Introduction
Colorectal carcinoma (CRC) is the third most common
cancer in Western countries and South Korea (1,2). CRC is
also one of the primary causes of cancer mortality worldwide
(1,3).
The pathogenesis of CRC has been reported to be complicated and
tightly controlled by various mechanisms, including genome
structural rearrangements, chromatin remodeling, genetic mutations
and epigenetic alterations (4,5).
MicroRNAs (miRNAs), which are small RNA molecules
that serve an essential role in fine-tuning gene expression,
regulate a range of biological processes, including cellular
development, differentiation, proliferation, K+ channel
modulation, stress responses, DNA repair, cell adhesion, cell
death, inflammation, metabolism and tumor development (6–9). It has
been identified that appropriate and physiological miRNA biogenesis
is controlled by an elaborate and well-regulated process, referred
to as the ‘miRNA machinery’ pathway (10). This signaling pathway is regulated by
various components, including Drosha, DiGeorge syndrome critical
region gene 8 (DGCR8), exportin-5 (Xpo5), Dicer,
transactivation-responsive RNA-binding protein (TRBP) and Argonaute
(AGO). Among these, Drosha and Dicer are important regulators of
miRNA biogenesis. In the nucleus, primary miRNAs (RNA extensions
several hundred nucleotides long) are processed into precursor
miRNAs (pre-miRNAs; stem-loop structures of between 70 and 100
nucleotides) by RNase III Drosha (11). These pre-miRNAs are then processed by
another RNase III, Dicer, into mature miRNAs within the cytoplasm
(12).
As disruption of the miRNA machinery pathway has
been suggested to be involved in cancer (13), a number of studies have demonstrated
the expression and the clinical implications of the components of
the miRNA machinery pathway in CRC. For instance, Faber et
al (14) identified that
overexpressed Dicer is significantly associated with poor survival
and decreased progression-free survival rates. Furthermore,
Papachristou et al (10)
demonstrated an association between upregulated Dicer and stage
III, but not stage II, tumors. However, downregulated Dicer reveals
statistically significant association with disease (World Health
Organization) stage, tumor stage, tumor grade and nodal metastasis
(15). By contrast, it has been
identified that dysregulated DGCR8 and AGO2 are not associated with
tumor-node-metastasis (TNM) stage or carcinoembryonic antigen (CEA)
titer in CRC (16). In spite of these
results, investigation of the mRNA expression levels of Drosha and
Dicer, and their clinicopathological associations in Korean
patients with CRC has been limited.
In the present study, the expression levels of
Drosha and Dicer mRNA in CRC tissues and their corresponding
adjacent non-neoplastic tissues from the same South Korean patients
were investigated, and the association between the expression
levels of Drosha and Dicer mRNA with various clinicopathological
characteristics, including sex, age, TNM stage, body mass index
(BMI) and CEA titer, were evaluated.
Materials and methods
Patients and tissues
In total, 77 patients (52 male and 25 female)
diagnosed with colorectal adenocarcinoma were included in the
present study. The mean age was 64.2±9.8 years. Colorectal
adenocarcinomas and adjacent non-neoplastic tissues were obtained
from the patients undergoing surgery in Dongsan Medical Center
(Daegu, Korea) between June 2008 and November 2010. Tissue
specimens were immediately frozen in liquid nitrogen and stored at
−80°C until RNA isolation. Tissue specimens were provided from the
Keimyung Human Bio-Resource Bank (Keimyung, Korea). The purpose of
the present study was explained to each patient who each provided
written informed consent. The present study was approved by the
Institutional Review Board of Keimyung University Dongsan Medical
Center (approval #2015-10-031-002).
RNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues using TRIzol
reagent (Molecular Research Center, Inc., Cincinnati, OH, USA).
RNAs were quantified using a NanoDrop 1000 instrument (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Each cDNA was
synthesized from 2 µg total RNA using Moloney murine leukemia virus
reverse transcriptase (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol. qPCR was performed on a
LightCycler® 480 Real-Time PCR system (Roche Diagnostics
GmbH, Mannheim, Germany) using SYBR Green Premix (Roche Diagnostics
GmbH) and specific primer pairs (listed in Table I). The following thermocycling
conditions were maintained: Pre-incubation, 5 min at 95°C;
amplification, 45 cycles with 10 sec at 95°C, 10 sec at 55°C and 15
sec at 72°C; melting curve, gradually from 65 to 95°C; cooling, 10
min at 37°C. β-actin was used as a loading control, and a
no-template sample was used as a negative control. qPCR data were
analyzed with the ΔCq values using Microsoft Excel (Microsoft
Corporation, Redmond, WA, USA) (17).
A total of three replicates per experiment were performed.
 | Table I.Primer sequences of microRNA machinery
components used in quantitative polymerase chain reaction. |
Table I.
Primer sequences of microRNA machinery
components used in quantitative polymerase chain reaction.
| Gene | Direction | Sequence |
|---|
| Drosha | Forward |
5′-CTGTCGATGCACCAGATT-3′ |
|
| Reverse |
5′-TGCATAACTCAACTGTGCAGG-3′ |
| Dicer | Forward |
5′-TTAACCTTTTGGTGTTTGATGAGTGT-3′ |
|
| Reverse |
5′-AGGACATGATGGACAATT-3′ |
| β-actin | Forward |
5′-CAGCCATGTACGTTGCTATCCAGG-3′ |
|
| Reverse |
5′-AGGTCCAGACGCAGGATGGCATG-3′ |
Statistical analysis
Statistical analysis was performed using SPSS
software (version 22.0; IBM SPSS, Armonk, NY, USA). Statistical
comparisons for significance were performed using Wilcoxon's
signed-rank test for paired samples. Differences between the groups
were analyzed statistically using the paired Students t-test. The
association between Drosha and Dicer expression was assessed using
Pearson's correlation coefficient analysis for continuous variables
and Fisher's exact test for categorical variables.
Clinicopathological associations with the mRNA expression levels of
Drosha and Dicer were analyzed using a linear-by-linear
association, Pearson's χ2 test and Fisher's exact test
for categorical variables. The mean value was used as threshold
value (low and high) for categorical variables. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression levels of Drosha and Dicer
mRNA in CRC tissues and adjacent non-neoplastic colorectal tissues
of patients with CRC
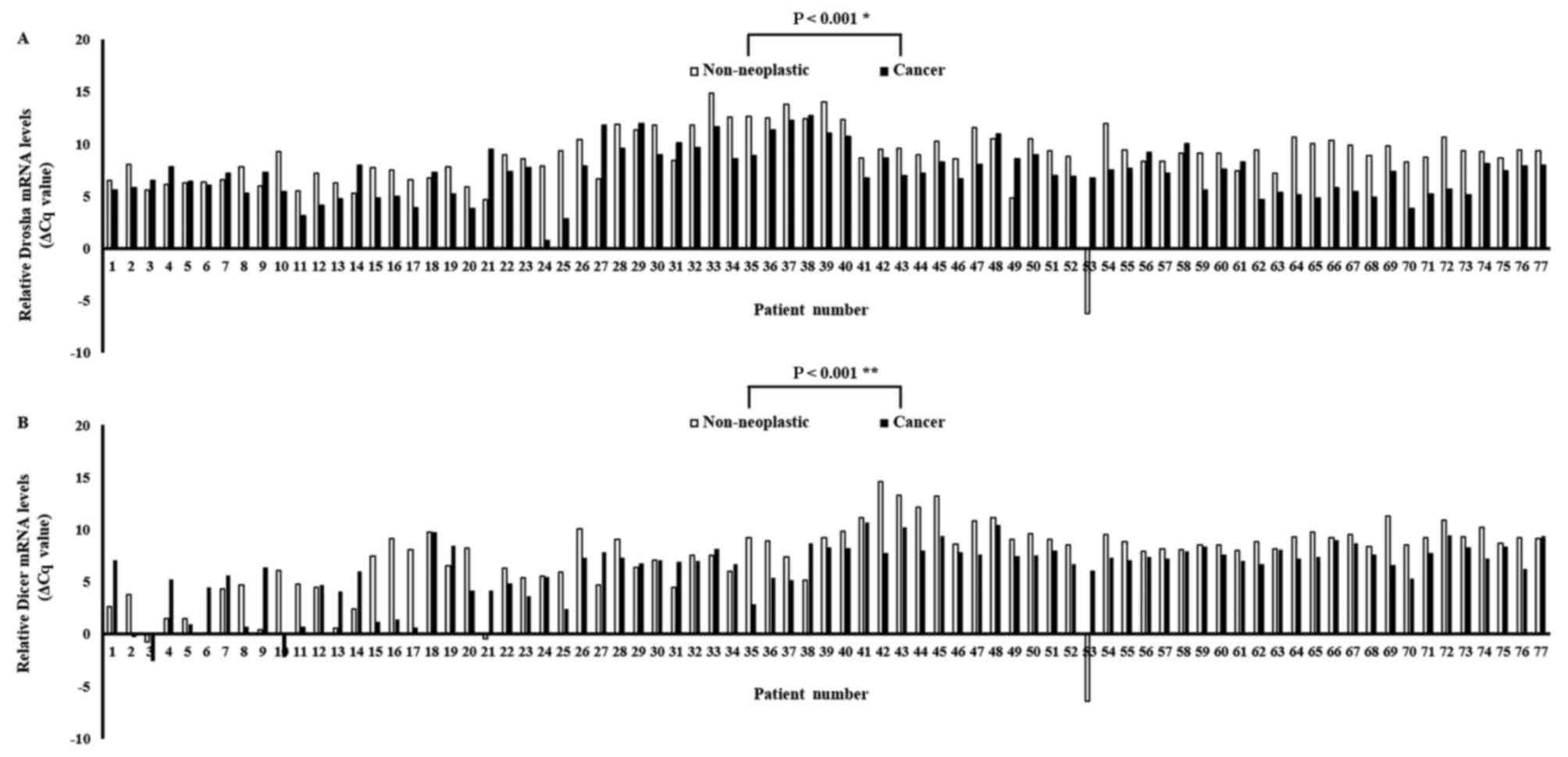
The expression levels of Drosha and Dicer mRNA were
quantified using qPCR in paired specimens of human cancerous
colorectal tissues and their corresponding non-neoplastic
colorectal tissues from 77 patients with CRC. The Drosha and Dicer
mRNA levels were normalized to the level of β-actin mRNA. The
results identified that Drosha mRNA expression was significantly
upregulated in carcinomatous tissues compared with in the
corresponding non-neoplastic tissues in 59/77 patients with CRC
(P<0.001; Fig. 1A). Furthermore,
Dicer mRNA expression was also significantly upregulated in
carcinomatous tissues compared with in the corresponding
non-neoplastic tissues in 59/77 patients with CRC (P<0.001;
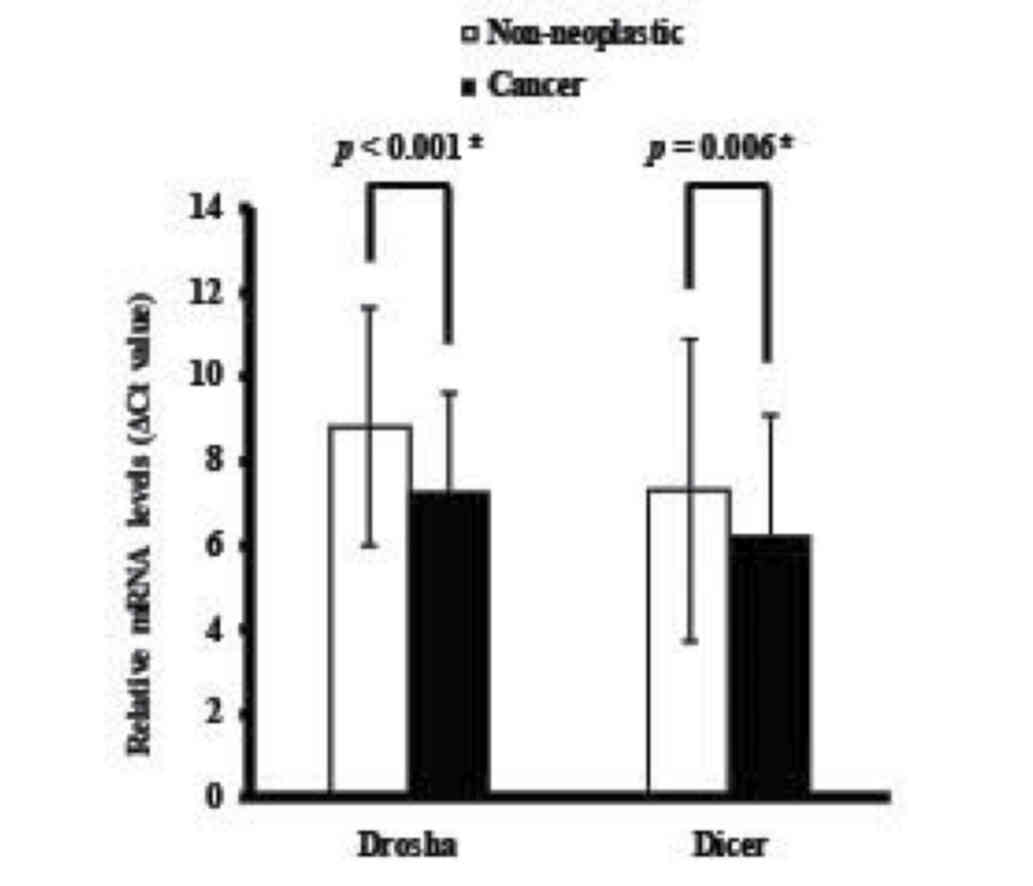
Fig. 1B). The mean levels of Drosha
and Dicer mRNA expression in cancerous tissues were significantly
upregulated compared with in non-neoplastic colorectal tissues
(P<0.001 and P=0.006, respectively; Fig. 2).
Association between Drosha and Dicer
mRNA expression levels and their clinicopathological features in
patients with CRC
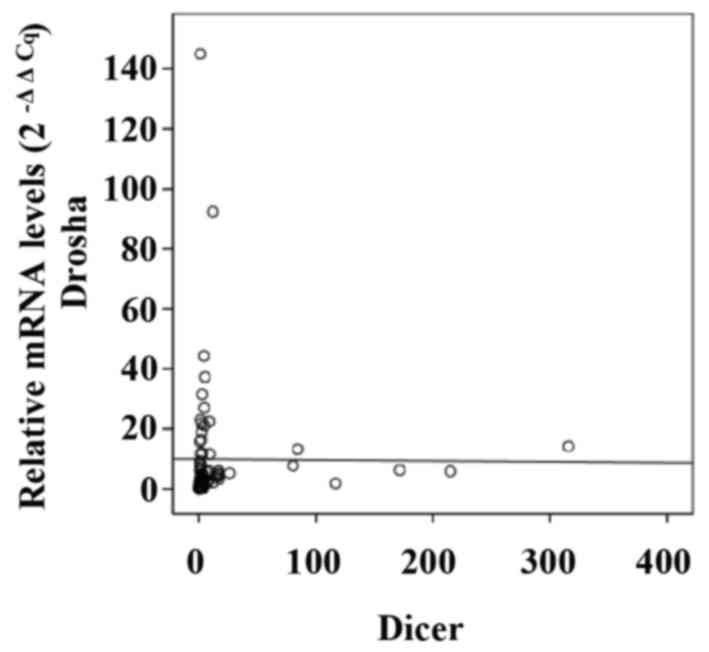
To investigate the association between mRNA levels
of Drosha and Dicer in CRC specimens, Pearson's correlation
coefficient analysis was performed. No significant association was
identified between Drosha and Dicer, with a Pearson's correlation
coefficient value of −0.007 (P=0.953; Fig. 3). Furthermore, the expression levels
of Drosha and Dicer mRNA were not associated with each other, as
determined by Fisher's exact test (Table
II). To investigate the biological roles of Drosha and Dicer in
CRC, the association between their mRNA expression levels and the
clinicopathological parameters that are used to describe tumor
progression and aggressiveness in CRC were evaluated. Prior to the
statistical analysis, the patients were classified according to
each clinical characteristic. The clinicopathological parameters in
the 77 patients with CRC according to Drosha and Dicer mRNA
expression levels are presented in Table
II. Drosha and Dicer mRNA expression levels were not
significantly associated with clinical parameters, including sex,
age, TNM stage, BMI and CEA titer in the CRC specimens.
 | Table II.Association between Drosha and Dicer
mRNA expression levels and clinicopathological parameters. |
Table II.
Association between Drosha and Dicer
mRNA expression levels and clinicopathological parameters.
|
| Drosha | Dicer |
|---|
|
|
|
|
|---|
| Characteristic | Low | High | P-value | Low | High | P-value |
|---|
| Sex |
|
| 0.070c |
|
| 0.092b |
| Male | 43 | 9 |
| 42 | 10 |
|
|
Female | 16 | 9 |
| 24 | 1 |
|
| Age, years |
|
| 0.190b |
|
| 0.056b |
|
≤50 | 7 | 0 |
| 4 | 3 |
|
|
>50 | 52 | 18 |
| 62 | 8 |
|
| T stage |
|
| 0.308a |
|
| 0.366a |
| T1 | 3 | 2 |
| 4 | 1 |
|
| T2 | 11 | 2 |
| 10 | 3 |
|
| T3 | 36 | 14 |
| 44 | 6 |
|
| T4 | 9 | 0 |
| 8 | 1 |
|
| N stage |
|
| 0.542a |
|
| 0.957a |
| N0 | 35 | 11 |
| 39 | 7 |
|
| N1 | 13 | 4 |
| 15 | 1 |
|
| N2 | 9 | 6 |
| 10 | 3 |
|
| N3 | 2 | 0 |
| 2 | 0 |
|
| M stage |
|
| 0.585b |
|
| 0.146b |
|
Negative | 54 | 18 |
| 63 | 9 |
|
|
Positive | 5 | 0 |
| 3 | 2 |
|
| BMI |
|
| 0.614a |
|
| 0.872a |
|
≤18.5 | 1 | 1 |
| 1 | 1 |
|
|
18.5–24.9 | 37 | 12 |
| 44 | 5 |
|
|
25-29.9 | 20 | 4 |
| 19 | 5 |
|
|
>30 | 1 | 1 |
| 2 | 0 |
|
| CEA (ng/ml) |
|
| 0.268c |
|
| 1.000b |
|
>5 | 15 | 7 |
| 19 | 3 |
|
| ≤5 | 44 | 11 |
| 47 | 8 |
|
| Dicer |
|
| 1.000b |
|
|
|
|
Low | 50 | 16 |
|
|
|
|
|
High | 9 | 2 |
|
|
|
|
| Drosha |
|
|
|
|
| 1.000b |
|
Low |
|
|
| 50 | 9 |
|
|
High |
|
|
| 16 | 2 |
|
Discussion
Gene expression is affected by various genetic and
epigenetic factors, including transcriptional or
post-transcriptional regulation, miRNA, long non-coding RNA, DNA
methylation, DNA-binding proteins and histone acetylation. Among
the factors involved in gene expression-regulating processes,
miRNAs mediate post-transcriptional gene silencing by the
degradation and/or translational suppression of target mRNA as a
result of binding to their complementary sequence in the 3′
untranslated region of target mRNA (18,19). The
biogenesis of miRNA is a well-organized, finely tuned and
complicated process, which is regulated by a number of miRNA
machinery components, including Drosha, DGCR8, Xpo5, Dicer, TRBP
and AGO (20). Although miRNAs and
their biogenesis serve important and diverse roles in development
and pathogenesis, the underlying molecular mechanisms by which
miRNA machinery components regulate miRNA production remain
unclear. Kim et al (21),
using a knockout procedure, demonstrated that Drosha, Xpo5 and
Dicer are required for the miRNA biogenesis pathway. In particular,
this study demonstrated an essential role for Drosha and a
contributory role for Dicer in the canonical miRNA signaling
pathway.
Dysregulation of the miRNA machinery components is
hypothesized to be associated with tumorigenic processes, including
tumor initiation, development and metastasis (13,22).
According to this aspect, the majority of the research in the miRNA
biogenesis field has focused on the regulation and clinical
association of miRNA machinery components in various human cancer
specimens (13,22). For example, the upregulated expression
of Drosha in gastric cancer is associated with patient survival
rates (23) and its downregulated
expression in endometrial cancer is associated with histological
grade (24). Furthermore, the
upregulated expression of Dicer in serous ovarian carcinoma is
associated with advanced tumor stages (25) and its downregulated expression in
breast cancer is associated with cancer progression and recurrence
(26). Particularly in CRC, previous
studies have identified that dysregulation of the miRNA machinery
may be involved in carcinogenesis (10,14,16,27,28).
Drosha mRNA levels were not identified to be significantly
associated with tumor stages (10).
However, contradictory results regarding the association between
the expression levels of Dicer and clinicopathological parameters
were identified (14,27,28).
These results were the motivation for the present
study exploring the expression levels of Drosha and Dicer mRNA in
CRC tissues compared with those in adjacent non-neoplastic
colorectal tissues and to evaluate their associations with specific
clinicopathological parameters. The expression levels of Drosha and
Dicer mRNA were identified to be significantly upregulated in 59/77
CRC specimens. However, no significant association was identified
between altered mRNA expression levels of Drosha and Dicer and any
clinicopathological characteristic in CRC, including sex, age, TNM
stage, BMI and CEA titer.
Accumulating evidence has identified that
interindividual miRNA biogenesis-associated components are
significantly associated with each other in paired samples of
cancer and adjacent non-neoplastic tissues: AGO2 and DGCR8 in CRC
specimens (16); Drosha and Dicer in
triple negative breast cancer specimens (29); and AGO2 and DGCR8 in invasive ductal
breast carcinoma specimens (30).
Therefore, the association between the expression levels of Drosha
and Dicer was investigated using Pearson's correlation coefficient
analysis and Fisher's exact test. In contrast with previous
studies, the results of the present study indicated that the
expression levels of Drosha and Dicer mRNA were not associated with
each other in CRC.
The results of the present study indicate that the
expression levels of Drosha and Dicer mRNA are significantly
upregulated in cases of CRC in the Korean population, suggesting
that increased expression of Drosha and Dicer may serve an
important role in the pathogenesis of CRC.
Acknowledgements
The present study was supported by Bumsuk Academic
Scholarship Foundation, 2013. The biospecimens for the present
study were provided by the Keimyung Human Bio-Resource Bank, a
member of the National Biobank of Korea, which is supported by the
Ministry of Health and Welfare.
Glossary
Abbreviations
Abbreviations:
|
miRNA
|
microRNA
|
|
CRC
|
colorectal cancer
|
|
DGCR8
|
DiGeorge syndrome critical region gene
8
|
|
Xpo5
|
exportin-5
|
|
TRBP
|
transactivation-responsive RNA-binding
protein
|
|
AGO
|
Argonaute
|
|
pre-miRNA
|
precursor miRNAs
|
References
|
1
|
Ministry of Health & Welfare KCCR,
National Cancer Center: Annual report of cancer statistics in Korea
in 2012. Journal. 2014.
|
|
2
|
Janakiram NB and Rao CV: Molecular markers
and targets for colorectal cancer prevention. Acta Pharmacol Sin.
29:1–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pilmore E and Hamilton KL: The role of
microRNAs in the regulation of K(+) channels in epithelial tissue.
Front Physiol. 6:3522015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tuna M, Machado AS and Calin GA: Genetic
and epigenetic alterations of microRNAs and implications for human
cancers and other diseases. Genes Chromosomes Cancer. 55:193–214.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horsham JL, Ganda C, Kalinowski FC, Brown
RA, Epis MR and Leedman PJ: MicroRNA-7: A miRNA with expanding
roles in development and disease. Int J Biochem Cell Biol.
69:215–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie Y, Zhang L, Gao Y, Ge W and Tang P:
The multiple roles of microrna-223 in regulating bone metabolism.
Molecules. 20:19433–19448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Papachristou DJ, Korpetinou A,
Giannopoulou E, Antonacopoulou AG, Papadaki H, Grivas P, Scopa CD
and Kalofonos HP: Expression of the ribonucleases Drosha, Dicer,
and Ago2 in colorectal carcinomas. Virchows Arch. 459:431–440.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee Y, Han J, Yeom KH, Jin H and Kim VN:
Drosha in primary microRNA processing. Cold Spring Harb Symp Quant
Biol. 71:51–57. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tijsterman M and Plasterk RH: Dicers at
RISC; the mechanism of RNAi. Cell. 117:1–3. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faber C, Horst D, Hlubek F and Kirchner T:
Overexpression of Dicer predicts poor survival in colorectal
cancer. Eur J Cancer. 47:1414–1419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faggad A, Kasajima A, Weichert W,
Stenzinger A, Elwali NE, Dietel M and Denkert C: Down-regulation of
the microRNA processing enzyme Dicer is a prognostic factor in
human colorectal cancer. Histopathology. 61:552–561. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim B, Lee JH, Park JW, Kwon TK, Baek SK,
Hwang I and Kim S: An essential microRNA maturing microprocessor
complex component DGCR8 is up-regulated in colorectal carcinomas.
Clin Exp Med. 14:331–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siomi H and Siomi MC: Posttranscriptional
regulation of microRNA biogenesis in animals. Mol Cell. 38:323–332.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mockenhaupt S, Schürmann N and Grimm D:
When cellular networks run out of control: Global dysregulation of
the RNAi machinery in human pathology and therapy. Prog Mol Biol
Transl Sci. 102:165–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim YK, Kim B and Kim VN: Re-evaluation of
the roles of DROSHA, Export in 5 and DICER in microRNA biogenesis.
Proc Natl Acad Sci USA. 113:E1881–E1889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hata A and Kashima R: Dysregulation of
microRNA biogenesis machinery in cancer. Crit Rev Biochem Mol Biol.
51:121–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tchernitsa O, Kasajima A, Schäfer R, Kuban
RJ, Ungethüm U, Györffy B, Neumann U, Simon E, Weichert W, Ebert MP
and Röcken C: Systematic evaluation of the miRNA-ome and its
downstream effects on mRNA expression identifies gastric cancer
progression. J Pathol. 222:310–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Torres A, Torres K, Paszkowski T,
Jodłowska-Jędrych B, Radomański T, Książek A and Maciejewski R:
Major regulators of microRNAs biogenesis Dicer and Drosha are
down-regulated in endometrial cancer. Tumour Biol. 32:769–776.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaksman O, Hetland TE, Trope CG, Reich R
and Davidson B: Argonaute, Dicer, and Drosha are up-regulated along
tumor progression in serous ovarian carcinoma. Hum Pathol.
43:2062–2069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan M, Huang HY, Wang T, Wan Y, Cui SD,
Liu ZZ and Fan QX: Dysregulated expression of dicer and drosha in
breast cancer. Pathol Oncol Res. 18:343–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Faggad A, Kasajima A, Weichert W,
Stenzinger A, Elwali NE, Dietel M and Denkert C: Down-regulation of
the microRNA processing enzyme Dicer is a prognostic factor in
human colorectal cancer. Histopathology. 61:552–561. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stratmann J, Wang CJ, Gnosa S, Wallin A,
Hinselwood D, Sun XF and Zhang H: Dicer and miRNA in relation to
clinicopathological variables in colorectal cancer patients. BMC
Cancer. 11:3452011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Passon N, Gerometta A, Puppin C, Lavarone
E, Puglisi F, Tell G, Di Loreto C and Damante G: Expression of
Dicer and Drosha in triple-negative breast cancer. J Clin Pathol.
65:320–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwon SY, Lee JH, Kim B, Park JW, Kwon TK,
Kang SH and Kim S: Complexity in regulation of microRNA machinery
components in invasive breast carcinoma. Pathol Oncol Res.
20:697–705. 2014. View Article : Google Scholar : PubMed/NCBI
|