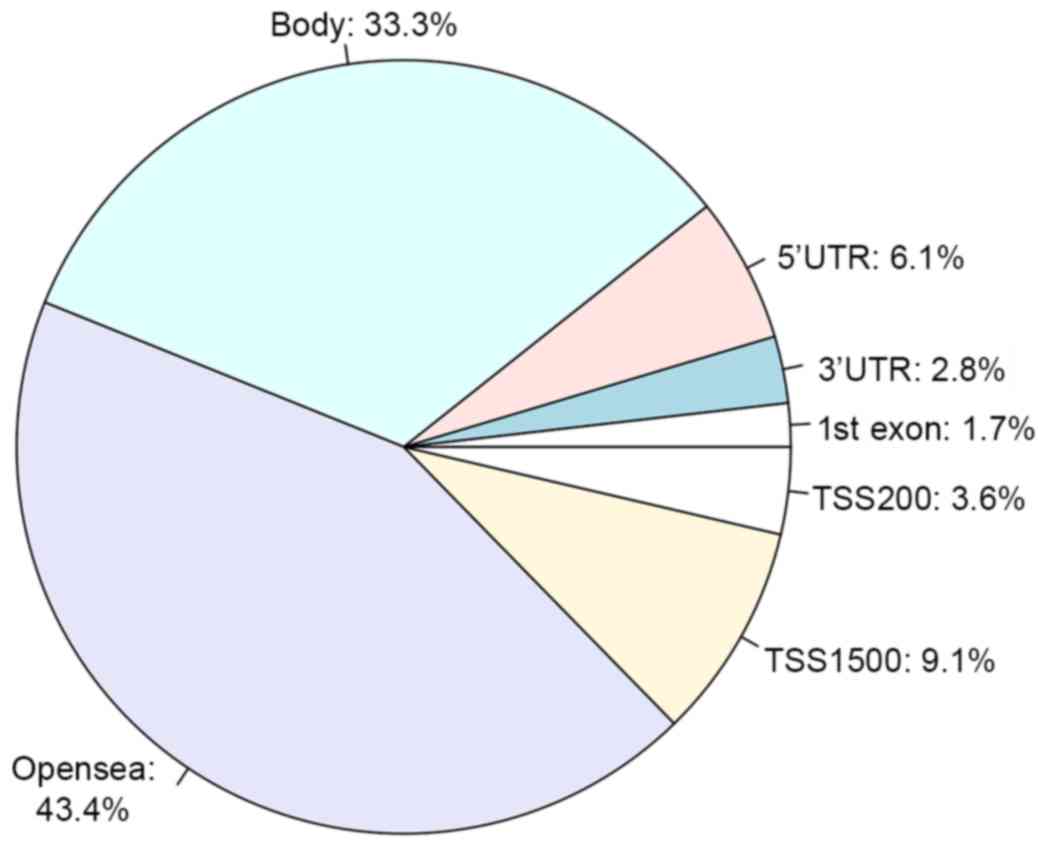
Additionally, a total of 3,902 differentially methylated sites were
obtained, including 3,715 downregulated methylated sites and 187
upregulated methylated sites. These methylated sites were mostly
located in the intergenic and gene-coding regions of genes
(Fig. 3).
Enriched GO terms and KEGG
pathways
In the present study, a total of 238 enriched GO
terms and 14 KEGG pathways were identified according to the
criteria P<0.05. The top 20 enriched GO terms are listed in
Table I. The majority of the enriched
GO terms were involved in the organic acid metabolic process. The
enriched KEGG pathways of the DEGs are shown in Table II. Certain KEGG pathways, for example
the chemical carcinogenesis pathway, mineral absorption pathway and
Bile secretion, were directly associated with HCA, and they may
affect the development of HCA.
 | Table I.Top 20 enriched GO terms for
differentially expressed genes. |
Table I.
Top 20 enriched GO terms for
differentially expressed genes.
| Category | GO ID | GO name | Gene number | P-value |
|---|
| BP | GO:0006082 | Organic acid
metabolic process | 54 |
1.89×10−23 |
| BP | GO:0019752 | Carboxylic acid
metabolic process | 51 |
2.08×10−23 |
| BP | GO:0043436 | Oxoacid metabolic
process | 51 |
2.88×10−21 |
| BP | GO:0032787 | Monocarboxylic acid
metabolic process | 36 |
3.97×10−20 |
| BP | GO:0042493 | Response to
drug | 26 |
4.53×10−14 |
| BP | GO:0006805 | Xenobiotic
metabolic process | 17 |
2.54×10−13 |
| BP | GO:0071466 | Cellular response
to xenobiotic stimulus | 17 |
2.82×10−13 |
| BP | GO:0010038 | Response to metal
ion | 21 |
3.60×10−13 |
| BP | GO:0009410 | Response to
xenobiotic stimulus | 17 |
5.16×10−13 |
| BP | GO:0055114 | Oxidation-reduction
process | 38 |
1.05×10−12 |
| BP | GO:0010035 | Response to
inorganic substance | 24 |
1.90×10−12 |
| MF | GO:0016491 | Oxidoreductase
activity | 31 |
2.60×10−12 |
| BP | GO:0006629 | Lipid metabolic
process | 40 |
1.54×10−11 |
| MF | GO:0004497 | Monooxygenase
activity | 13 |
2.02×10−11 |
| BP | GO:0071294 | Cellular response
to zinc ion | 7 |
4.12×10−11 |
| CC | GO:0005615 | Extracellular
space | 39 |
4.44×10−11 |
| BP | GO:0044282 | Small molecule
catabolic process | 19 |
4.76×10−11 |
| BP | GO:0008202 | Steroid metabolic
process | 19 |
1.20×10−10 |
| BP | GO:0071248 | Cellular response
to metal ion | 12 |
2.21×10−10 |
| MF | GO:0020037 | Heme binding | 13 |
4.63×10−10 |
 | Table II.Enriched KEGG pathways for
differentially expressed genes. |
Table II.
Enriched KEGG pathways for
differentially expressed genes.
| KEGG pathway
name | Gene number | P-value |
|---|
| Chemical
carcinogenesis | 11 |
7.14×10−09 |
| Mineral
absorption | 9 |
1.84×10−08 |
| Bile secretion | 10 |
3.21×10−08 |
| Tryptophan
metabolism | 8 |
4.18×10−08 |
| Linoleic acid
metabolism | 7 |
7.54×10−08 |
| Retinol
metabolism | 9 |
1.44×10−07 |
| Drug
metabolism-cytochrome P450 | 9 |
2.47×10−07 |
| Arginine and
proline metabolism | 8 |
7.43×10−07 |
| Steroid hormone
biosynthesis | 8 |
7.43×10−07 |
| Metabolism of
xenobiotics by |
| cytochrome
P450 | 8 |
5.61×10−06 |
| Serotonergic
synapse | 8 |
1.31×10−04 |
| Arachidonic acid
metabolism | 6 |
1.94×10−04 |
| Carbon
metabolism | 6 |
1.44×10−03 |
|
Glycolysis/gluconeogenesis | 5 |
1.78×10−03 |
Key genes in hepatocellular
adenoma
In total, 12 DEGs that contained differentially
methylated sites were identified in the case groups compared with
the control groups (Fig. 4). Among
the DEGs, 8 genes with inverse associations between gene expression
level and DNA methylation level were identified, consisting of
annexin A2 (ANXA2), chitinase 3-like 1 (CHI3L1),
fibroblast growth factor receptor 4 (FGFR4), mal, T-cell
differentiation protein like (MALL), palladin, cytoskeletal
associated protein (PALLD), plasmalemma vesicle associated
protein (PLVAP), pregnancy zone protein (PZP) and
solute carrier family 22 member 1 (SLC22A1).
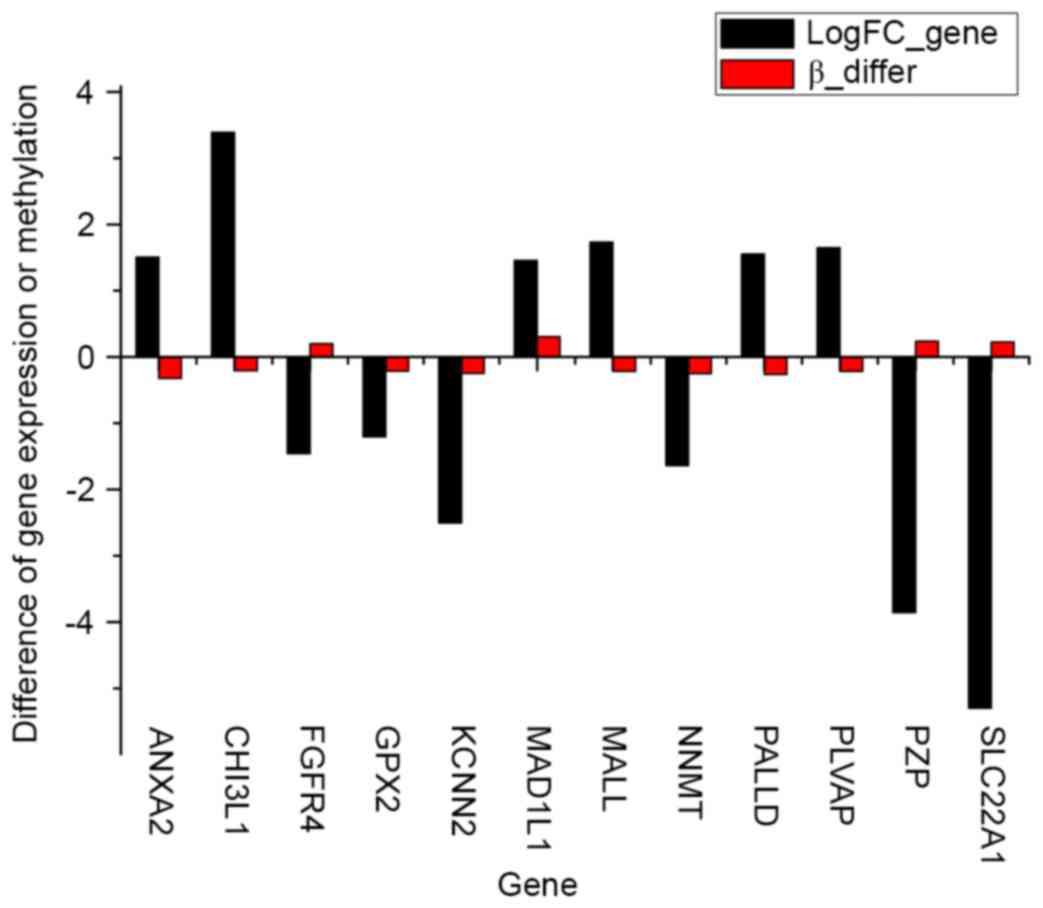 | Figure 4.In total, 12 genes were
differentially expressed and methylated in the case groups compared
with the control groups. LogFC_Gene, fold-change in gene
differential expression; Beta_differ, fold-change in gene
methylation level; ANXA2, annexin A2; CHI3L1, chitinase 3-like 1;
FGFR4, fibroblast growth factor receptor 4; MALL, mal, T-cell
differentiation protein like; PALLD, palladin, cytoskeletal
associated protein; PLVAP, plasmalemma vesicle associated protein;
PZP, pregnancy zone protein; SLC22A1, solute carrier family 22
member 1. |
Discussion
The development of human cancers is associated with
two factors: Gradual accumulation and mutual interactions of
genetic and epigenetic alterations (30). As one of the major characteristics in
human cancers, epigenetic alterations may suggest the molecular
mechanisms underlying malignant transformation (30–33). DNA
methylation is one of these epigenetic mechanisms, and is widely
found in human cancers, including HCA. At present, studies have
reported that a family of DNA methyltransferase enzymes (DNMTs)
mediates DNA methylation. DNMT1 was found to maintain methylation,
whereas DNMT3A and DNMT3B induced de novo methylation
(34). Promoter hypermethylation was
associated with gene expression and resulted in transcriptional
inhibition and loss of gene function. Certain studies have revealed
that dysregulation of the removal and establishment of DNA
methylation was involved in hepatocarcinogenesis (35,36). In
HCC, hypermethylation mainly affects the expression of certain
tumor suppressor genes, particularly the genes involving in cell
differentiation, cell proliferation, cell adhesion, cellular
metabolism, and DNA repair. Hypermethylated genes, including
adenomatosis polyposis coli, Ras association domain family member 1
and suppressor of cytokine signaling 1, have been identified in
chronic hepatitis and cirrhosis (20,37–40). Also,
genes such as glutathione S-transferase pi 1, cyclin dependent
kinase inhibitor 2A, cytochrome c oxidase subunit II, HIC ZBTB
transcriptional repressor 1, and runt related transcription factor
3, which were frequently methylated, have been identified in
dysplastic liver nodules (20,41–43).
Previously, the emergence of new diagnostic methods
such as ultrasound, and novel treatment methods such as
liver-directed therapy, has improved the prognosis of HCA (44,45).
However, a limit remains in terms of its early diagnosis and
curative potential. The present study used microarray technology to
identify key factors, such as the genes, biological process and
signal pathways, involved in HCA.
In the present study, DNA expression and methylation
profiles were obtained by bioinformatics to identify the
differentially methylated sites and DEGs in HCA compared with
normal liver tissues. A total of 182 DEGs (53 upregulated and 129
downregulated) and 3,902 differentially methylated sites (187
upregulated and 3,715 downregulated) were identified. In addition,
8 overlapped genes with inverse correlations between methylation
levels and gene expression levels were identified, including
PZP and SLC22A1.
Important genes may be potential targets for HCA
diagnosis or treatment. PZP is a major pregnancy-associated
plasma protein that is strongly associated with α 2-macroglobulin
(46). PZP is considered an
auxiliary index for the identification of gynecological tumors.
Berne (47) revealed that estrogen
induced PZP expression, and PZP was found in the serum of women who
usually took oral contraceptives containing estrogen. As the
long-term use of oral contraceptives may lead to HCA, this
indicates that PZP may be a major gene in HCA. Polyspecific
organic cation transporters are involved in the uptake of
positively charged and neutral small molecules, certain drugs and
environmental toxins into organs including the liver, kidney and
intestine (48). In 1967, the human
organic cation transporter 1, which is encoded by SLC22A1,
was first reported to remove >70% of the serotonin in the portal
blood via filtration and metabolism in the liver (49). Solute carrier family 22 (organic
cation transporter), member 1 is the major active influx protein
responsible for the transport of imatinib mesylate into cells
(50). The present study hypothesized
that SLC22A1 may play a critical role in removing endogenous
substances, drugs and other toxins associated with HCA.
According to the functional enrichment of DEG
analysis, certain GO terms associated with metabolic process and
response to drugs were identified, and the KEGG pathways closely
associated with chemical carcinogenesis and mineral absorption were
obtained. Chemical carcinogenesis has become synonymous with
genotoxic events, which lead to DNA damage and genetic mutations
(51). In addition, epigenetic
effects, such as aberrant DNA methylation, have been identified as
one of the key contributors to carcinogenesis. Aberrant DNA
methylation has been widely studied in carcinogenesis (19,52,53).
Although certain animal models, which reflect the association
between exposure to chemical carcinogens and epigenetic effects,
have been established, the specific mechanism of this process has
yet to be clarified (54).
Overall, using bioinformatics analysis, DEGs,
differentially methylated sites, significant GO terms and KEGG
pathways were obtained. It was found that DEGs were mainly involved
in acid metabolic processing and chemical carcinogenesis in HCA.
Based on comprehensive bioinformatics analysis, 8 important DEGs,
consisting of SLC22A1, PZP, ANXA2, CHI3L1,
FGFR4, MALL, PALLD and PLVAP, were
identified. These DEGs, which are related to HCA, may potentially
act as biomarkers for detection, prognosis, monitoring and
predicting therapeutic responses in HCA. However, additional
experiments are required to confirm their function in HCA.
References
|
1
|
Nault JC, Bioulac-Sage P and Zucman-Rossi
J: Hepatocellular benign tumors-from molecular classification to
personalized clinical care. Gastroenterology. 144:888–902. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bioulac-Sage P, Balabaud C and
Zucman-Rossi J: Focal nodular hyperplasia, hepatocellular adenomas:
Past, present, future. Gastroenterol Clin Biol. 34:355–358. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Aalten SM, Witjes CD, de Man RA,
Ijzermans JN and Terkivatan T: Can a decision-making model be
justified in the management of hepatocellular adenoma? Liver Int.
32:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi BY and Nguyen MH: The diagnosis and
management of benign hepatic tumors. J Clin Gastroenterol.
39:401–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buell JF, Tranchart H, Cannon R and Dagher
I: Management of benign hepatic tumors. Surg Clin North Am.
90:719–735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bioulac-Sage P, Laumonier H, Couchy G, Le
Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G,
Trillaud H, et al: Hepatocellular adenoma management and phenotypic
classification: The Bordeaux experience. Hepatology. 50:481–489.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Micchelli ST, Vivekanandan P, Boitnott JK,
Pawlik TM, Choti MA and Torbenson M: Malignant transformation of
hepatic adenomas. Mod Pathol. 21:491–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zucman-Rossi J, Jeannot E, Nhieu JT,
Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis
V, Michalak S, et al: Genotype-phenotype correlation in
hepatocellular adenoma: New classification and relationship with
HCC. Hepatology. 43:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shanbhogue A, Shah SN, Zaheer A, Prasad
SR, Takahashi N and Vikram R: Hepatocellular adenomas: Current
update on genetics, taxonomy, and management. J Comput Assist
Tomogr. 35:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Velazquez I and Alter BP: Androgens and
liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J
Hematol. 77:257–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carrasco D, Barrachina M, Prieto M and
Berenguer J: Clomiphene citrate and liver-cell adenoma. N Engl J
Med. 310:1120–1121. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vazquez JJ and Marigil MA: Liver-cell
adenoma in an epileptic man on barbiturates. Histol Histopathol.
4:301–303. 1989.PubMed/NCBI
|
|
13
|
Espat J, Chamberlain RS, Sklar C and
Blumgart LH: Hepatic adenoma associated with recombinant human
growth hormone therapy in a patient with Turner's syndrome. Dig
Surg. 17:640–643. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bioulac-Sage P, Rebouissou S, Thomas C,
Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G,
Imbeaud S, et al: Hepatocellular adenoma subtype classification
using molecular markers and immunohistochemistry. Hepatology.
46:740–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manichon AF, Bancel B, Durieux-Millon M,
Ducerf C, Mabrut JY, Lepogam MA and Rode A: Hepatocellular adenoma:
Evaluation with contrast-enhanced ultrasound and MRI and
correlation with pathologic and phenotypic classification in 26
lesions. HPB Surg. 2012:4187452012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bluteau O, Jeannot E, Bioulac-Sage P,
Marqués JM, Blanc JF, Bui H, Beaudoin JC, Franco D, Balabaud C,
Laurent-Puig P and Zucman-Rossi J: Bi-allelic inactivation of TCF1
in hepatic adenomas. Nat Genet. 32:312–315. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pelletier L, Rebouissou S, Paris A,
Rathahao-Paris E, Perdu E, Bioulac-Sage P, Imbeaud S and
Zucman-Rossi J: Loss of hepatocyte nuclear factor 1alpha function
in human hepatocellular adenomas leads to aberrant activation of
signaling pathways involved in tumorigenesis. Hepatology.
51:557–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antequera F and Bird A: Number of CpG
islands and genes in human and mouse. Proc Natl Acad Sci USA.
90:11995–11999. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baylin SB and Jones PA: A decade of
exploring the cancer epigenome-biological and translational
implications. Nat Rev Cancer. 11:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Um TH, Kim H, Oh BK, Kim MS, Kim KS, Jung
G and Park YN: Aberrant CpG island hypermethylation in dysplastic
nodules and early HCC of hepatitis B virus-related human multistep
hepatocarcinogenesis. J Hepatol. 54:939–947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pogribny IP and Rusyn I: Role of
epigenetic aberrations in the development and progression of human
hepatocellular carcinoma. Cancer Lett. 342:223–230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki MM and Bird A: DNA methylation
landscapes: Provocative insights from epigenomics. Nat Rev Genet.
9:465–476. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP
and Kouzarides T: The methyl-CpG-binding protein MeCP2 links DNA
methylation to histone methylation. J Biol Chem. 278:4035–4040.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim BH, Cho NY, Shin SH, Kwon HJ, Jang JJ
and Kang GH: CpG island hypermethylation and repetitive DNA
hypomethylation in premalignant lesion of extrahepatic
cholangiocarcinoma. Virchows Arch. 455:343–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang YJ, Wu HC, Yazici H, Yu MW, Lee PH
and Santella RM: Global hypomethylation in hepatocellular carcinoma
and its relationship to aflatoxin B(1) exposure. World J Hepatol.
4:169–175. 2012. View Article : Google Scholar PubMed/NCBI
|
|
26
|
Tangkijvanich P, Hourpai N, Rattanatanyong
P, Wisedopas N, Mahachai V and Mutirangura A: Serum LINE-1
hypomethylation as a potential prognostic marker for hepatocellular
carcinoma. Clin Chim Acta. 379:127–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu HC, Wang Q, Yang HI, Tsai WY, Chen CJ
and Santella RM: Global DNA methylation levels in white blood cells
as a biomarker for hepatocellular carcinoma risk: A nested
case-control study. Carcinogenesis. 33:1340–1345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graziadei I, Finkenstedt A, Stauber RE,
Müller C, Trauner M and Vogel W: 78 Imatinib treatment for patients
with advanced stage hepatocellular carcinoma: A multicenter phase
II trial. Gastroenterology. 138:(Suppl 1). S775–S776. 2010.
View Article : Google Scholar
|
|
29
|
Pilati C, Letouzé E, Nault JC, Imbeaud S,
Boulai A, Calderaro J, Poussin K, Franconi A, Couchy G, Morcrette
G, et al: Genomic profiling of hepatocellular adenomas reveals
recurrent FRK-activating mutations and the mechanisms of malignant
transformation. Cancer Cell. 25:428–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
You JS and Jones PA: Cancer genetics and
epigenetics: Two sides of the same coin? Cancer cell. 22:9–20.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anwar SL and Lehmann U: DNA methylation,
microRNAs, and their crosstalk as potential biomarkers in
hepatocellular carcinoma. World J Gastroenterol. 20:7894–7913.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knudson AG: Cancer genetics. Am J Med
Genet. 111:96–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rodriguez-Paredes M and Esteller M: Cancer
epigenetics reaches mainstream oncology. Nat Med. 17:330–339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cedar H and Bergman Y: Programming of DNA
methylation patterns. Annu Rev Biochem. 81:97–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee HS, Kim BH, Cho NY, Yoo EJ, Choi M,
Shin SH, Jang JJ, Suh KS, Kim YS and Kang GH: Prognostic
implications of and relationship between CpG island
hypermethylation and repetitive DNA hypomethylation in
hepatocellular carcinoma. Clin Cancer Res. 15:812–820. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park HJ, Yu E and Shim YH: DNA
methyltransferase expression and DNA hypermethylation in human
hepatocellular carcinoma. Cancer Lett. 233:271–278. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Csepregi A, Röcken C, Hoffmann J, Gu P,
Saliger S, Müller O, Schneider-Stock R, Kutzner N, Roessner A,
Malfertheiner P and Ebert MP: APC promoter methylation and protein
expression in hepatocellular carcinoma. J Cancer Res Clin Oncol.
134:579–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lehmann U, Berg-Ribbe I, Wingen LU,
Brakensiek K, Becker T, Klempnauer J, Schlegelberger B, Kreipe H
and Flemming P: Distinct methylation patterns of benign and
malignant liver tumors revealed by quantitative methylation
profiling. Clin Cancer Res. 11:3654–3660. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hua D, Hu Y, Wu YY, Cheng ZH, Yu J, Du X
and Huang ZH: Quantitative methylation analysis of multiple genes
using methylation-sensitive restriction enzyme-based quantitative
PCR for the detection of hepatocellular carcinoma. Exp Mol Pathol.
91:455–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang L, Wang WL, Zhang Y, Guo SP, Zhang J
and Li QL: Epigenetic and genetic alterations of PTEN in
hepatocellular carcinoma. Hepatol Res. 37:389–396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee S, Lee HJ, Kim JH, Lee HS, Jang JJ and
Kang GH: Aberrant CpG island hypermethylation along multistep
hepatocarcinogenesis. Am J Pathol. 163:1371–1378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nishida N, Kudo M, Nagasaka T, Ikai I and
Goel A: Characteristic patterns of altered DNA methylation predict
emergence of human hepatocellular carcinoma. Hepatology.
56:994–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shim YH, Yoon GS, Choi HJ, Chung YH and Yu
E: p16 Hypermethylation in the early stage of hepatitis B
virus-associated hepatocarcinogenesis. Cancer Lett. 190:213–219.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boas FE, Do B, Louie JD, Kothary N, Hwang
GL, Kuo WT, Hovsepian DM, Kantrowitz M and Sze DY: Optimal imaging
surveillance schedules after liver-directed therapy for
hepatocellular carcinoma. J Vasc Interv Radiol. 26:69–73. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kong WT, Wang WP, Huang BJ, Ding H, Mao F
and Si Q: Contrast-enhanced ultrasound in combination with color
doppler ultrasound can improve the diagnostic performance of focal
nodular hyperplasia and hepatocellular adenoma. Ultrasound Med
Biol. 41:944–951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Christensen U, Simonsen M, Harrit N and
Sottrup-Jensen L: Pregnancy zone protein, a proteinase-binding
macroglobulin. Interactions with proteinases and methylamine.
Biochemistry. 28:9324–9331. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Berne BH: Alpha-2 pregnoglobulin
(pregnancy zone protein)-An estrogen-dependent macroglobulin
elevated in pregnancy and oral contraception. Clin Chem.
19:6571973.
|
|
48
|
Boxberger KH, Hagenbuch B and Lampe JN:
Common drugs inhibit human organic cation transporter 1
(OCT1)-mediated neurotransmitter uptake. Drug Metab Dispos.
42:990–995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thomas DP and Vane JR: 5-hydroxytryptamine
in the circulation of the dog. Nature. 216:335–338. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cao C, Li X, Liu T, Zhang L, Shen K and
Zhu H: Human organic cation transporter 1 protein levels of
granulocytes can optimize imatinib therapy in patients with chronic
myeloid leukemia. Acta Haematol. 133:199–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Loeb LA and Harris CC: Advances in
chemical carcinogenesis: A historical review and prospective.
Cancer Res. 68:6863–6872. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fraga MF, Herranz M, Espada J, Ballestar
E, Paz MF, Ropero S, Erkek E, Bozdogan O, Peinado H, Niveleau A, et
al: A mouse skin multistage carcinogenesis model reflects the
aberrant DNA methylation patterns of human tumors. Cancer Res.
64:5527–5534. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yamamoto E, Yamano HO, Suzuki H, Kamimae
S, Imai K, Shinomura Y and Toyota M: The role of aberrant DNA
methylation in carcinogenesis of colorectal tumor with K-ras
mutation. Japan J Mol Tumor Marker Res. 25:37–38. 2010.(In
Japanese).
|
|
54
|
Pogribny IP and Rusyn I: Environmental
toxicants, epigenetics, and cancer. Adv Exp Med Biol. 754:215–232.
2013. View Article : Google Scholar : PubMed/NCBI
|