Introduction
Bladder cancer, the most common malignancy of the
urinary tract and the seventh most prevalent cancer worldwide
(1), is diagnosed based on the
combination of urethrocystoscopy and voided urine cytology
(2). While voided urine cytology has
an extremely low sensitivity (3)
considering its dependence on tumor grades, what makes
urethrocystoscopy the ‘golden standard’ for the screening and
follow-up of bladder cancer patients (2). However, the procedure of
urethrocystoscopy is invasive and discomfort for patients.
Therefore, it is important to develop better non-invasive detection
methods for the early diagnosis of bladder cancer (4). Several urinary tests including Bladder
tumor antigen (BTA) and Nuclear matrix protein-22 (NMP-22) have
been approved by FDA (5). Recently
additional possible urinary biomarkers have been investigated
(6) including Cyfra 21-1 (7), Matrix metalloproteinase-7 (MMP-7)
(8) as well as survivin.
Survivin, a 16.5 kDa protein, is one member of the
inhibitor of apoptosis protein (IAP) family and has a unique role
in apoptosis and control of cell division (9–11).
Survivin is expressed in various tumors while in normal adults it
is only expressed in tissues as thymus, endothelium and placenta
(12–14). Given its differential expression
between normal and tumor tissues, survivin is considered to be a
unique marker for the diagnosis of cancer. Studies have shown that
survivin is associated with poor prognosis in certain kinds of
tumors including bladder cancer (15,16). In
bladder cancer, survivin is expressed in epithelium and its
expression could be detected by immunohistochemistry (IHC). What's
more, as a malignancy of the urinary tract, survivin is expressed
in urine that could be detected at the protein and mRNA levels
(17). Certain studies found that the
measurement of survivin mRNA in urine seemed to perform better than
voided urine cytology (15). Recent
increasing attention has been focused on the value of urinary
survivin detection in bladder cancer, while its clinical utility
still remains to be elucidated partly due to the limitation of
detection methods.
ELISA method is considered accurate and reliable,
which has also been used for the detection of urinary survivin
(18,19). But the assay is time consuming with
poor reproducibility. Thus alternative methods with better
performance are needed. Chemiluminescence immunoassay (CLIA) is a
much more effective measurement with the advantages of high
sensitivity, uniformity and broad dynamic range (20,21). In
this study, we tried to develop a microplate magnetic CLIA with two
anti-survivin monoclonal antibodies (McAbs) prepared by our
laboratory. Besides, in order to evaluate the potential application
of the established method, urinary survivin levels of bladder
cancer patients and healthy controls were examined.
Materials and methods
Animals
Female Balb/c mice weighing 18–22 g were purchased
from the Laboratory Animal Centre of Chinese Academy of Medical
Sciences. The Animal Care Committee of Peking University approved
the animal experiments. We performed animal studies in accordance
with the Experimental Animal Management Ordinance approved by the
Scientific and technological committee of China.
Apparatus
Protein-A/G sepharose (HiTrap Protein G HP, 1 ml)
was purchased from GE Healthcare Life Sciences (Buckinghamshire,
UK). ALC-B6 peristaltic pump was purchased from Alcott Biotech Co.,
Ltd. (Shanghai, China). The chemiluminescence detection was carried
out using SpectraMax L microplate reader from Molecular Devices,
LLC (Sunnyvale, CA, USA). The IKA® MS3 Digital (Staufen,
Germany) was employed to blend the solutions in microplates. The
incubation procedure at 37°C was carried out at an electric heat
constant temperature incubator. The white opaque 96-well
flat-bottomed microplates were from Thermo Fisher Scientific
(Waltham, MA, USA). A magnetic separation device for 96-well
microplate purchased from Beaver Beads Co., Ltd. (Suzhou, China)
was used for the separation procedure.
Chemicals and solutions
Incomplete freund's adjuvant (IFA), PEG, horseradish
peroxidase (HRP; H1759), sodium borohydride (NaBH4;
10H3440), sodium m-periodate (NaIO4) (38F-0860) and
(+)-biotin-N-hydroxysuccinimide (NHSB) (HMBD0595 V) were from
Sigma-Aldrich Co. (St. Louis, MO, USA).
Hypoxanthine-aminopterin-thymidine (HAT) and hypoxanthine-thymidine
(HT) were from Corning Inc. (Corning, NY, USA). BCA protein assay
kit was obtained from Thermo Fisher Scientic. Chemiluminescent (CL)
substrates were purchased from Ke Yue Zhong Kai Co., Ltd. (Beijing,
China). The magnetic particles (MPs, 10 mg/ml) coated with
streptavidin and suspended in solution were purchased from Beaver
Beads Co., Ltd. Phosphate-buffered saline (PBS) buffer and bovine
serum albumin (BSA) were from ZSGB-Bio (Beijing, China). The
washing buffer was PBS containing 0.05% (v/v) Tween-20 (PBST). PBST
containing 1% (w/v) BSA served as dilution buffer for HRP-labeled
antibody, biotinylated antibody and standard series. The
microplates were pre-coated with 300 µl 1% (w/v) BSA in PBS buffer
at 4°C for 12 h and washed three times before use.
Generation of hybridoma cell
lines
The mouse was immunized with 150 µg purified
recombinant human sequence survivin protein MS2-survivin
and the same volume of IFA every two weeks until the serum antibody
titer was up to 1:10,000. Then 150 µg MS2-survivin was
administered into the intra-peritoneal cavity without IFA as the
final immunogen boost, 3 days before mice's spleen was harvested
for cell fusion. Isolated splenocytes were mixed with myeloma cells
at the ratio of 10:1 with the presence of polyethylene glycol (PEG)
and HAT medium. Six to eight days passed and hybrid colonies
survived. The colonies were screened by ELISA with
MS2-survivin and MS2-PAI for three times.
Positive clonal cells were transferred and expanded. Then the
hybridoma cell line was established. Cells were passaged in HT
medium after HAT selection is completed (2–3 weeks).
Preparation and purification of McAbs
to survivin and standard protein
Hybridoma cell lines (C6 and E6) secreting
anti-survivin McAbs with high signals on ELISA were expanded as
ascetic fluids in BALB/c mice. The McAbs were purified by protein-G
affinity chromatography from the ascetic fluids. Antibody
concentrations were determined by using the BCA protein assay kit.
Our laboratory has already done experiments before comparing the
newly generated antibodies with commercial antibodies. We have
detected survivin expression in cancer by several methods such as
IHC and western blot analysis, using the newly generated antibodies
and the commercial antibody (an anti-survivin monoclonal antibody
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
simultaneously. Results showed that the newly generated antibodies
were comparable with the commercial antibody (22). Hence, our newly generated antibodies
have been identified to be reliable. Recombinant human sequence
survivin protein MS2-survivin produced by our laboratory
was used as the standard protein (23). The standard series were prepared by
diluting MS2-Survivin stock with dilution buffer to
target values of 0, 10, 50, 100, 500, 1,000 ng/ml, assigning to S0,
S1, S2, S3, S4 and S5, respectively.
Preparation of HRP-labeled
antibody
Anti-survivin McAbs (C6, E6) were labeled with HRP,
respectively. Briefly, purified McAbs were dialyzed against several
changes of carbonate buffer [0.1 M sodium carbonate buffer
(NaHCO3/Na2CO3)] pH 9.5 at 4°C
overnight. The HRP protein dissolved in deionized water at a
concentration of 5 mg/ml was pretreated with NaIO4
stirring for 20 min at room temperature in dark, and then was
dialyzed against CH3COONa (1 mmol/l sodium acetate
buffer) pH 4.4 at 4°C overnight. Equivalent pretreated McAbs and
HRP solution were blended and incubated at room temperature for 2 h
with gentle stirring in dark. Then NaH4B was added,
stirring at 4°C for 2 h. The reaction solution was dialyzed against
several changes of PBS buffer (0.01 M sodium phosphate, 0.15 M
sodium chloride, pH 7.4) at 4°C overnight. After dialyzing, the
reaction mixture was applied to a Sephacryl S-200 column to remove
unlabeled HRP. The HRP-conjugated McAbs were stored at −20°C until
use.
Preparation of biotinylated
antibody
Anti-survivin McAbs (C6, E6) were coupled with
biotin, respectively using a standard protocol. Briefly, 1 mg of
anti-survivin monoclonal antibody dissolved in 1 ml of 0.1 mol/l
carbonate buffer (pH 8.0) was dialyzed against carbonate buffer (pH
8.0) at 4°C for 2 h. 1 mg of NHSB was dissolved in 1 ml of DMSO. 1
ml McAbs solution was added into 120 µl of NHS-D-biotin solution
with gentle stirring and incubated at room temperature for 4 h.
Then 9.6 µl of 1 mol/l NH4Cl was added, stirring at room
temperature for 10 min. The reaction solution was dialyzed against
several changes of PBS buffer (0.01 M sodium phosphate, 0.15 M
sodium chloride, pH 7.4) at 4°C to remove unlabeled biotin. The
dialyzed biotinylated McAbs were stored at −20°C until use.
ELISA procedure
E6 (100 µl, 2.5 µg/ml) was coated onto a 96-well
microplate at 4°C overnight. After blocking with 200 µl 5% skimmed
milk in PBS for 1 h at 37°C and washing 3 times with PBST, 100 µl
survivin standards and urine samples were added into the wells.
After incubating at 37°C for 1 h and washing 3 times, 100 µl
HRP-labeled C6 was added to each well. The microplate was incubated
at 37°C for 1 h and washed 3 times. Then substrate solution was
added to the wells and every pore's absorption was determined at
450 nm. McAbs (C6 and E6) used in this ELISA procedure have been
identified to be reliable as mentioned above.
Microplate magnetic CLIA
procedure
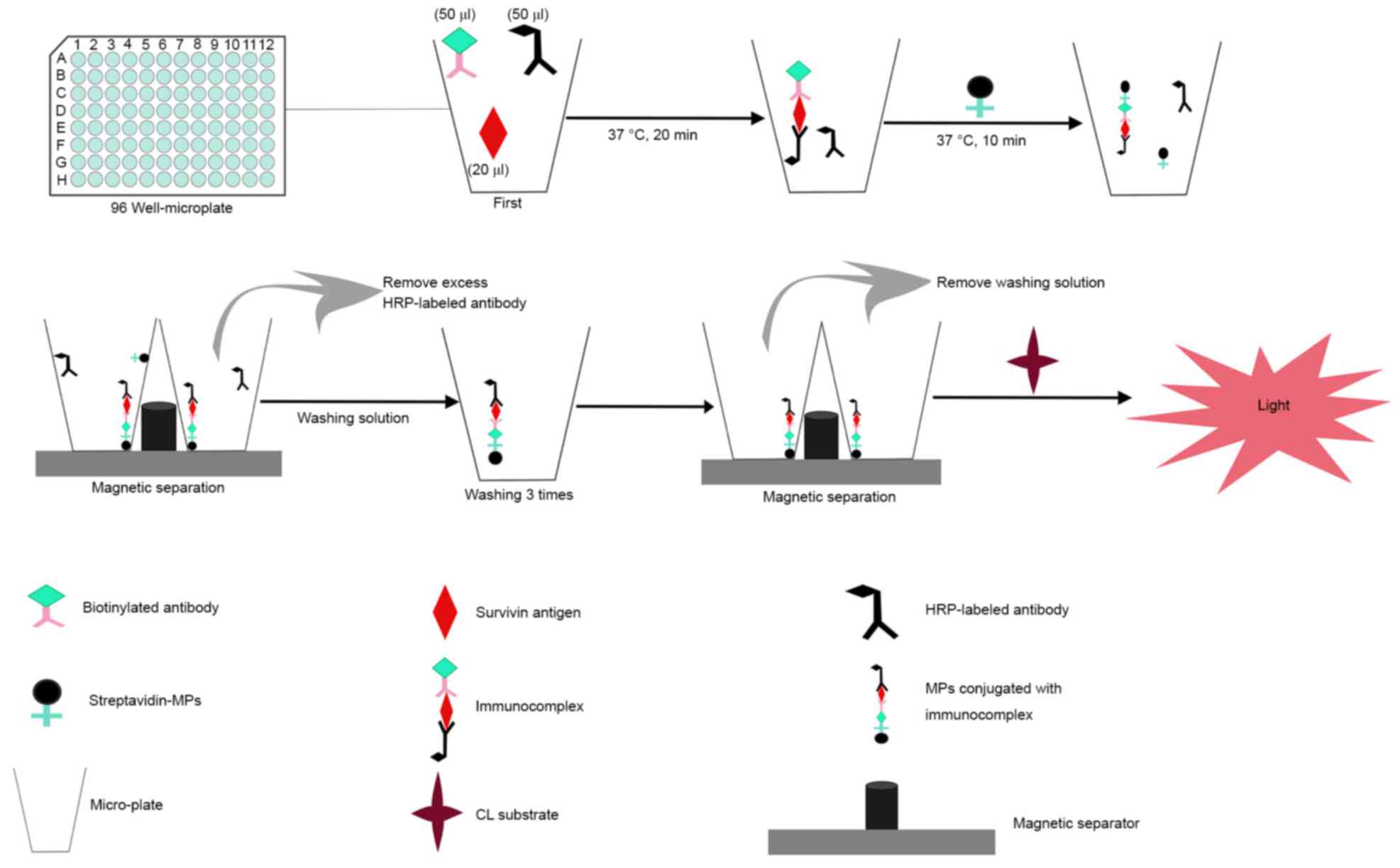
The immunoassay procedure of microplate magnetic
CLIA in this study is displayed in Fig.
1 and the detailed steps were as follows: First, 50 µl
HRP-labeled anti-survivin McAb, 50 µl biotinylated anti-survivin
McAb and 20 µl survivin standard solution were added into the
micro-well and incubated for 20 min at 37°C. After the sandwich
reaction, 1 µl streptavidin MPs were added to react with
immunoassay reagents for another 10 min (capture time) at 37°C.
Subsequently, the separation procedure was carried out, during
which the magnets attracted streptavidin MPs and any specific
captured materials to the bottom of micro-wells. The micro-wells
were washed with 200 µl of washing solution three times after
removing unwanted materials. Finally, 150 µl of CL substrate was
added and the relative light unit (RLU) was measured in the
dark.
Human specimens collection and
detection
All human specimens were obtained from Peking
University Cancer Hospital. All of the cancer patients were
diagnosed histopathologically and staged according to the tumor
node metastasis (TNM) classification released by the American Joint
Committee on Cancer (AJCC 7th edition, 2010). Healthy controls with
a negative cystoscopy were chosen at the medical examination
center. A total of 130 urine samples of bladder cancer patients and
113 urine samples of healthy controls were collected from January
to July 2016. All urine samples were collected at the day and
centrifuged immediately at 3,000 r/min for 5 min, and the
supernatant was aliquoted, and stored at −40°C until detection. The
samples were tested for survivin levels using the established
method directly without any pretreatment. All patients and healthy
controls were informed consent for participation in this study. The
study was approved by the Ethics Committee of Peking University
Cancer Hospital and Institute. All study procedures were in
accordance with the Helsinki Declaration.
Data analysis
Standards and samples were measured, and CL
intensity values were integrated. Standard curves were obtained by
plotting the logarithm of CL intensity (in RLUs) against the
logarithm of standard concentration and fitting to a linear
equation. Student's t-test was used for the comparison of the
variables between groups. Data was expressed as mean values ±
standard deviation. Enumeration data was expressed as percentages
analyzing by χ2 test. A p-value of <0.05 was
considered to be significant. Cutoff value was determined by the
optimal Youden's index (sensitivity + specificity-1). All the
statistical analysis was performed using the SPSS statistical
software (SPSS for Mac, version 20).
Results
Determination of the proper antibody
combination
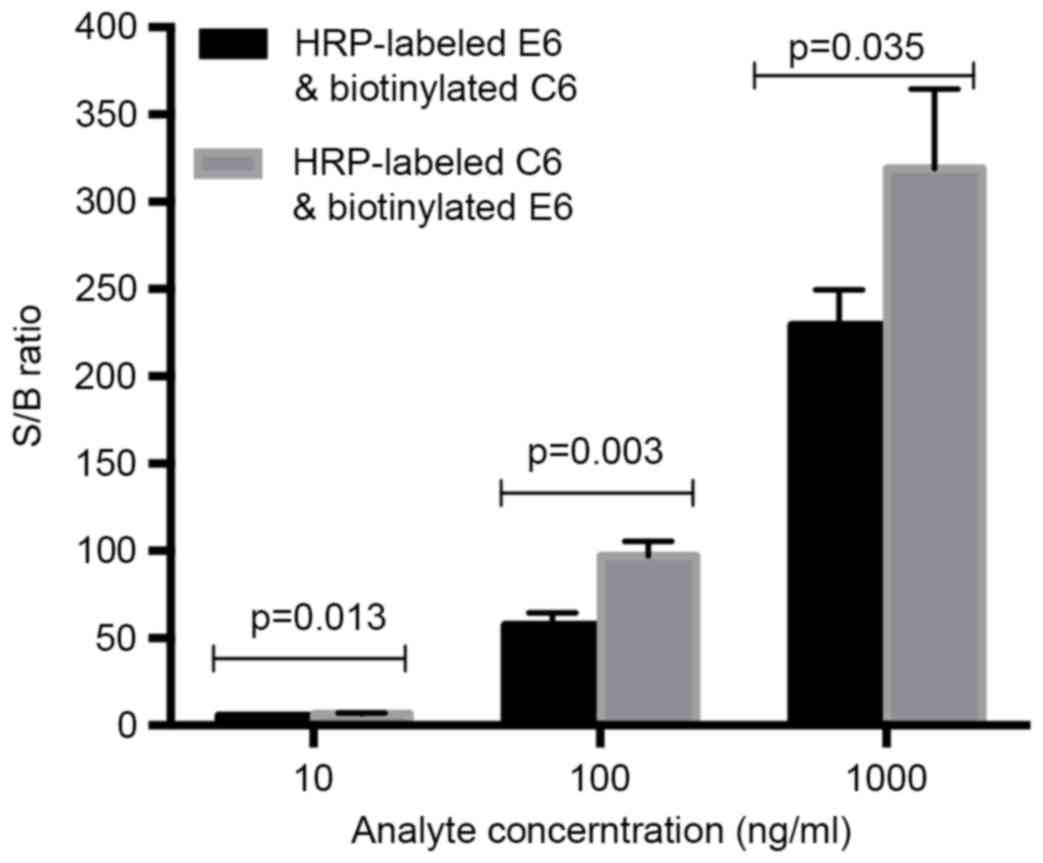
The antibody pair used in this assay has been tested
in a sandwiched ELISA assay. One of the two antibodies was labeled
with HRP and the other was to be biotinylated. Since there were two
possible combinations, the first development step was the selection
of the optimal antibody combination. In order to test whether
biotinylated E6 and HRP-labeled C6 or biotinylated C6 and
HRP-labeled E6 combined better; three different concentrations (10,
100, 1,000 ng/ml) of the survivin standard and a negative control
(dilution buffer) were analyzed under both combinations. Then the
signal-to-background (S/B) ratio was calculated and compared for
the determination of the better antibody combination. Student's
t-test was used for data comparison. According to Fig. 2, HRP-labeled C6 and biotinylated E6
were determined as the proper antibody pair which providing a
higher S/B ratio (P<0.05).
Influence and optimization of
immunoassay reagents
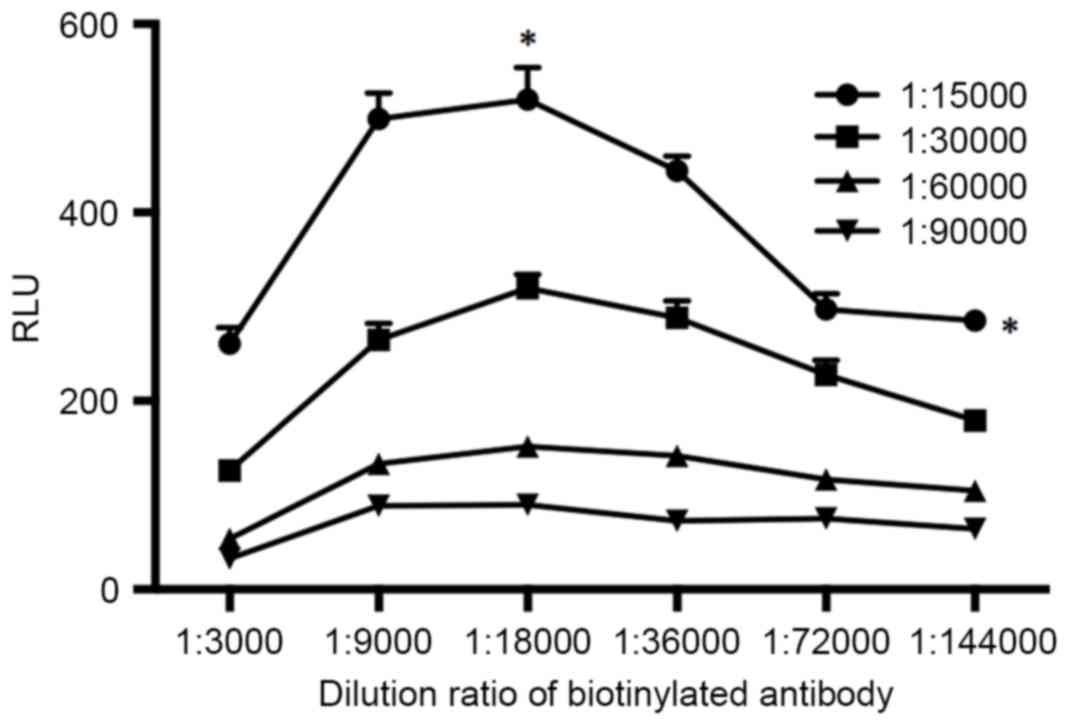
The immunoreaction reagent is an important parameter
affecting the sensitivity and accuracy of immunoassay, especially
in a sandwich immunoassay. In this experiment, the dilution ratios
of HRP-labeled antibody and biotinylated antibody were studied and
optimized. The HRP-labeled antibody and biotinylated antibody were
diluted with dilution buffers to a series of dilution ratios with
the standard survivin concentration of 100 ng/ml. Data was analyzed
using Student's t-test. As shown in Fig.
3, the RLUs increased when the dilution ratios of HRP-labeled
antibody increased from 1:90,000 to 1:15,000 at all dilution ratios
of the examined biotinylated antibody (P<0.05). As for the
biotinylated antibody, the RLU was approximately a peak with the
dilution ratio of 1:18,000 (P<0.05). Considering both the
sensitivity and the assay cost, we selected the dilution ratios of
1:15,000 and 1:18,000 for HRP-labeled antibody and biotinylated
antibody respectively.
Influence and optimization of
physicochemical parameters
Influence of immunoassay incubation time
Incubation time of the immunoreagents may have a
direct effect on the sensitivity of the immunoassay. Incubation
time from 10 to 60 min (10 min as the interval) was studied. As
shown in Table I, RLUs increased with
increasing reaction time, while RLUS1/RLUS0
(reflecting sensitivity) and RLUS5/RLUS0
(reflecting linear range) increased with time up to 20 min and
after 20 min the values tended to decrease. Based on all this and
considering nonspecific absorption would improve with a longer
incubation time, incubation time of 20 min was selected.
 | Table I.Effects of immunoassay incubation
time. |
Table I.
Effects of immunoassay incubation
time.
|
| RLU |
|---|
|
|
|
|---|
| Incubationtime
(min) | S0 | S1 | S5 | S1/S0 | S5/S0 |
|---|
| 10 | 4.439 | 20.063 | 997.43 | 4.519711647 | 224.6970038 |
| 20 | 4.771 | 34.796 | 1182.4 | 7.293229931 | 247.8306435 |
| 30 | 15.11 | 99.722 | 1234.9 | 6.599735275 | 81.72733289 |
| 40 | 17.304 | 79.333 | 1338.2 | 4.584662506 | 77.3347203 |
| 50 | 21.694 | 133 | 1341.2 | 6.13072739 | 61.82354568 |
| 60 | 22.932 | 105.42 | 1400.9 | 4.597069597 | 61.08930752 |
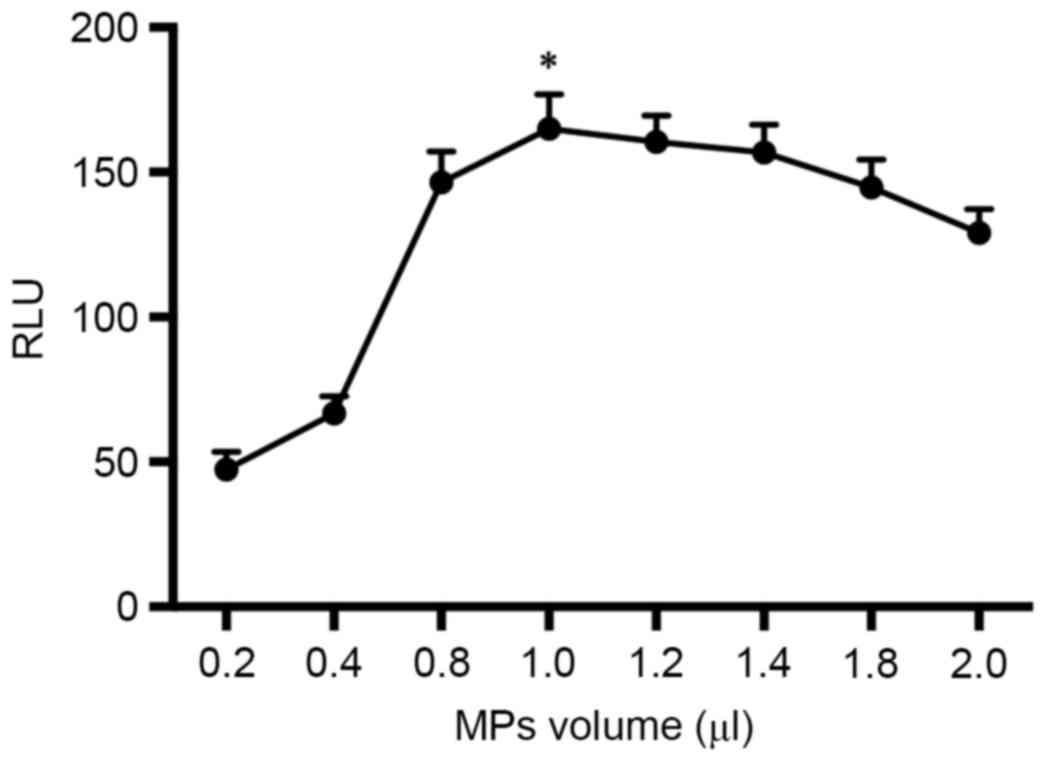
Influence of the volume of MPs
The quantity of streptavidin MPs was critical for
the immunoassay system. The volumes of the MPs (10 mg/ml) were
optimized from 0.2 to 2.0 µl. Student's t-test was used for data
comparison. As shown in Fig. 4, the
RLUs increased with the volume of MPs from 0.2 to 1.0 µl and then
decreased gradually as the volume increased from 1.0 to 2.0 µl. An
excess of MPs might absorb the emitted light (24). Therefore the optimal volume of MPs was
set to 1.0 µl (P<0.05).
Influence of capture time
In this experiment, MPs were used as the separation
agent. After adding MPs, immunoassay regents were captured. Capture
time from 10 to 60 min (10 min as the interval) was also explored
to make clear its effect on the sensitivity of this immunoassay. As
shown in Table II, RLUs increased
weakly with increasing capture time, meanwhile
RLUS1/RLUS0 (reflecting sensitivity) and
RLUS5/RLUS0 (reflecting linear range) tended
to decrease as time gone by. Thus, capture time of 10 min was
selected.
 | Table II.Effects of immunoassay capture
time. |
Table II.
Effects of immunoassay capture
time.
|
| RLU |
|---|
|
|
|
|---|
| Capturing time
(min) | S0 | S1 | S5 | S1/S0 | S5/S0 |
|---|
| 10 | 4.406 | 39.744 | 1176.4 | 9.020426691 | 266.9995461 |
| 20 | 7.916 | 70.159 | 1234.9 | 8.862935826 | 156.0005053 |
| 30 | 8.74 | 73.929 | 1338.2 | 8.458695652 | 153.1121281 |
| 40 | 14.858 | 105.74 | 1341.2 | 7.116704805 | 90.26786916 |
| 50 | 17.935 | 94.731 | 1400.9 | 5.281906886 | 78.10984109 |
| 60 | 20.983 | 86.384 | 1524.4 | 4.116856503 | 72.64928752 |
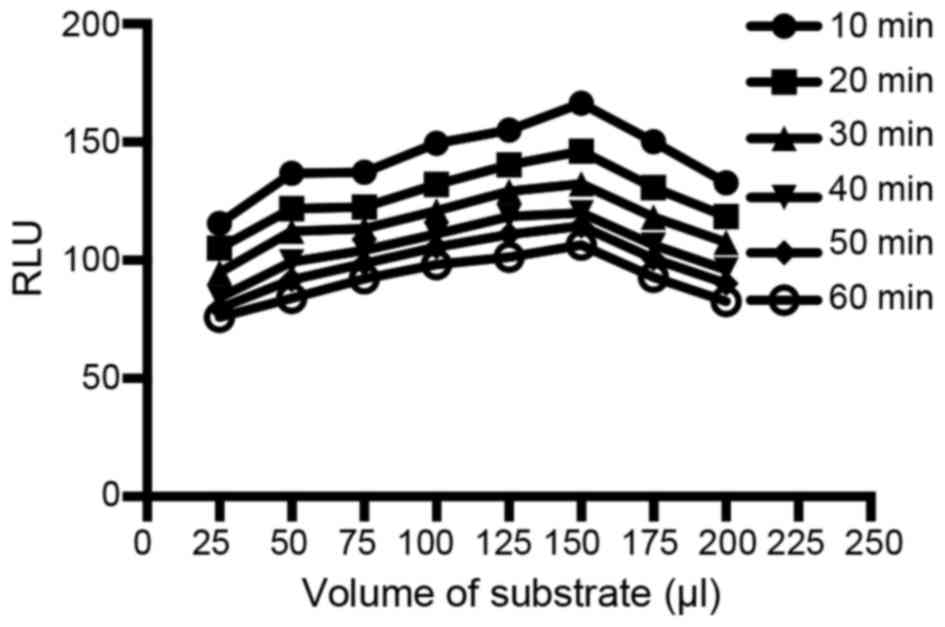
Influence of the volume of the CL substrate and
chemiluminescence reaction time
The CL substrate was a pivotal factor related to the
CL intensity and the sensitivity of the assay. In this experiment,
the CL substrate volume from 0 to 200 µl (25 µl as the interval)
and the chemiluminescence reaction time from 10 to 60 min (10 min
as the interval) were examined. Data was compared by Student's
t-test. As shown in Fig. 5, the RLUs
increased with increasing volume of CL substrate up to a maximum at
150 µl and then gradually decreased (P<0.05). As to the
chemiluminescence reaction time, RLUs decreased over time
(P<0.05). Thus, 150 µl of CL substrate and 10 min of
chemiluminescence reaction time were chosen.
Method evaluation
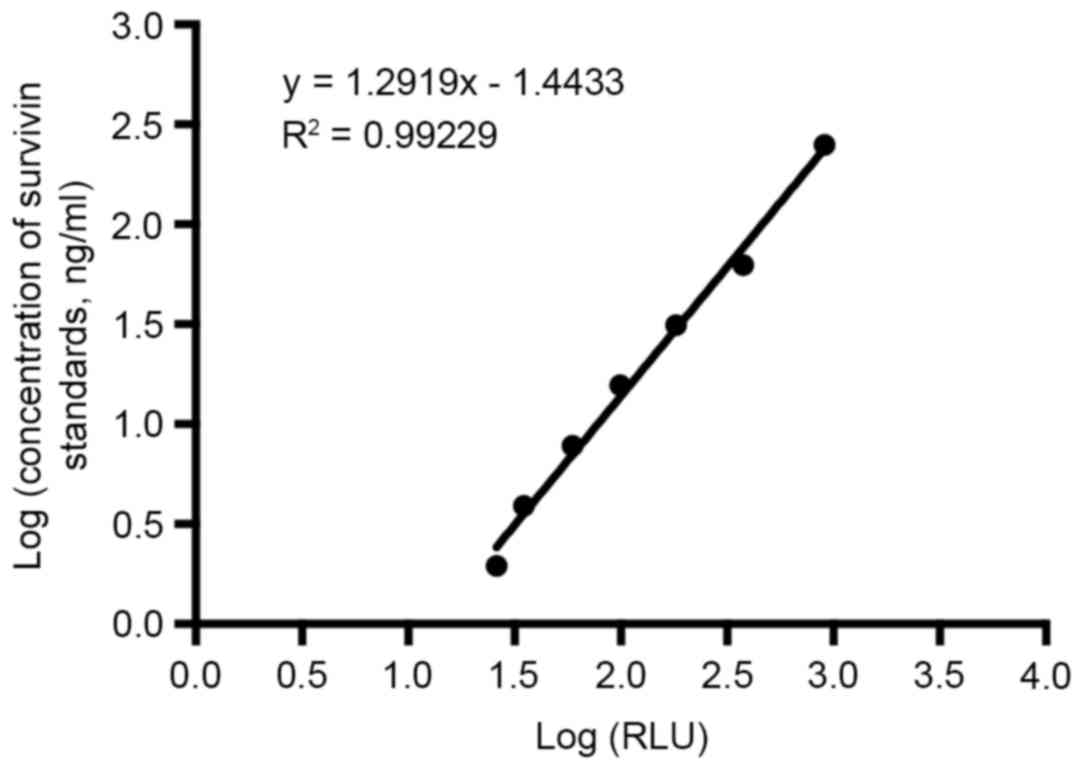
Calibration curve and sensitivity
Under the optimal conditions, dose-response curve
(Fig. 6) was obtained. By first
obtaining the average RLU signals for 10 replicates of
S0 and then adding 2 standard deviations (SDs) into the
dose-response curve the detection limit was calculated. The
obtained detection limit for survivin was 0.83 ng/ml.
Precision
In order to obtain the intra-assay precision, three
different concentrations of standards were measured 10 times within
one assay. Similarly, these standards were analyzed on 5 different
days using the same protocol (2 replicates per run) to obtain the
inter-assay variation. As shown in Table III, intra-assay and inter-assay CVs
were <8 and <11%, respectively.
 | Table III.Intra- and inter-assay variability
for survivin. |
Table III.
Intra- and inter-assay variability
for survivin.
|
| Intra-assay | Inter-assay |
|---|
|
|
|
|
|---|
| Sample no. | Times of
replication | Concentration
(ng/ml) | CV (%) | Days of
replication | Concentration
(ng/ml) | CV (%) |
|---|
| 1 | 10 | 331.5 | 0.7 | 5 | 330.7 |
1.62 |
| 2 | 10 | 128.76 | 3.7 | 5 |
127.83 |
7.87 |
| 3 | 10 |
22.91 |
7.54 | 5 |
23.14 | 10.46 |
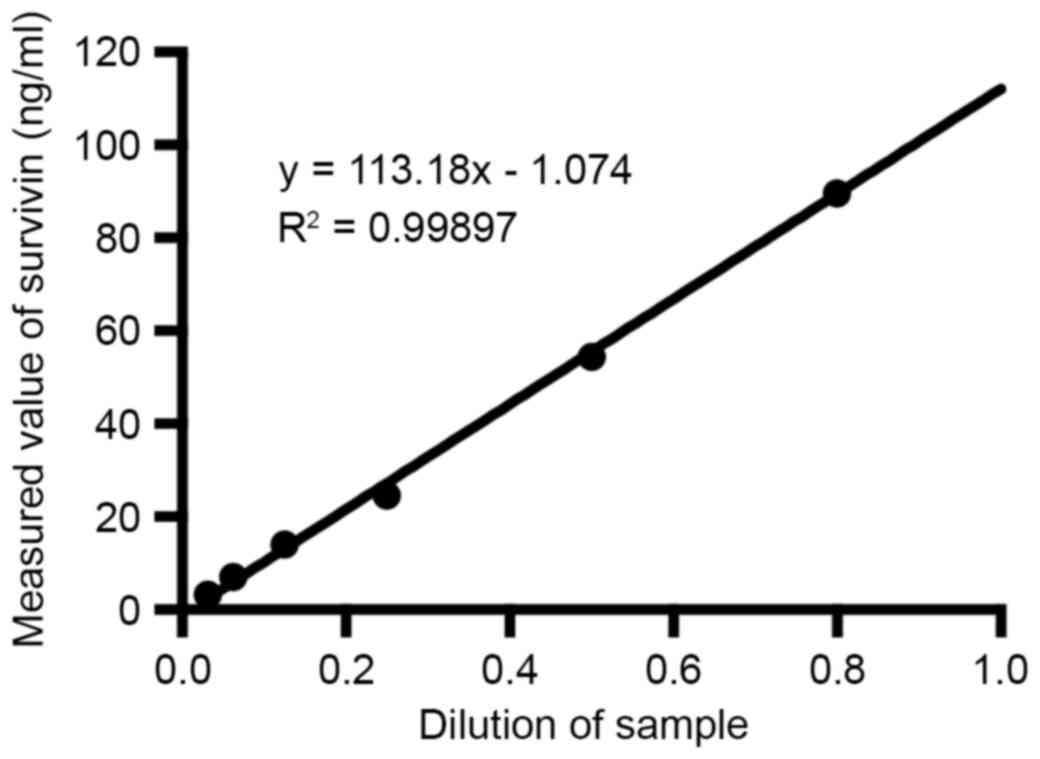
Linearity-dilution effect
The linearity-dilution effect was studied by
selecting a certain human sample with relatively high
concentration. This sample was then diluted to a series of
concentrations with dilution buffer. The results were shown in
Fig. 7.
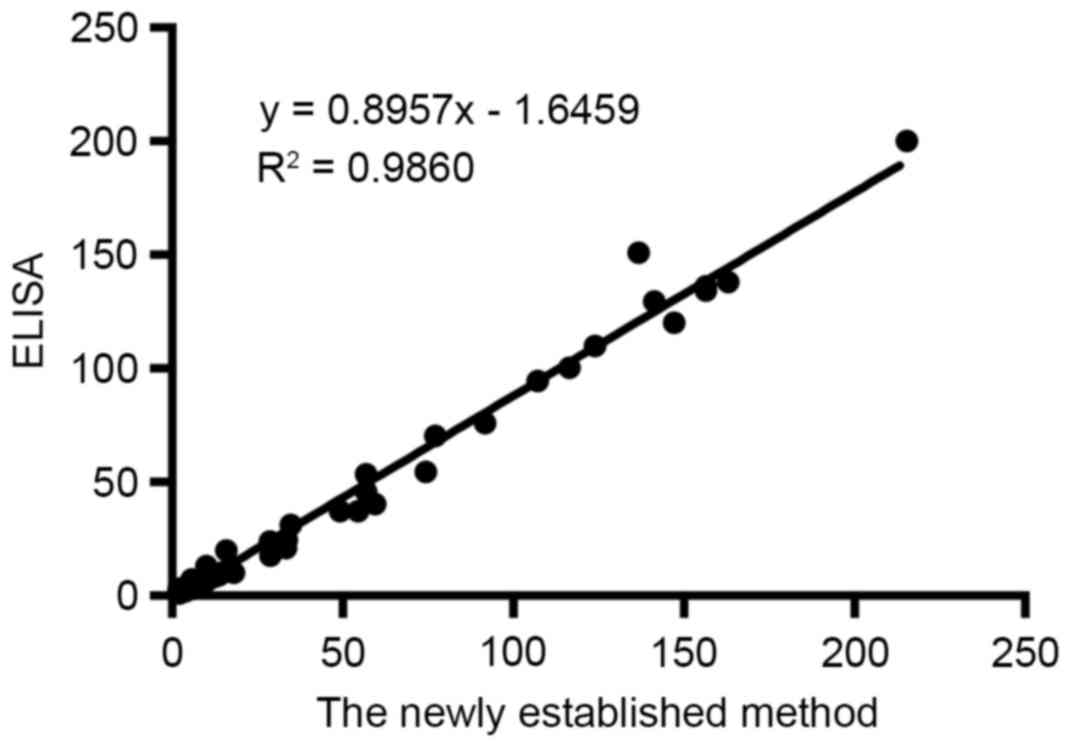
Comparison with ELISA
Survivin levels of 60 urine samples of bladder
cancer patients were determined simultaneously using ELISA and the
newly established method. As can be seen in Fig. 8, two methods were compared and there
was a good agreement with the correlation coefficient of 0.9860
(P<0.01).
Sample analysis
General data of groups
A total of 130 bladder cancer patients and 113
healthy controls were enrolled in the present study. Table IV summarized the general
characterization of two groups (χ2 test).
 | Table IV.General data of bladder cancer
patients and healthy controls. |
Table IV.
General data of bladder cancer
patients and healthy controls.
| Variable | Patients
(n=130) | Controls
(n=113) | P-value |
|---|
| Gender, n (%) |
|
| 0.331 |
|
Female | 36 (27.7) | 38 (33.6) |
|
|
Male | 94 (72.3) | 75 (66.4) |
|
| Age, n (%) |
|
| 0.239 |
|
>62 | 74 (56.9) | 73 (64.6) |
|
|
≤62 | 56 (43.1) | 40 (35.4) |
|
Analysis of urine survivin levels between
groups
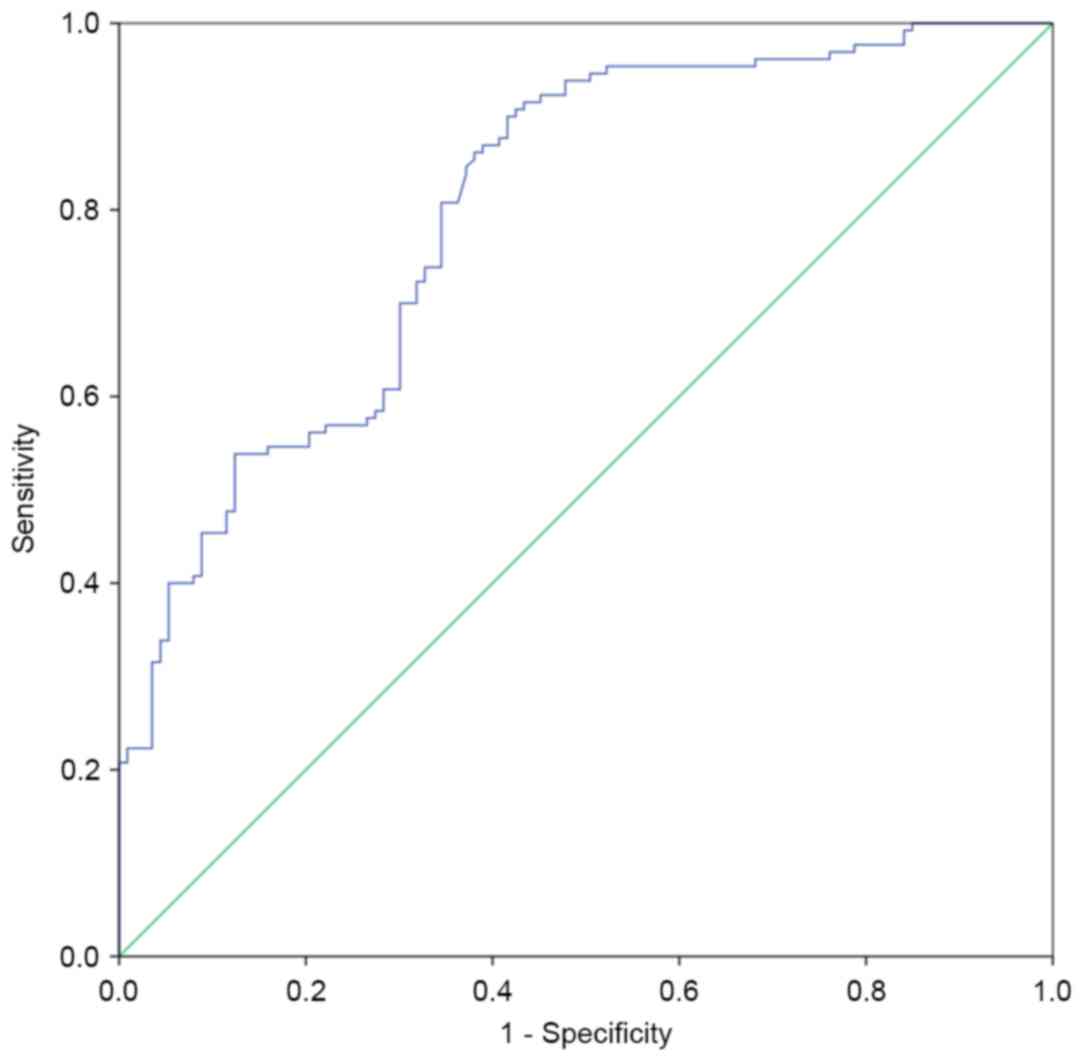
Receiver operating characteristic (ROC) curve based
on the detection of 130 bladder cancer patients and 113 healthy
people was shown in Fig. 9. The area
under the curve was 0.799. When survivin concentration was 2.0884
ng/ml, sensitivity and specificity were 86.9 and 61.9%,
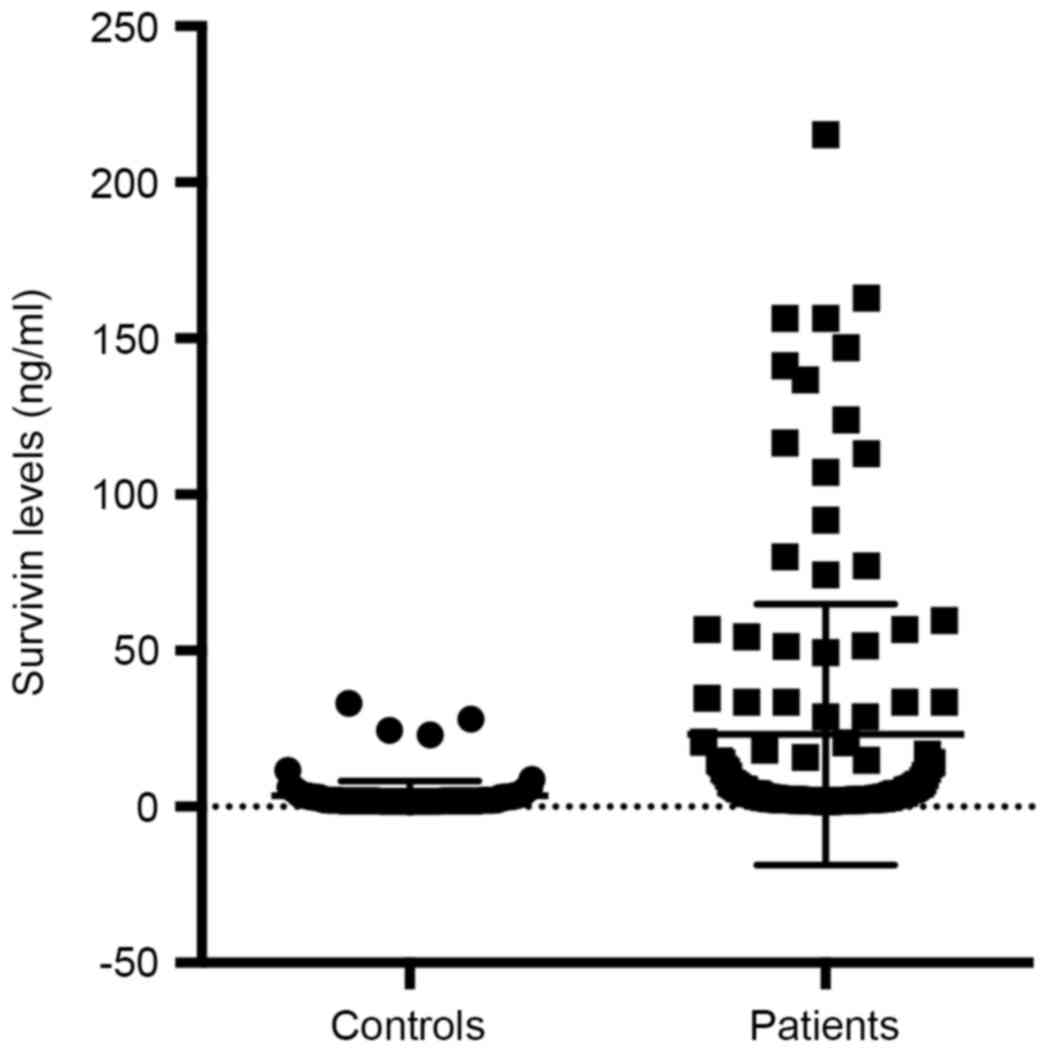
respectively. We compared the urinary survivin levels between
bladder cancer patients and healthy controls (Student's t-test).
The urinary survivin levels were significantly higher in bladder
cancer patients than in healthy controls (P<0.001). Table V and Fig.
10 gave the results of comparison and the scatter plot of
survivin levels between groups.
 | Table V.Comparison of survivin levels between
groups. |
Table V.
Comparison of survivin levels between
groups.
|
|
| Survivin levels
(ng/ml) |
|
|---|
|
|
|
|
|
|---|
| Group | Number | Mean ± SD | P-value |
|---|
| Patients | 130 |
23.1372±41.73024 | <0.001 |
| Controls | 113 | 3.4419±4.85624 |
|
Comparison of urine survivin levels in different
clinicopathological categories of bladder cancer patients
Clinicalpathological features including age, gender,
smoke, hypertension, metastasis stage, lymph node status, TNM
stage, histological stage, tumor size, tumor thrombus and primary
or not were obtained. We compared the urinary survivin levels
between different clinicopathological categories of bladder cancer
patients using Student's t-test. Results showed that among all the
factors, urinary survivin levels associated with metastatic stage,
histological stage and recurrence (P<0.01). The comparison
results were summarized in Table
VI.
 | Table VI.Comparison of survivin levels in
different clinicopathological categories of bladder cancer
patients. |
Table VI.
Comparison of survivin levels in
different clinicopathological categories of bladder cancer
patients.
|
|
| Survivin levels
(ng/ml) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | Number | Mean ± SD | P-value |
|---|
| Age (years) |
|
| 0.192 |
|
>62 | 74 | 27.10±47.36 |
|
|
≤62 | 56 | 17.90±32.53 |
|
| Gender |
|
| 0.83 |
|
Female | 36 | 24.41±38.55 |
|
|
Male | 94 | 22.65±43.07 |
|
| Smoke |
|
| 0.515 |
|
Yes | 56 | 20.34±42.01 |
|
| No | 72 | 25.23±42.08 |
|
| Hypertension |
|
| 0.995 |
|
Yes | 45 | 22.97±43.30 |
|
| No | 84 | 22.92±41.29 |
|
| Metastatic
stage |
|
| <0.01 |
| M1 | 108 | 51.88±50.78 |
|
| M0 | 22 | 17.28±37.27 |
|
| Lymph node
status |
|
| 0.058 |
|
Positive | 19 | 39.91±43.70 |
|
|
Negative | 111 | 20.27±40.90 |
|
| Histological
Stage |
|
| <0.01 |
| G3 | 63 | 33.14±48.89 |
|
|
G1/G2 | 54 | 9.15±13.95 |
|
| Size (cm) |
|
| 0.768 |
| ≥3 | 28 | 15.80±26.97 |
|
|
<3 | 59 | 18.10±36.70 |
|
| Tumor thrombus |
|
| 0.636 |
|
Visible | 16 | 18.48±23.11 |
|
|
Invisible | 114 | 23.79±43.74 |
|
| Recurrence |
|
| <0.01 |
|
Primary | 91 | 12.58±31.11 |
|
|
Recurrent | 39 | 47.77±52.25 |
|
| TNM stage |
|
| 0.063 |
|
I–II | 94 | 18.59±39.27 |
|
|
III–IV | 36 | 35.02±46.05 |
|
Discussion
Survivin is expressed during fatal development and
involved in blocking caspases (25–27) as
well as favoring aberrant progression through mitosis (28). Studies have found that overexpression
of survivin in human malignancies could be associated with
carcinoma metastasis and progression (29). High expression of survivin in bladder
cancer is associated with several unfavorable prognostic factors
such as increasing recurrence rates, progression and resistance to
therapy (30). Though a series of
studies have evaluated urinary survivin as a biomarker for bladder
cancer, the exact role of urinary survivin is still unclear partly
due to the suboptimal measurements (31,32).
Several methods have been used to detect survivin
expression including IHC, real-time-PCR, Reverse
transcription-polymerase chain reaction (RT-PCR) and ELISA
(32–34). However, RT-PCR and Real-time PCR are
costly which requires professional facilities. IHC is accurate
while tissue specimens are difficult to get. Though ELISA is
considered accurate and sensitive, it is to some content limited as
time consuming with a bad uniformity. Here in this research, a new
measurement was established for the detection of urinary
survivin.
In the present study, a microplate magnetic CLIA was
established for the analysis of urinary survivin levels. By
combining magnetic separation with chemiluminescence detection
system, this research first applied a novel sandwich immunoassay to
the determination of urinary survivin. The dilution ratios of
immunoreagents as well as physicochemical parameters were optimized
during the method development.
With the established and refined measurement, 130
samples of bladder cancer patients and 113 samples of healthy
controls were detected for urinary survivin levels. The results
showed that urinary survivin levels of bladder cancer patients were
significantly higher than that of healthy controls, which is
consistent with some other studies (13,19,35) and
indicating urinary survivin as a potential biomarker for the
diagnosis of bladder cancer. Besides, by analyzing the correlation
of urinary survivin levels with clinicopathological factors, the
currently study found that the urinary survivin levels were
positively associated with metastatic stage, histological stage and
recurrence, which is consistent with some studies (19,32) while
disaccord with another study (18).
Compared with ELISA, a main advantage of the novel
measurement in this experiment is its rapidity. Besides, by
utilizing microplates, a high flux of analysis and a good
uniformity are realized (20,36). At the same time, the microplates were
pre-coated with 1% (w/v) BSA in PBS buffer to avoid the high
non-specific absorption of the plates. The microplate magnetic
technologies were rarely reported (21,37,38) and
even few applications were found for the detection of survivin.
Here in this study, streptavidin MPs were used as separation
reagents, combined with the magnetic separation device, greatly
simplifying the separation procedure. Furthermore, the new
measurement reduces the amount of immunoassay regents and the
volume of samples, showing great potential in the future.
Meanwhile, it should be noted that this research has
some limitations. On one hand, the use of MPs in this research was
expected to provide many more active binding sites and increase the
sensitivity as well as facilitate larger linear range in the
detection (39), but results were not
positive. When the cutoff survivin level was 2.0884 ng/ml,
sensitivity and specificity were 86.9 and 61.9% respectively, not
superior to some other reports of different methods (18,32,40). This
may be partly due to the heterogeneity between different studies as
well as different cut-off values. While the complexity of urine
components and variability of urine pH may be the main factor
hinders the development of more sensitive methods (41,42). On
the other hand, the linearity-dilution effect was unstable and
varied a lot between different samples using this method. This may
be attributed to the imperfect diluent used in this research, which
is unable to deal with the complexity of urine components.
The main objective of our study was the
establishment of a novel immunoassay for urinary survivin
detection; for this purpose urine sample of patients treated
recently (from January 2016 to July 2016) in our hospital were
collected. It is too close to track the long-term prognosis
information of these patients. Since long-term prognosis
information of patients is important for clinical course
evaluation, our study is limited to some extent considering the
deficiency of the information. This study showed that urinary
survivin level was correlated with metastatic stage, histological
stage and recurrence, which implied survivin as a potential marker
of disease progression. It could be better if we could obtain the
long-term prognosis information to further evaluate the value of
survivin. In the next years, we will follow-up the bladder cancer
patients included in this study to do further investigation
according to your suggestion. Moreover, we established a new method
and compared it with ELISA, while it is not the end. There are
other methods for the evaluation of survivin levels such as methods
for mRNA detection and IHC (31,43).
Though we have obtained data of these methods from other papers, it
is not so accurate considering sample differences (31,43–45).
Therefore it is significant for us to collect more specimens and
perform different methods to make better comparisons in the
future.
This research implies urinary survivin as a
potential tumor marker of metastasis, progression and recurrence in
addition to diagnosis for bladder cancer with the novel established
method. While considering the small number of patients in this
study, a larger series of samples and further studies are needed in
order to understand the role of survivin in bladder cancer better.
Besides, since the molecular mechanism of bladder cancer is
complicated, it may be much more predictive to combine other
urinary markers with survivin (46,47). Last
but not least, the method itself is not perfect given the
unsatisfactory sensitivity and linear range. Considering the
complexity of urine matrix (48), it
is difficult to develop immunoassays with good performance for
urinary biomarkers. Therefore much more work should be done to
explore a better measurement for urinary survivin detection based
on this research.
References
|
1
|
Youssef RF and Lotan Y: Predictors of
outcome of non-muscle-invasive and muscle-invasive bladder cancer.
ScientificWorldJournal. 11:369–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babjuk M, Böhle A, Burger M, Capoun O,
Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M,
et al: EAU guidelines on non-muscle-invasive urothelial carcinoma
of the bladder: Update 2016. Eur Urol. 71:447–461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lokeshwar VB, Habuchi T, Grossman HB,
Murphy WM, Hautmann SH, Hemstreet GP III, Bono AV, Getzenberg RH,
Goebell P, Schmitz-Dräger BJ, et al: Bladder tumor markers beyond
cytology: International consensus panel on bladder tumor markers.
Urology. 66:(6 Suppl 1). S35–S63. 2005. View Article : Google Scholar
|
|
4
|
Frantzi M, Latosinska A, Flühe L, Hupe MC,
Critselis E, Kramer MW, Merseburger AS, Mischak H and Vlahou A:
Developing proteomic biomarkers for bladder cancer: Towards
clinical application. Nat Rev Urol. 12:317–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gogalic S, Sauer U, Doppler S and
Preininger C: Bladder cancer biomarker array to detect aberrant
levels of proteins in urine. Analyst. 140:724–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamat AM, Hegarty PK, Gee JR, Clark PE,
Svatek RS, Hegarty N, Shariat SF, Xylinas E, Schmitz-Dräger BJ,
Lotan Y, et al: ICUD-EAU International consultation on bladder
cancer 2012: Screening, diagnosis, and molecular markers. Eur Urol.
63:4–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nisman B, Yutkin V, Peretz T, Shapiro A,
Barak V and Pode D: The follow-up of patients with
non-muscle-invasive bladder cancer by urine cytology, abdominal
ultrasound and urine CYFRA 21-1: A pilot study. Anticancer Res.
29:4281–4285. 2009.PubMed/NCBI
|
|
8
|
Jäger T, Tschirdewahn S, Vom Dorp F,
Piechotta G, Rübben H and Szarvas T: Siliconchiptechnology-based
MMP-7 analysis in urine: An option for preoperative identification
of lymph node metastasis in bladder cancer. Urologe A. 52:853–858.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altieri DC: Survivin in apoptosis control
and cell cycle regulation in cancer. Prog Cell Cycle Res.
5:447–452. 2003.PubMed/NCBI
|
|
10
|
Uren AG, Wong L, Pakusch M, Fowler KJ,
Burrows FJ, Vaux DL and Choo KH: Survivin and the inner centromere
protein INCENP show similar cell-cycle localization and gene
knockout phenotype. Curr Biol. 10:1319–1328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giodini A, Kallio MJ, Wall NR, Gorbsky GJ,
Tognin S, Marchisio PC, Symons M and Altieri DC: Regulation of
microtubule stability and mitotic progression by survivin. Cancer
Res. 62:2462–2467. 2002.PubMed/NCBI
|
|
12
|
Zaffaroni N, Pennati M and Daidone MG:
Survivin as a target for new anticancer interventions. J Cell Mol
Med. 9:360–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spaulding B, Pan D, Ghadersohi A, Nielsen
G, Jensen S, Gellert F, Ling X, Zhang M, Black A and Li F:
Characterization of the 12C4 survivin monoclonal antibody and
insight into the expression of survivin in human adult tissues.
Histopathology. 49:622–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muschol-Steinmetz C, Friemel A, Kreis NN,
Reinhard J, Yuan J and Louwen F: Function of survivin in
trophoblastic cells of the placenta. PLoS One. 8:e733372013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weikert S, Christoph F, Schrader M, Krause
H, Miller K and Müller M: Quantitative analysis of survivin mRNA
expression in urine and tumor tissue of bladder cancer patients and
its potential relevance for disease detection and prognosis. Int J
Cancer. 116:100–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karam JA, Lotan Y, Ashfaq R, Sagalowsky AI
and Shariat SF: Survivin expression in patients with
non-muscle-invasive urothelial cell carcinoma of the bladder.
Urology. 70:482–486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Margulis V, Lotan Y and Shariat SF:
Survivin: A promising biomarker for detection and prognosis of
bladder cancer. World J Urol. 26:59–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Wang Y, Xu J and Zhang Q: Sandwich
ELISA for detecting urinary survivin in bladder cancer. Chin J
Cancer Res. 25:375–381. 2013.PubMed/NCBI
|
|
19
|
Srivastava AK, Singh PK, Srivastava K,
Singh D, Dalela D, Rath SK, Goel MM and Bhatt Brahma ML: Diagnostic
role of survivin in urinary bladder cancer. Asian Pac J Cancer
Prev. 14:81–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schneider C, Schöler HF and Schneider RJ:
Direct sub-ppt detection of the endocrine disruptor
ethinylestradiol in water with a chemiluminescence enzyme-linked
immunosorbent assay. Analyt Chim Acta. 551:92–97. 2005. View Article : Google Scholar
|
|
21
|
Gundersen SG, Haagensen I, Jonassen TO,
Figenschau KJ, de Jonge N and Deelder AM: Magnetic bead antigen
capture enzyme-linked immunoassay in microtitre trays for rapid
detection of schistosomal circulating anodic antigen. J Immunol
Methods. 148:1–8. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen D, Xu J and Zhang Q: Detection of
survivin expression in bladder cancer and renal cell carcinoma with
its specific monoclonal antibodies. Oncology Reports In Press.
|
|
23
|
Wang Y, Zhang QY and Wang YM: Cloning of
survivin gene and preparation its monoclonal antibodies as well as
checking survivin expression in liver carcinoma cells. Chinese
Journal of Laboratory Medicine. 2006.
|
|
24
|
Li Z, Zhang Q, Zhao L, Li Z, Hu G, Lin J
and Wang S: Micro-plate magnetic chemiluminescence immunoassay and
its applications in carcinoembryonic antigen analysis. Sci China
Chem. 53:812–819. 2010. View Article : Google Scholar
|
|
25
|
Herman MP, Svatek RS, Lotan Y, Karakiewizc
PI and Shariat SF: Urine-based biomarkers for the early detection
and surveillance of non-muscle invasive bladder cancer. Minerva
Urol Nefrol. 60:217–235. 2008.PubMed/NCBI
|
|
26
|
Budman LI, Kassouf W and Steinberg JR:
Biomarkers for detection and surveillance of bladder cancer. Can
Urol Assoc J. 2:212–221. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Domnanich P, Sauer U, Pultar J and
Preininger C: Protein microarray for the analysis of human melanoma
biomarkers. Sensors Actuators B Chem. 139:2–8. 2009. View Article : Google Scholar
|
|
28
|
Li F and Ling X: Survivin study: An update
of ‘what is the next wave’? J Cell Physiol. 208:476–486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eissa S, Shabayek MI, Ismail MF, El-Allawy
RM and Hamdy MA: Diagnostic evaluation of apoptosis inhibitory gene
and tissue inhibitor matrix metalloproteinase-2 in patients with
bladder cancer. IUBMB Life. 62:394–399. 2010.PubMed/NCBI
|
|
30
|
Qin C, Cao Q, Li P, Ju X, Wang M, Chen J,
Wu Y, Meng X, Zhu J, Zhang Z, et al: Functional promoter −31G>C
variant in survivin gene is associated with risk and progression of
renal cell cancer in a Chinese population. PLoS One. 7:e288292012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeon C, Kim M, Kwak C, Kim HH and Ku JH:
Prognostic role of survivin in bladder cancer: A systematic review
and meta-analysis. PLoS One. 8:e767192013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shariat SF, Casella R, Khoddami SM,
Hernandez G, Sulser T, Gasser TC and Lerner SP: Urine detection of
survivin is a sensitive marker for the noninvasive diagnosis of
bladder cancer. J Urol. 171:626–630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kenney DM, Geschwindt RD, Kary MR, Linic
JM, Sardesai NY and Li ZQ: Detection of newly diagnosed bladder
cancer, bladder cancer recurrence and bladder cancer in patients
with hematuria using quantitative rt-PCR of urinary survivin.
Tumour Biol. 28:57–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moussa O, Abol-Enein H, Bissada NK, Keane
T, Ghoneim MA and Watson DK: Evaluation of survivin reverse
transcriptase-polymerase chain reaction for noninvasive detection
of bladder cancer. J Urol. 175:2312–2316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abd El-Hakim TF, El-Shafie MK, Abdou AG,
Azmy RM, El-Naidany SS and El-Din Badr MO: Value of urinary
survivin as a diagnostic marker in bladder cancer. Anal Quant
Cytopathol Histpathol. 36:121–127. 2014.PubMed/NCBI
|
|
36
|
Zhao L, Lin JM, Li Z and Ying X:
Development of a highly sensitive, second antibody format
chemiluminescence enzyme immunoassay for the determination of
17β-estradiol in wastewater. Anal Chim Acta. 558:290–295. 2006.
View Article : Google Scholar
|
|
37
|
Cudjoe KS, Hagtvedt T and Dainty R:
Immunomagnetic separation of Salmonella from foods and their
detection using immunomagnetic particle (IMP)-ELISA. Int J Food
Microbiol. 27:11–25. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu H, Ahmed H and Vasta GR: Development of
a magnetic microplate chemifluorimmunoassay for rapid detection of
bacteria and toxin in blood. Anal Biochem. 261:1–7. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Q, Wang X, Li Z and Lin JM:
Evaluation of alpha-fetoprotein (AFP) in human serum by
chemiluminescence enzyme immunoassay with magnetic particles and
coated tubes as solid phases. Anal Chim Acta. 631:212–217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Xi X, Kong X, Huang G and Ge G:
The expression and significance of survivin mRNA in urinary bladder
carcinomas. J Cancer Res Clin Oncol. 130:487–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu J, Chen YD and Gu W: Urinary proteomics
as a novel tool for biomarker discovery in kidney diseases. J
Zhejiang Univ Sci B. 11:227–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fichorova RN, Richardson-Harman N, Alfano
M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti
CS, Donoval B, et al: Biological and technical variables affecting
immunoassay recovery of cytokines from human serum and simulated
vaginal fluid: A multicenter study. Anal Chem. 80:4741–4751. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Johnen G, Gawrych K, Bontrup H, Pesch B,
Taeger D, Banek S, Kluckert M, Wellhäußer H, Eberle F, Nasterlack
M, et al: Performance of survivin mRNA as a biomarker for bladder
cancer in the prospective study UroScreen. PLoS One. 7:e353632012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Horstmann M, Bontrup H, Hennenlotter J,
Taeger D, Weber A, Pesch B, Feil G, Patschan O, Johnen G, Stenzl A
and Brüning T: Clinical experience with survivin as a biomarker for
urothelial bladder cancer. World J Urol. 28:399–404. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun YW, Xuan Q, Shu QA, Wu SS, Chen H,
Xiao J, Xiang P, Zhu YP, Wang FL and Zhao ST: Correlation of tumor
relapse and elevated expression of survivin and vascular
endothelial growth factor in superficial bladder transitional cell
carcinoma. Genet Mol Res. 12:1045–1053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Karam JA, Lotan Y, Karakiewicz PI, Ashfaq
R, Sagalowsky AI, Roehrborn CG and Shariat SF: Use of combined
apoptosis biomarkers for prediction of bladder cancer recurrence
and mortality after radical cystectomy. Lancet Oncol. 8:128–136.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Als AB, Dyrskjøt L, von der Maase H, Koed
K, Mansilla F, Toldbod HE, Jensen JL, Ulhøi BP, Sengeløv L, Jensen
KM and Orntoft TF: Emmprin and survivin predict response and
survival following cisplatin-containing chemotherapy in patients
with advanced bladder cancer. Clin Cancer Res. 13:4407–4414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Adachi J, Kumar C, Zhang Y, Olsen JV and
Mann M: The human urinary proteome contains more than 1500
proteins, including a large proportion of membrane proteins. Genome
Biol. 7:R802006. View Article : Google Scholar : PubMed/NCBI
|