Introduction
The human epidermal growth factor receptor 2 (HER2,
ErbB2/neu), a member of the ErbB/HER family proteins, is
overexpressed in approximately 20% of human breast cancers and is
positively associated with the aggressiveness of the disease and
with poor prognosis (1–4). Therefore, HER2-targeted therapy is
considered a very rational strategy for HER2-overexpressing breast
cancer. Trastuzumab, the first humanized anti-HER2 monoclonal
antibody, binds to domain IV of the HER2 extracellular domain (ECD)
and inhibits ligand-independent HER2/HER3 signaling and HER2
shedding (5,6). Trastuzumab also has the ability to
trigger antibody-dependent cell-mediated cytotoxicity (ADCC) by
binding to the Fcγ receptor of immune cells such as natural killer
cells through its Fc region (7,8).
Trastuzumab has been approved for the treatment of both early and
metastatic HER2-overexpressing breast cancer. Although trastuzumab
improves the survival of patients with advanced HER2-overexpressing
cancer (9,10), most patients eventually experience
progressive disease. Therefore, a new treatment modality including
trastuzumab was needed for advanced HER2-overexpressing cancer.
Pertuzumab is a humanized anti-HER2 monoclonal
antibody that binds to a distinct epitope of HER2 (domain II)
(11). Because domain II of HER2 is a
region necessary for dimerization with other HER family receptors
and signaling, pertuzumab inhibits ligand-induced dimerization and
its downstream signaling (11,12). In
previous preclinical studies, we found that pertuzumab and
trastuzumab bind to HER2 without competing with each other
(13), and we and others have
reported that the combination of pertuzumab plus trastuzumab exerts
enhanced antitumor activity as compared to single-agent treatment
(13,14). In a Phase III clinical trial in
patients with HER2-positive metastatic breast cancer (the CLEOPATRA
study) it was demonstrated that the triple-drug combination of
pertuzumab plus trastuzumab plus docetaxel, as compared to the
combination of trastuzumab plus docetaxel, significantly improved
progression-free survival and overall survival (15,16). On
the basis of that result, pertuzumab was firstly approved for
HER2-positive metastatic breast cancer in combination with
trastuzumab and chemotherapy.
Nowadays, the triple-drug combination of pertuzumab
plus trastuzumab plus docetaxel is becoming a first-line therapy
for HER2-positive metastatic breast cancer. It is of enormous
clinical importance, therefore, to give further thought to how the
combination damages tumor cells and alters the tumor
microenvironment. Preclinical studies using mouse xenograft models
are a simple and effective way to investigate these questions. The
aim of the present study was to assess the antitumor effect of the
triple-drug combination from the aspect of cancer cell death and
host immune cell response by using a human breast cancer
xenografted mouse model.
Materials and methods
Test agents
Trastuzumab and pertuzumab were provided by F.
Hoffmann-La Roche (Basel, Switzerland) as a fine powder and a
liquid, respectively. Trastuzumab was dissolved in distilled water.
The two antibodies were then diluted with saline for the in
vivo experiments and culture medium for the in vitro
experiments. Human immunoglobulin G (HuIgG) was purchased from MP
Biomedicals, LLC (Solon, OH, USA) and was reconstituted with
distilled water and diluted with saline. Docetaxel was purchased
from Sanofi K.K. (Tokyo, Japan) and was diluted with saline just
before administration. Paclitaxel was purchased from Wako Pure
Chemical Industries, Ltd. (Osaka, Japan) as a fine powder.
Paclitaxel was reconstituted with Cremophor EL-ethanol solution
(1:1) and diluted tenfold with saline just before
administration.
Animals
Female, 5-week-old BALB-nu/nu mice
(CAnN.Cg-Foxn1<nu>/CrlCrlj nu/nu) were obtained from Charles
River Laboratories Japan, Inc. (Yokohama, Japan). All animals were
allowed to acclimatize and recover from shipping-related stress for
1 week prior to the study. The health of the mice was monitored by
daily observation. The animals were allowed free access to
chlorinated water and irradiated food, and the animals were kept
under a controlled light-dark cycle (12–12 h). All animal
experiments were reviewed and approved by the Institutional Animal
Care and Use Committee at Chugai Pharmaceutical Co. Ltd.
Cell line and culture conditions
The HER2-positive human breast cancer cell line
KPL-4 was kindly provided by Dr. J Kurebayashi (Kawasaki Medical
School, Kurashiki, Japan). KPL-4, which is sensitive to trastuzumab
in vivo (17) and is estrogen
receptor-negative (18), was
maintained in Dulbecco's modified Eagle's medium (D-MEM, 1 g/l
glucose; Sigma-Aldrich Co. LLC., St. Louis, MO, USA) supplemented
with 5% FBS at 37°C under 5% CO2.
In vivo tumor growth inhibition
studies
Each mouse was inoculated subcutaneously into the
second mammary fat pad with 5×106 cells/mouse of KPL-4.
When tumor volumes reached approximately 0.2 to 0.3 cm3,
the mice were randomly allocated to control and treatment groups,
and treatment with the antitumor agents was started (Day 1).
Docetaxel at 10 mg/kg or vehicle was administered intravenously on
the first day of treatment. Paclitaxel was administered at 15 mg/kg
intravenously once a week for 3 weeks. Trastuzumab at 10 mg/kg,
pertuzumab at 20 mg/kg, or HuIgG were administered
intraperitoneally once a week for 3 weeks. In a separate
experiment, trastuzumab was administered at 30 mg/kg. To evaluate
the antitumor activity and tolerability of the test agents, tumor
volume and body weight were measured twice a week. The tumor volume
(TV) was estimated from the equation V = ab2 / 2,
where a and b are tumor length and width, respectively. The
percentage of tumor growth inhibition (TGI%) was calculated as
follows: TGI% = [1 - (TV of treatment group on evaluation day - TV
of treatment group on Day 1) / (TV of control group at evaluation
day - TV of control group on Day 1)] × 100. Tumor growth rate was
calculated as follows: tumor growth rate = (TV on evaluation day) /
(tumor volume on Day 1).
Hematoxylin-eosin staining
Hematoxylin-eosin staining was used for assessment
of mitotic tumor cells and mononuclear cells. KPL-4 tumor xenograft
tissues were collected 4 days after the initiation of treatment.
The tissues were fixed with 10% neutral buffered formalin and
embedded in paraffin. Slide specimens were prepared by sectioning
the tissue and staining with hematoxylin-eosin stain. Then, the
number of mitotic tumor cells in every 1,000 cells was counted
under a microscope. Mononuclear cells infiltrating into tumor
tissues were scored as-or 0, no change; ± or 1, very slight; + or
2, slight; ++ or 3, moderate; or +++ or 4, marked.
Terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick end labeling (TUNEL) assay
Apoptotic cells were assessed by TUNEL assay. KPL-4
tumor xenograft tissues were collected 4 days after the initiation
of treatment. The tissues were fixed with 10% neutral buffered
formalin and embedded in paraffin. TUNEL assay was performed and
the number of apoptotic cells in every 1,000 tumor cells was
counted by Sapporo General Pathology Laboratory Co., Ltd. (Sapporo,
Japan).
Ki-67 staining
Proliferating cells were assessed with Ki-67
staining. KPL-4 tumor xenograft tissues were collected 4 days after
the initiation of treatment. The tissues were fixed with 10%
neutral buffered formalin and embedded in paraffin. Ki-67 staining
was performed and the number of Ki-67-positive cells in every 1,000
tumor cells was counted by Sapporo General Pathology Laboratory
Co., Ltd.
Western blotting
KPL-4 tumor xenograft tumors were collected 4 days
after the initiation of treatment and immediately frozen in liquid
nitrogen and stored at −80°C. Tumor samples were homogenized with
Cell Lysis Buffer (Cell Signaling Technology, Inc., Beverly, MA,
USA) including 10 mM NaF, 1 µg/ml aprotinin, and 1 mM
phenylmethylsulfonyl fluoride (PMSF). After centrifugation, the
resultant supernatant was used for the assays. The lysate was
separated on sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gel and transferred onto polyvinylidene
fluoride (PVDF) membrane. The membrane was primarily treated with
antibodies against p-EGFR, EGFR, p-HER2, HER2, p-HER3, p-ERK, ERK,
p-AKT, AKT (Cell Signaling Technology, Inc.), HER3 (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), and β-actin (Sigma-Aldrich
Co. LLC., St. Louis, MO, USA). These proteins were detected by
horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa
Cruz Biotechnology, Inc.). For HER3, HRP-conjugated anti-rabbit IgG
(Cell Signaling Technology, Inc.) was used as the secondary
antibody.
Flow cytometry analysis
To examine the cell cycle of KPL-4 cells in
vivo, KPL-4 tumor xenograft tumors were collected 4 days after
the initiation of treatment and dissociated with a Tumor
Dissociation kit, Human (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany). Then, tumor cells were isolated with a Mouse Cell
Depletion kit (Miltenyi Biotec GmbH). The cell cycle of the tumor
cells was examined by BD Cycletest Plus DNA Reagent kit (BD
Biosciences, San Jose, CA, USA). The DNA content in each cell
nucleus was determined by FACSVerse (Becton-Dickinson, Franklin
Lakes, NJ, USA), and the cell cycle was analyzed by using ModFit LT
Version 4 (Verity Software House, Topsham, ME, USA).
Statistical analysis
To analyze the data, Student's t-test was used. For
multiple comparisons, significance was determined by hierarchical
testing. Firstly, the statistical significance between the control
group and the triple-drug combination group (pertuzumab plus
trastuzumab plus docetaxel/paclitaxel) was analyzed; secondly, the
statistical significance between the docetaxel/paclitaxel group and
the triple-drug combination group were analyzed. Finally,
Bonferroni correction was applied to establish a threshold for
statistical significance between docetaxel/paclitaxel plus
trastuzumab group or docetaxel/paclitaxel plus pertuzumab group and
the triple-drug combination group. Statistical analyses were
conducted using JMP (SAS Institute Japan Ltd., Tokyo, Japan).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Establishment of the pertuzumab plus
trastuzumab plus docetaxel triple-drug combination treatment
model
To examine the internal changes in tumors treated
with pertuzumab plus trastuzumab plus docetaxel, we first
established a mouse xenograft model by using KPL-4, a HER2-positive
human breast cancer cell line, in which the treatment could show
sufficient efficacy. In this model, no significant (P<0.05)
anti-tumor effect was observed with docetaxel (10 mg/kg),
pertuzumab (20 mg/kg) or trastuzumab (10 mg/kg). However, the
triple-drug combination of pertuzumab plus trastuzumab plus
docetaxel showed a dramatically stronger antitumor activity
compared to either pertuzumab plus docetaxel or trastuzumab plus
docetaxel (Fig. 1A). Five out of six
mice receiving the triple-drug combination achieved a complete
tumor regression 21 days after the treatment started, whereas no
mice were cured in either of the double-drug combination groups.
The TV, TGI%, and incidence of tumor-free mice are summarized in
Table I. We also examined the
efficacy of pertuzumab plus trastuzumab plus paclitaxel as another
triple-drug combination. This combination also showed significantly
enhanced antitumor activity compared to either pertuzumab plus
paclitaxel or trastuzumab plus paclitaxel (Fig. 1B).
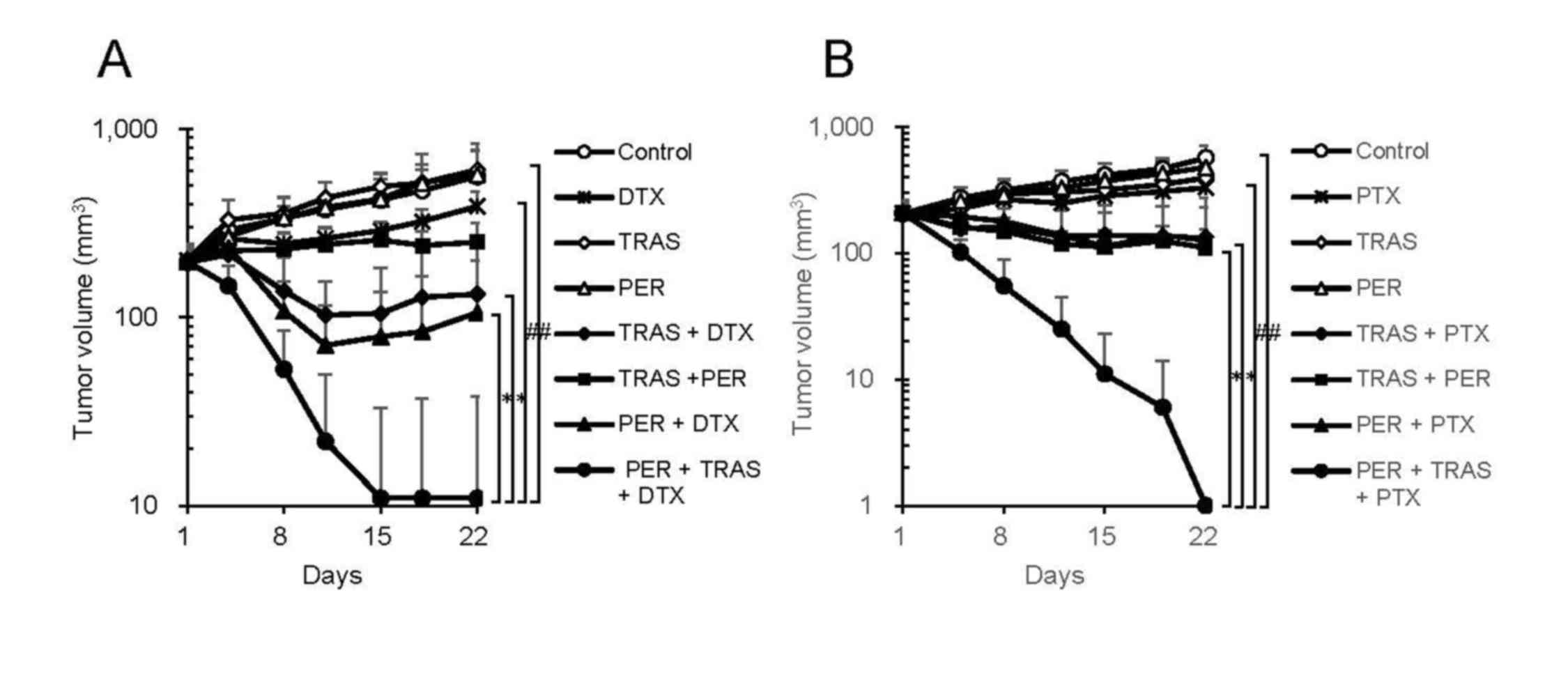 | Figure 1.In vivo efficacy of triple-drug
combination. (A) Mice bearing KPL-4 tumors were randomly divided
into eight groups (n=6 per group) and were treated with
trastuzumab, pertuzumab, docetaxel, trastuzumab + docetaxel,
trastuzumab + pertuzumab, pertuzumab + docetaxel, or trastuzumab +
docetaxel + pertuzumab. As a control, human IgG and vehicle of
docetaxel were administered. (B) Mice bearing KPL-4 tumors were
randomly divided into eight groups (n=6 per group) and
treated with trastuzumab, pertuzumab, paclitaxel, trastuzumab +
paclitaxel, trastuzumab + pertuzumab, pertuzumab + paclitaxel, or
trastuzumab + paclitaxel + pertuzumab. As a control, human IgG and
vehicle of paclitaxel were administered. Data points are mean +
standard deviation of the tumor volume (mm3).
Statistically significant differences are shown as
#P<0.05 and *P<0.025. TRAS, trastuzumab; PER,
pertuzumab; DTX, docetaxel; PTX, paclitaxel. |
 | Table I.Antitumor activity in the KPL-4
HER2-positive breast cancer xenograft model. |
Table I.
Antitumor activity in the KPL-4
HER2-positive breast cancer xenograft model.
|
| Tumor volume
(mm3) |
|
|
|---|
|
|
|
|
|
|---|
| Treatment | Day 1 | Day 22 | TGI% on Day 22 | Tumor-free mice on
Day 22 |
|---|
| Control | 196±24 | 560±206 | − | 0/6 |
| Trastuzumab | 202±42 | 606±168 | −11 | 0/6 |
| Pertuzumab | 197±30 | 574±262 | −3 | 0/6 |
| Docetaxel | 201±38 | 389±76 | 48 | 0/6 |
|
Trastuzumab+docetaxel | 196±28 | 133±102 | 117 | 0/6 |
|
Pertuzumab+docetaxel | 202±44 | 106±95 | 126 | 0/6 |
|
Trastuzumab+pertuzumab | 196±25 | 251±66 | 85 | 0/6 |
|
Pertuzumab+trastuzumab+docetaxel | 198±32 |
11±27 | 151 | 5/6 |
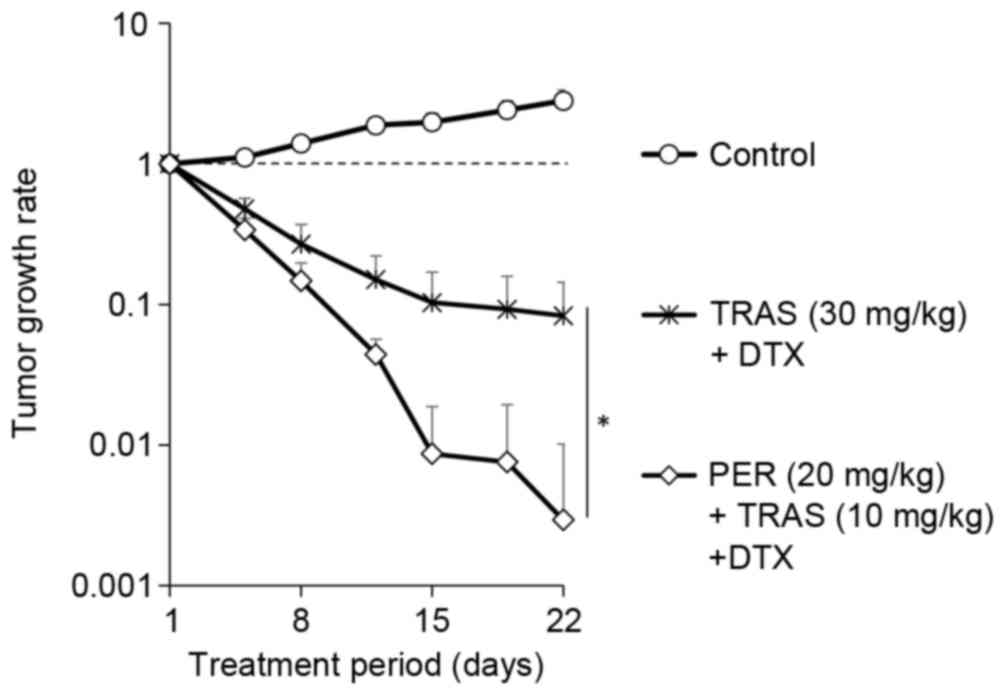
In order to eliminate the possibility that the
higher antitumor activity of the triple-drug combination was due to
the higher overall dosage of anti-HER2 antibodies, the tumor growth
rate under the combination of docetaxel plus trastuzumab was
compared with the tumor growth rate under the combination of
docetaxel plus pertuzumab plus trastuzumab, in which the total
dosage of anti-HER2 antibodies in each treatment was equivalent.
Tumor regression with the combination of 20 mg/kg pertuzumab plus
10 mg/kg trastuzumab plus docetaxel was significantly higher than
that with the combination of 30 mg/kg trastuzumab plus docetaxel
(Fig. 2).
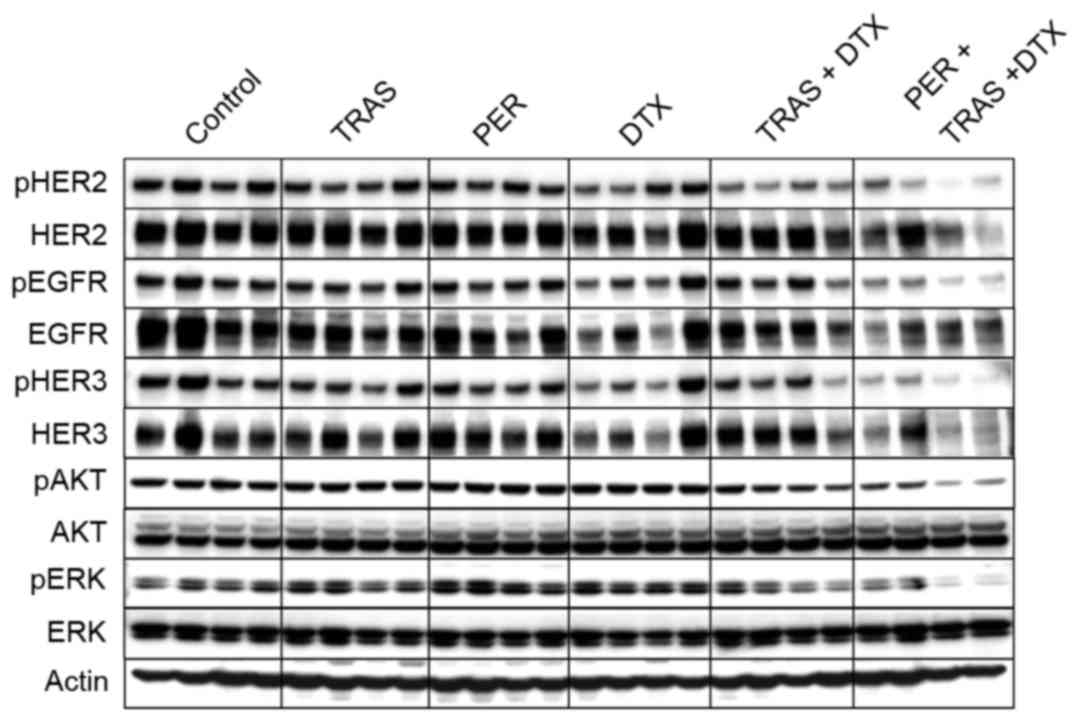
Inhibition of HER2 signaling in KPL-4
tumor tissue following treatment with the triple-drug
combination
Because trastuzumab and pertuzumab bind to different
domains of the HER2 molecule and suppress different aspects of HER2
signaling, i.e. ligand-independent and ligand-induced, trastuzumab
and pertuzumab used in combination is expected to inhibit HER2
signaling more effectively. Therefore, we examined HER2 signaling
after treatment. Trastuzumab, pertuzumab, or docetaxel alone
exhibited little effect on the HER2-related signal transduction. In
contrast with the weak suppression by single- or double-drug
treatments, the triple-drug combination strongly inhibited the
phosphorylation of HER2, EGFR, HER3, ERK, and AKT in tumor tissues
(Fig. 3).
Effect of triple-drug combination on
apoptosis of tumor cells in vivo
Evaluation of apoptotic cells was performed on tumor
tissues obtained 4 days after initiation of treatment. The
triple-drug combination significantly enhanced the number of
apoptotic cells as compared to the combination of docetaxel plus
trastuzumab or docetaxel plus pertuzumab (Fig. 4A and B). We also assessed the number
of tumor cells in the mitotic phase, because docetaxel was a
tubulin depolymerization inhibitor. As shown in Fig. 4C, combination of trastuzumab,
pertuzumab, or both of them did not increase the cell number of the
mitotic phase induced by docetaxel. These results indicated that
trastuzumab and pertuzumab enhanced the induction of apoptosis when
combined with docetaxel in spite of the increase of mitotic arrest.
In accordance with the apoptosis, proliferating cells assessed by
counting the Ki-67 positivity were significantly decreased by the
triple-drug combination as compared to the double-drug combinations
of docetaxel plus trastuzumab or docetaxel plus pertuzumab
(Fig. 4D).
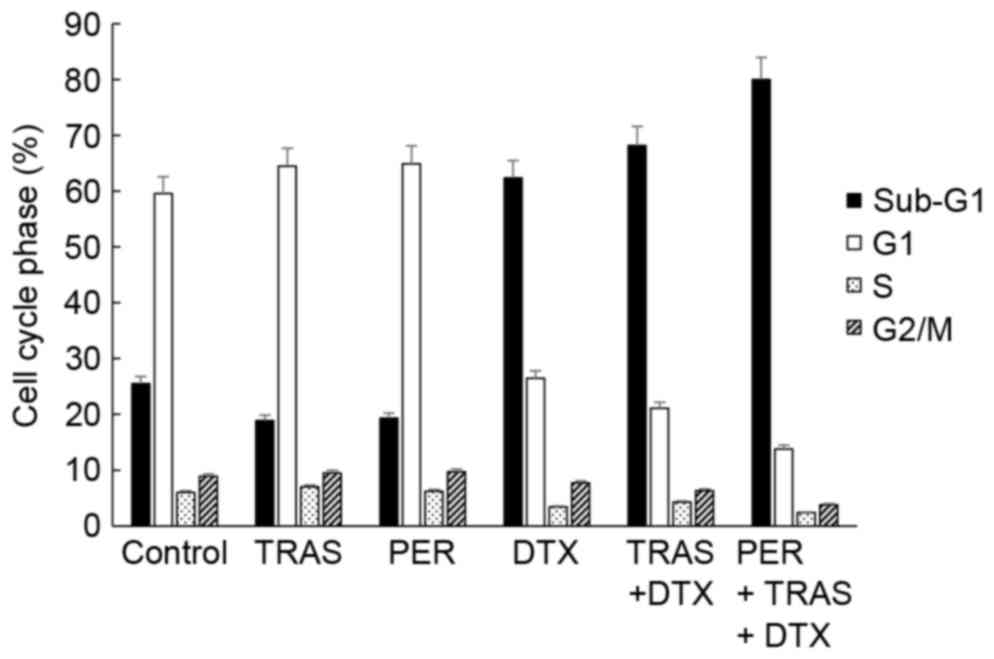
Cell cycle analysis of KPL-4 cells in
tumor tissues following treatment with the triple-drug
combination
The triple-drug combination might affect the cell
cycle in the tumors because it is well known that anti-HER2
antibodies induce G1 arrest and docetaxel induces M arrest in
cancer cells treated in vitro (19–22).
Therefore, we isolated cancer cells from xenografted tumors treated
with trastuzumab, pertuzumab, docetaxel, trastuzumab plus
docetaxel, or pertuzumab plus trastuzumab plus docetaxel and
analyzed the cell cycle distribution (Fig. 5). Trastuzumab or pertuzumab treatment
did not cause a substantial change in the percentage of G0/G1 phase
cells or other phase cells. Docetaxel treatment did not increase
the percentage of G2/M phase cells, but augmented the percentage of
sub-G1 phase cells. In agreement with the number of TUNEL-positive
cells (Fig. 4B), treatment with the
combination of docetaxel plus pertuzumab plus trastuzumab had a
tendency to increase in the percentage of sub-G1 phase cells
(74.2%) as compared to treatment with docetaxel alone or docetaxel
plus trastuzumab (62.2 and 60.9%, respectively). On the other hand,
addition of trastuzumab or pertuzumab plus trastuzumab to docetaxel
did not affect the G2/M phase population (Fig. 5).
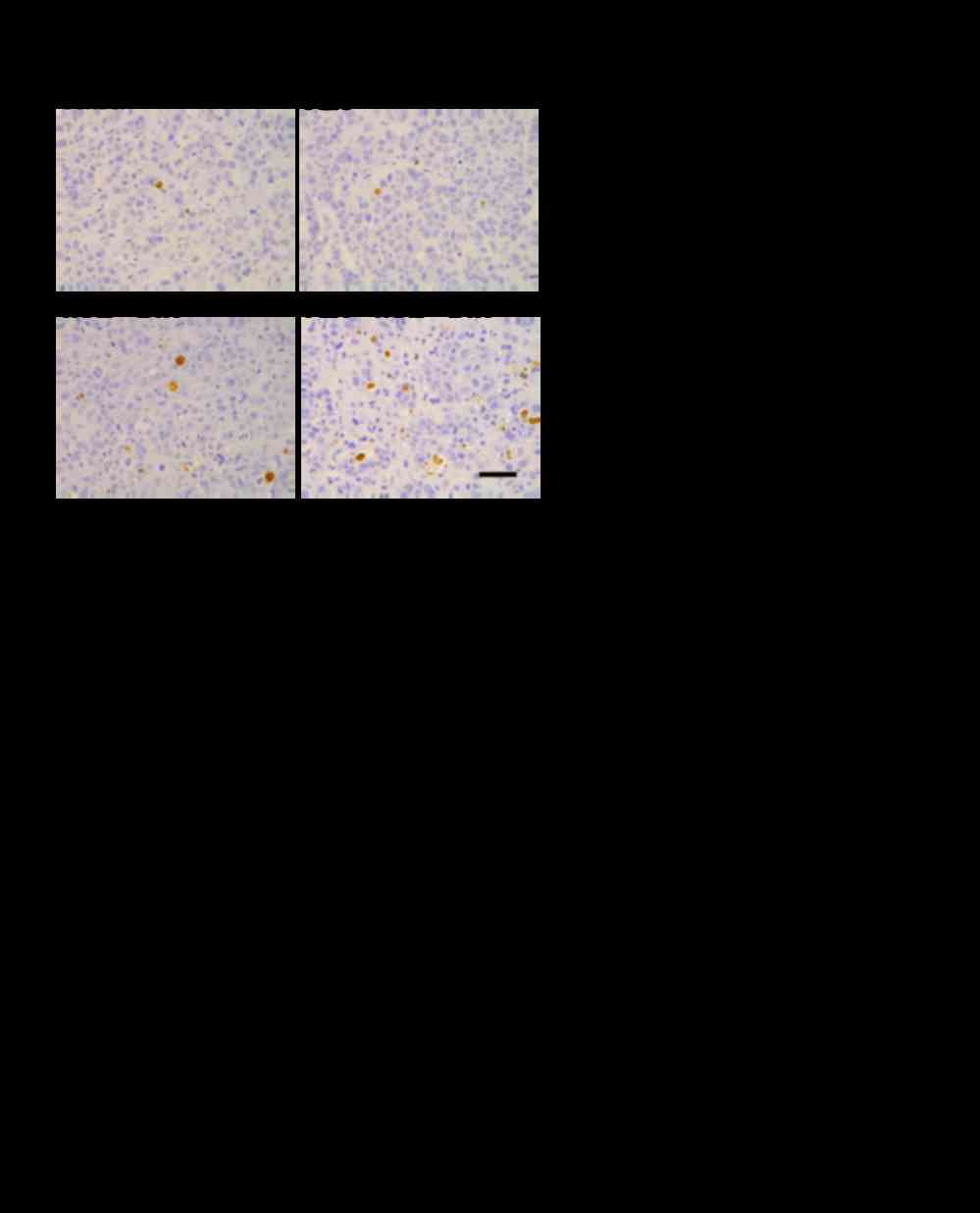
Invasion of mononuclear cells in tumor
tissues following treatment with the triple-drug combination of
pertuzumab plus trastuzumab plus docetaxel
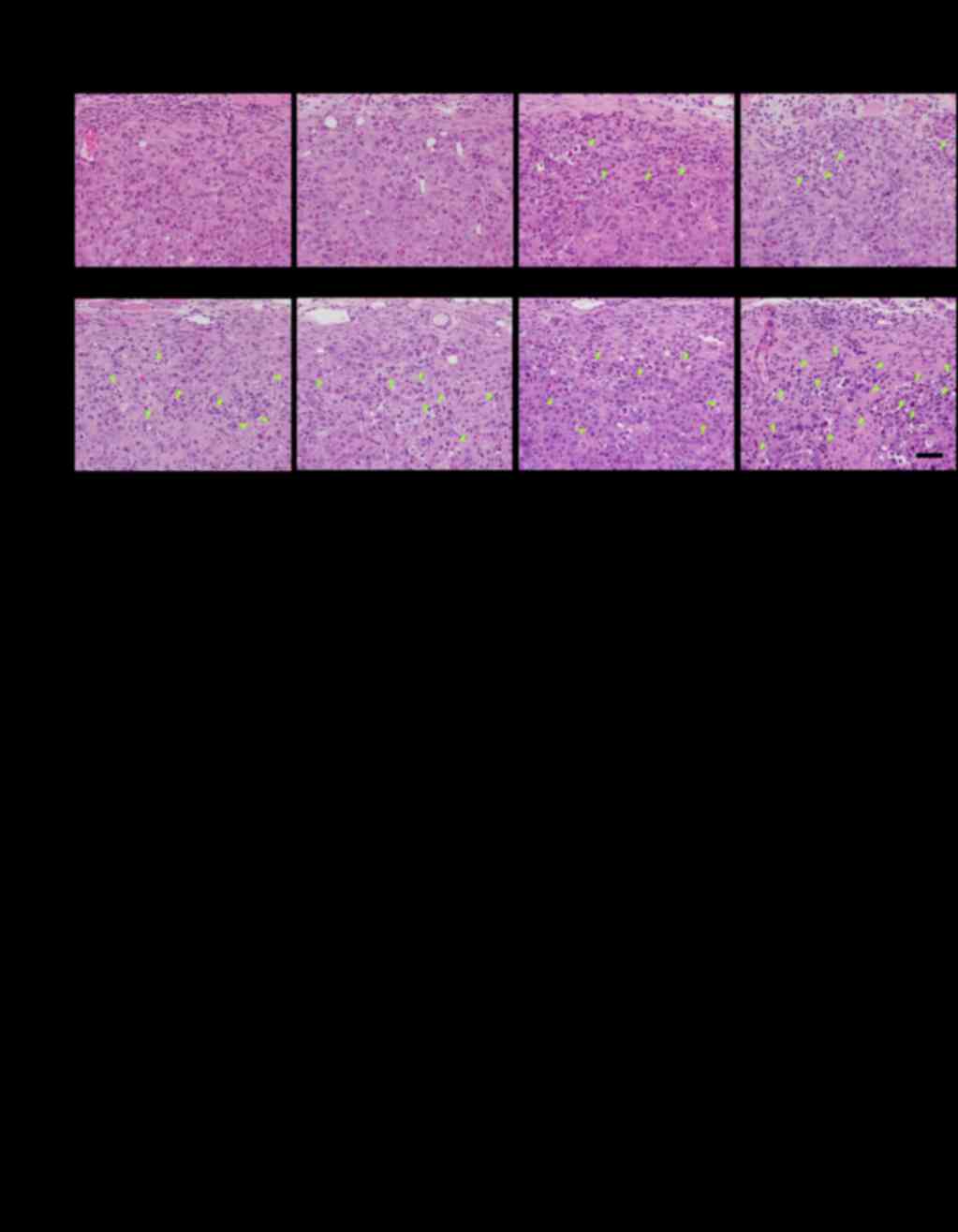
To examine innate immune responses, we checked the
differences in tumor-infiltrating mononuclear cells (MNCs) in
xenografted tumors in nude mice. The invasion of MNCs in the KPL-4
tumor tissues was analyzed 4 days after initiation of treatment
(Fig. 6A and B, Table II). Very slight infiltration of MNCs
was observed around the tumor cells from xenografted mice treated
with trastuzumab or pertuzumab alone, whereas no infiltration was
observed around the tumor cells from xenografted mice treated with
docetaxel or control. A significant increase in tumor-infiltrating
MNCs was observed in the trastuzumab plus pertuzumab group. The MNC
infiltration markedly increased with the combination treatment of
docetaxel plus trastuzumab plus pertuzumab compared to the
trastuzumab plus pertuzumab treatment (Fig. 6A and B). In accordance with the MNC
infiltration around the tumor cells, a remarkable increase in
single-cell necrosis or apoptosis of the tumor cells and
replacement of tumor cell area by connective tissues was observed
in the triple-drug combination group (Table II). The MNC infiltration induced by
the triple-drug combination was observed as early as the day after
the first treatment (Fig. 6B).
 | Table II.Histopathological analysis of the
tumor tissues 4 days after starting treatment. |
Table II.
Histopathological analysis of the
tumor tissues 4 days after starting treatment.
|
| Control group | TRAS group | PER group | DTX group |
|---|
|
|
|
|
|
|
|---|
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| MNC infiltration
around tumor cells | − | − | − | − | − | − | − | − | − | ± | ± | ± | − | − | − | − | ± | ± | − | − | − | − | − | − |
| Increase of single
cell necrosis/apoptosis of tumor cells | − | − | − | − | − | − | − | − | − | ± | ± | ± | − | − | − | − | ± | ± | ± | ± | ± | ± | ± | ± |
| Replacement of
tumor cell area by connective tissues | − | − | − | − | − | − | − | − | − | − | ± | ± | − | − | − | − | − | ± | − | − | − | − | − | − |
|
|
| TRAS + DTX
group | PER + DTX
group | TRAS + PER
group | PER + TRAS + DTX
group |
|
|
|
|
|
|
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|
| MNC infiltration
around tumor cells | ± | ± | ± | ± | + | + | − | ± | ± | ± | + | + | ± | ± | ± | ± | + | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Increase of single
cell necrosis/apoptosis of tumor cells | ± | + | + | + | + | + | ± | + | + | + | + | + | ± | ± | ± | ± | + | + | + | ++ | ++ | ++ | ++ | ++ | +++ |
| Replacement of
tumor cell area by connective tissues | ± | ± | ± | ± | ± | + | − | + | + | + | + | ++ | ± | ± | ± | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
Discussion
To elucidate the mechanism of action of pertuzumab
and trastuzumab in combination with docetaxel, in the present study
we established a mouse xenograft model in which the combination
treatments exhibited marked antitumor efficacy even though the
dosage of each drug was set to a dosage that had no or weak
efficacy on its own (Fig. 1A). The
efficacy of the triple-drug combination was significantly higher
than the double-drug combinations of trastuzumab plus docetaxel or
pertuzumab plus docetaxel (Fig. 1A).
Of note, complete tumor regression was observed in five out of six
mice during treatment with the triple-drug combination. Similar
results were obtained when paclitaxel was used as a combination
partner with pertuzumab and trastuzumab (Fig. 1B), indicating that similar combination
effects may be obtained with other chemotherapeutic agents besides
docetaxel as was reported in the clinical trial (23). In addition, by examining the effect of
docetaxel plus trastuzumab in comparison with the effect of
docetaxel plus pertuzumab plus trastuzumab when each combination
contained an equivalent total dosage of anti-HER2 antibodies, it
was shown that the remarkable antitumor effect of the triple-drug
combination was not merely due to the higher overall dosage of
anti-HER2 antibodies but was due to the synergistic biological
effects of pertuzumab, trastuzumab, and docetaxel (Fig. 2). Based on these findings, we used the
newly established xenograft model to investigate the mechanisms of
action of the triple-drug combination from the aspects of HER2
signaling inhibition, cell cycle distribution, and infiltration of
MNCs into tumor tissues.
Firstly, we analyzed signal transduction relevant to
the two antibodies. It was found that the combination of pertuzumab
plus trastuzumab plus docetaxel reduced phosphorylation of EGFR,
HER3 and their downstream factors AKT and ERK in the tumor tissues
more strongly than did either agent alone or the combination of
trastuzumab plus docetaxel. The combination of pertuzumab plus
trastuzumab has been shown to complementarily suppress HER3-AKT
signaling by inhibiting both ligand-induced and ligand-independent
HER2-HER3 complex formation (5). It
is also reported that suppression of the HER3-AKT pathway activates
caspase-3 and induces apoptosis in HER2-positive breast and gastric
cancer cell lines (5,24,25) and
that EGFR-ERK pathway inhibition induces G1-arrest (26). Consequently, in the present model,
apoptotic cells (Fig. 4B) were
increased and Ki-67 positive cells (Fig.
4D) were decreased by the triple-drug treatment. These results
suggest that the strong antitumor activity and pro-apoptotic
activity is at least in part due to enhanced inhibition of HER3-AKT
and EGFR-ERK signaling caused by the triple-drug treatment.
Secondly, we analyzed cell cycle distribution of the
tumor cells because enhancement of taxane-induced cell cycle arrest
could be another mechanism of action. The present study
demonstrated that docetaxel, a tubulin depolymerization inhibitor,
dramatically increased the number of cells in the sub-G1 phase 4
days after initiation of treatment, and that addition of pertuzumab
together with trastuzumab to docetaxel greatly increased numbers of
sub-G1 cells and TUNEL-positive cells as compared to numbers
following docetaxel or docetaxel plus trastuzumab treatment
(Figs. 4A and B, and 5). However, the numbers of cells in the
mitotic phase were unchanged between docetaxel and the triple-drug
combination groups, suggesting that the triple-drug combination
promotes the induction of apoptosis immediately after mitotic
arrest.
Thirdly, we analyzed infiltration of MNCs into the
tumor tissues because tumor-infiltrating lymphocytes (TILs) have
been proved to be a predictive therapeutic marker in early breast
cancers (27) as well as in
HER2-positive breast cancers (28).
Although higher levels of TILs have been shown to be associated
with greater trastuzumab benefit in the FinHER trial (29), changes in the number of TILs following
trastuzumab treatment as well as changes in the infiltration of
MNCs such as NK cells or macrophages into tumor tissues have not
yet been clearly analyzed. Here, we demonstrated for the first time
that trastuzumab plus pertuzumab enhanced MNC infiltration around
the tumor cells 4 days after initiation of treatment. Furthermore,
the triple-drug combination dramatically increased the MNC
infiltration compared with the trastuzumab plus pertuzumab
combination. Of note, the MNC infiltration following the
triple-drug combination was observed as early as the day after the
first treatment, before tumor growth inhibition had become
apparent. These results suggest that the enhanced recruitment of
MNCs into tumor tissues contributes to inhibition of tumor growth
in the triple-drug combination, possibly through ADCC (13,14).
Indeed, it has been shown that the number of KPL-4 cells killed by
NK cells in vitro depends on the amount of antibodies
(14). In addition, docetaxel has
been reported to increase serum IL-2 level and enhance NK cell
activity in patients with breast cancer (30). Thus, we consider that the triple-drug
combination of trastuzumab plus docetaxel plus pertuzumab could
cooperatively enhance ADCC activity and contribute to tumor
shrinkage in the KPL-4 xenografted mouse model. Although athymic
nude mice do retain NK cells, it has to be admitted that xenograft
models using nude mice as hosts are inadequate in regard to the
immune system. In order to investigate the mechanism of action of
the triple-drug combination more precisely in terms of immune
reactions, models appropriate for the evaluation, such as models
using humanized mice, should be utilized.
In conclusion, the synergistic efficacy of the
triple-drug combination of pertuzumab in combination with
trastuzumab and docetaxel against a HER2-positive breast cancer
model was considered to be produced by the integration of two
mechanisms: the inhibition of HER2 signaling pathways by anti-HER2
antibodies promoted the apoptosis evoked after docetaxel-induced
mitotic arrest; docetaxel enhanced the infiltration of tumor
tissues by mononuclear cells, and this increased infiltration may
have upregulated the antibody-dependent cellular cytotoxicity. This
is the first report to reveal the mechanism of action of the
superior antitumor effect of the triple-drug combination
therapy.
Acknowledgements
We greatly appreciate Hiromi Sawamura, Masako
Miyazaki, and Kumiko Kondo (Product Research department, Chugai)
for their excellent technical assistance; and Dr Kaori
Fujimoto-Ouchi, Dr Mieko Yanagisawa, and Dr Yasushi Yoshimura
(Product Research department, Chugai) for helpful discussion and
comments regarding the study.
References
|
1
|
Ravdin PM and Chamness GC: The c-erbB-2
proto-oncogene as a prognostic and predictive marker in breast
cancer: A paradigm for the development of other macromolecular
markers-a review. Gene. 159:19–27. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dawood S, Broglio K, Buzdar AU, Hortobagyi
GN and Giordano SH: Prognosis of women with metastatic breast
cancer by HER2 status and trastuzumab treatment: An
institutional-based review. J Clin Oncol. 28:92–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: Ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Junttila TT, Akita RW, Parsons K, Fields
C, Lewis Phillips GD, Friedman LS, Sampath D and Sliwkowski MX:
Ligand-independent HER2/HER3/PI3K complex is disrupted by
trastuzumab and is effectively inhibited by the PI3K inhibitor
GDC-0941. Cancer Cell. 15:429–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Molina MA, Codony-Servat J, Albanell J,
Rojo F, Arribas J and Baselga J: Trastuzumab (Herceptin), a
humanized anti-Her2 receptor monoclonal antibody, inhibits basal
and activated Her2 ectodomain cleavage in breast cancer cells.
Cancer Res. 61:4744–4749. 2001.PubMed/NCBI
|
|
7
|
Beano A, Signorino E, Evangelista A, Brusa
D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L and
Matera L: Correlation between NK function and response to
trastuzumab in metastatic breast cancer patients. J Transl Med.
6:252008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barok M, Isola J, Pályi-Krekk Z, Nagy P,
Juhász I, Vereb G, Kauraniemi P, Kapanen A, Tanner M, Vereb G and
Szöllösi J: Trastuzumab causes antibody-dependent cellular
cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1
breast cancer xenografts despite intrinsic drug resistance. Mol
Cancer Ther. 6:2065–2072. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marty M, Cognetti F, Maraninchi D, Snyder
R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A,
et al: Randomized phase II trial of the efficacy and safety of
trastuzumab combined with docetaxel in patients with human
epidermal growth factor receptor 2-positive metastatic breast
cancer administered as first-line treatment: The M77001 study
group. J Clin Oncol. 23:4265–4274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franklin MC, Carey KD, Vajdos FF, Leahy
DJ, de Vos AM and Sliwkowski MX: Insights into ErbB signaling from
the structure of the ErbB2-pertuzumab complex. Cancer Cell.
5:317–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agus DB, Akita RW, Fox WD, Lewis GD,
Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K,
et al: Targeting ligand-activated ErbB2 signaling inhibits breast
and prostate tumor growth. Cancer Cell. 2:127–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashita-Kashima Y, Iijima S, Yorozu K,
Furugaki K, Kurasawa M, Ohta M and Fujimoto-Ouchi K: Pertuzumab in
combination with trastuzumab shows significantly enhanced antitumor
activity in HER2-positive human gastric cancer xenograft models.
Clin Cancer Res. 17:5060–5070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scheuer W, Friess T, Burtscher H,
Bossenmaier B, Endl J and Hasmann M: Strongly enhanced antitumor
activity of trastuzumab and pertuzumab combination treatment on
HER2-positive human xenograft tumor models. Cancer Res.
69:9330–9336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baselga J, Cortés J, Kim SB, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast
cancer. N Engl J Med. 366:109–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Swain SM, Kim SB, Cortés J, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Knott A, et al: Pertuzumab, trastuzumab, and docetaxel for
HER2-positive metastatic breast cancer (CLEOPATRA study): Overall
survival results from a randomised, double-blind,
placebo-controlled, phase 3 study. Lancet Oncol. 14:461–471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurebayashi J, Otsuki T, Tang CK, Kurosumi
M, Yamamoto S, Tanaka K, Mochizuki M, Nakamura H and Sonoo H:
Isolation and characterization of a new human breast cancer cell
line, KPL-4, expressing the Erb B family receptors and
interleukin-6. Br J Cancer. 79:707–717. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kunisue H, Kurebayashi J, Otsuki T, Tang
CK, Kurosumi M, Yamamoto S, Tanaka K, Doihara H, Shimizu N and
Sonoo H: Anti-HER2 antibody enhances the growth inhibitory effect
of anti-oestrogen on breast cancer cells expressing both oestrogen
receptors and HER2. Br J Cancer. 82:46–51. 2000.PubMed/NCBI
|
|
19
|
Le XF, McWatters A, Wiener J, Wu JY, Mills
GB and Bast RC Jr: Anti-HER2 antibody and heregulin suppress growth
of HER2-overexpressing human breast cancer cells through different
mechanisms. Clin Cancer Res. 6:260–270. 2000.PubMed/NCBI
|
|
20
|
Lane HA, Beuvink I, Motoyama AB, Daly JM,
Neve RM and Hynes NE: ErbB2 potentiates breast tumor proliferation
through modulation of p27Kip1-Cdk2 complex formation: Receptor
overexpression does not determine growth dependency. Mol Cell Biol.
20:3210–3223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yakes FM, Chinratanalab W, Ritter CA, King
W, Seelig S and Arteaga CL: Herceptin-induced inhibition of
phosphatidylinositol-3 kinase and Akt is required for
antibody-mediated effects on p27, cyclin D1, and antitumor action.
Cancer Res. 62:4132–4141. 2002.PubMed/NCBI
|
|
22
|
Ringel I and Horwitz SB: Studies with RP
56976 (taxotere): A semisynthetic analogue of taxol. J Natl Cancer
Inst. 83:288–291. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dang C, Iyengar N, Datko F, D'Andrea G,
Theodoulou M, Dickler M, Goldfarb S, Lake D, Fasano J, Fornier M,
et al: Phase II study of paclitaxel given once per week along with
trastuzumab and pertuzumab in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer. J Clin Oncol.
33:442–447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piechocki MP, Yoo GH, Dibbley SK and
Lonardo F: Breast cancer expressing the activated HER2/neu is
sensitive to gefitinib in vitro and in vivo and acquires resistance
through a novel point mutation in the HER2/neu. Cancer Res.
67:6825–6843. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamashita-Kashima Y, Shu S, Harada N and
Fujimoto-Ouchi K: Enhanced antitumor activity of trastuzumab
emtansine (T-DM1) in combination with pertuzumab in a HER2-positive
gastric cancer model. Oncol Rep. 30:1087–1093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Y, Yu T, Fu X, Chen J, Liu Y, Li C,
Xia Y, Zhang Z and Li L: EGFR inhibition prevents in vitro tumor
growth of salivary adenoid cystic carcinoma. BMC Cell Biol.
14:132013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Melichar B, Študentova H, Kalábová H,
Vitásková D, Čermáková P, Hornychová H and Ryška A: Predictive and
prognostic significance of tumor-infiltrating lymphocytes in
patients with breast cancer treated with neoadjuvant systemic
therapy. Anticancer Res. 34:1115–1125. 2014.PubMed/NCBI
|
|
28
|
Zardavas D, Fouad TM and Piccart M:
Optimal adjuvant treatment for patients with HER2-positive breast
cancer in 2015. Breast. 24:(Suppl 2). S143–S148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loi S, Michiels S, Salgado R, Sirtaine N,
Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V,
Desmedt C, et al: Tumor infiltrating lymphocytes are prognostic in
triple negative breast cancer and predictive for trastuzumab
benefit in early breast cancer: Results from the FinHER trial. Ann
Oncol. 25:1544–1550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsavaris N, Kosmas C, Vadiaka M,
Kanelopoulos P and Boulamatsis D: Immune changes in patients with
advanced breast cancer undergoing chemotherapy with taxanes. Br J
Cancer. 87:21–27. 2002. View Article : Google Scholar : PubMed/NCBI
|