Introduction
Lung cancer is a major challenge to global public
health due to its high epidemiologic incidence (1,2). It is
estimated that ~1.8 million patients are diagnosed with lung cancer
annually, 80–85% of whom are diagnosed with non-small cell lung
cancer (NSCLC), including squamous cell carcinoma, adenocarcinoma
and large cell carcinoma (3–5).
Although there have been advances in treatment
strategies, the survival rate for lung cancer remains low (6) Surgery is the most effective option for
patients with lung cancer; however, in the majority of patients,
lung cancer is diagnosed at the advanced stage, and only 30% of
patients are eligible for curative resection (7) Therefore, chemotherapy and radiotherapy
are considered as alternative options for these patients. However,
modern chemotherapy using various antitumor drugs either
incompletely kills the malignant cells or causes fatal dysfunction
and serious systemic toxicity (8). In
addition, tumor drug resistance is a major problem associated with
chemotherapy and involves numerous factors, including anticancer
drug potency, tumor cell-drug reaction, the tumor microenvironment
and tumor cell heterogeneity (9).
Numerous apoptosis-inducing agents are also substrates and inducers
of drug transporters, including P-glycoprotein (P-gp), multidrug
resistance-associated protein 1 (MRP1) and breast cancer resistance
protein (BCRP) (10,11). These drug transporters recognize
numerous functionally and structurally independent anticancer drugs
and, therefore, can expel intracellular drugs efficiently. The
overexpression of these proteins can confer cancer cells with
multidrug resistance (12,13). The development of strategies to
overcome the side effects and drug resistance of antitumor drugs is
an ongoing challenge for medical workers. As a broad-spectrum
anticancer drug, Adriamycin (AD) is used in chemotherapy to treat a
variety of tumor types. However, in addition to bone marrow
suppression, myocardial injury and other side effects, drug
resistance is an important factor that limits its use (14). Furthermore, the efficacy of AD is
associated with drug concentration, which is also associated with
drug resistance. The inhibition of tumor cell efflux can
effectively increase the sensitivity of tumor cells to
chemotherapeutic drugs (15,13). Therefore, agents with high efficacy
and few side effects are urgently required in clinical
practice.
A number of previous studies have aimed to extract
and screen the active components of traditional Chinese herbs in
order to develop effective and relatively safe drugs for cancer
treatment (16,17). Shikonin (SHK) is the major active
ingredient isolated from the dried roots of Lithospermum
erythrorhizon, and possesses a molecular weight of 288 kDa.
The anticancer effects of SHK have been investigated
in numerous prior studies (18–24). SHK
is not toxic to normal cells (25),
but exerts cytotoxic effects against various neoplastic cells, and
has not been demonstrated to promote anticancer drug resistance
(26). However, its effects against
lung cancer remain unclear. The aim of the present study was to
investigate the effects of SHK on lung adenocarcinoma cells and the
underlying molecular mechanisms of these effects, which may provide
an understanding of its novel antitumor functions.
Materials and methods
Reagents
SHK was purchased from Shanghai Shifeng Biological
Technology Co., Ltd. (Shanghai, China). AD was purchased from
Zhejiang Hisun Chemical Co., Ltd. (Taizhou, China). MTT was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
JC-1 and propidium iodide (PI) assay kits were purchased from
Beyotime Institute of Biotechnology (Haimen, China), and the ATP
assay kit was obtained from Sigma-Aldrich (Merck KGaA). Monoclonal
antibodies against MRP-1 (cat. no. sc-13960) and β-actin were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
The anti-BCRP antibody (cat. no. MAB4155F) was obtained from EMD
Millipore (Billerica, MA, USA) and the anti-P-gp antibody (cat. no.
ab98322) was from Abcam (Cambridge, UK).
Cell lines and culture
The human A549 lung adenocarcinoma cancer cell line
was obtained from the Central Laboratory of the Central South
University (Changsha, China), and cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS) without
mycoplasma (Sigma-Aldrich; Merck KGaA), penicillin (100 µg/ml) and
streptomycin (100 µg/ml; North China Pharmacy Co., Ltd.,
Shijiazhuang, China) at 37°C in a humidified incubator with 5%
CO2. The culture solution was replaced every 2 days, and
cell morphology and vitality were monitored using an inverted
microscope (Olympus Corporation, Tokyo, Japan).
Cell viability assay
A549 cells were counted using a cell counting plate
prior to being seeded into 96-well tissue culture plates at a
density of 1×104 cells/well, and cultured at 37°C with
5% CO2 overnight. Subsequently, the cells were exposed
to various concentrations of SHK (0.8, 1.6, 3.2, 6.4, 12.8 or 25.6
µmol/l) or AD (0.375, 0.75, 1.5, 3, 6 or 12 mg/l) and then cultured
for 24 and 48 h, respectively control cells were incubated with
medium only. The cells were further incubated with PBS containing 5
mg/ml MTT for 4 h at 37°C, then the MTT solution was removed and
replaced with 150 µl dimethyl sulfoxide/well. Following this, the
absorbance of the reaction solution was measured using a plate
reader at 490 nm.
Colony formation assays
A549 cells were seeded in 6-well plates at a density
of 4×103 cells/well and treated with 1.6 µmol/l SHK,
0.75 mg/l AD or both (1.6 µmol/l SHK and 0.75 mg/l AD) for 24 h.
Following drug treatment, the cells were inoculated onto the 6-hole
culture plate. The number of cells was adjusted to 100 cells/hole.
The culture plate was placed in saturated humidity at 37°C and 5%
CO2 environment for 7 days. Following this, medium was
removed and the plates were rinsed with PBS 2 times. Methanol was
used to fix the plates for 15 min and air dried once the methanol
was discarded. A total of 200 ml/pore 0.5% crystal violet stain was
added, and after 20 min at room temperature, the plates were rinsed
under water to remove the dye solution. The colonies were
visualized under an inverted microscope (Olympus Corporation,
Tokyo, Japan), violet and the cell number was counted in 10 fields
of view.
PI staining
A549 cells were cultured in 6-well plates
(2×106 cells/well) for 24 h and allowed to attain
exponential growth. Then the cells were treated with 1.6 µmol/l
SHK, 0.75 mg/l AD, or both for 24 h, stained with 600 µl PI/well
for 2 h and then evaluated using flow cytometry (Accuri C6: US; BD
Biosciences, Franklin Lakes, NJ, USA). SPSS 19.0 (IBM Corp.,
Arkmonk, NY, USA) was used to analyse the results of the
results.
Intracellular ATP measurement
A549 cells were seeded at 2×105
cells/well in a culture plate for 24 h. Cellular ATP levels were
determined using the CellTiter-Glo® Luminescent Cell
Viability Assay kit (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol. The luminescence levels
were measured using the luminometer mode on a microplate
reader.
Mitochondrial membrane potential
A549 cells were cultured in 12-well plates at a
density of 2×105 cells/well for 24 h and allowed to
attain exponential growth prior to treatment. Changes in the
mitochondrial membrane potential were evaluated using the JC-1
Mitochondrial Membrane Potential Fluorescence Probe kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) according to the
manufacturer's protocol. The cells stained with JC-1 solution were
visualized using an inverted fluorescence microscope (IX-71:
Olympus Corporation).
Live cell imaging
A549 cells were cultured in 6-well plates
(2×105 cells/well) for 24 h to allow attainment of
exponential growth. Then, the cells were treated with 1.6 µmol/l
SHK followed by 0.75 mg/l AD for 1 h each. The drug-induced
fluorescence in the cells was visualized using a live cell imaging
system (Olympus Corporation Live Cell Imaging Workstation
IX83).
Western blot analysis
A549 cells were rinsed with ice-cold PBS and
lysed with radioimmunoassay precipitation buffer (Shanghai Fankang
Biotechnology Co., Ltd., Shanghai, China) for 30 min on ice. The
cell lysates were centrifuged at 12,000 × g for 30 min at 4°C. The
supernatant proteins were separated using 6% SDS-PAGE and
subsequently transferred to PVDF membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membranes were blocked with 5%
skimmed milk + TBST for 1 h at room temperature, and then washed
with TBST 3 times. Membranes were then incubated with primary
antibodies overnight at 4°C, followed by the horseradish
peroxidase-labeled rabbit anti-mouse IgG (cat. no. PA128568;
Invitrogen; Thermo Fisher Scientific, Inc.), while β-actin was used
as the loading control. TBST was used to rinse the polyvinylidene
fluoride (PVDF) membranes 3 times for 10 min each time. The PVDF
membranes were dipped in methanol for l0 min, and the membranes
were allowed to develop in the dark. Following this, the membranes
were analyzed using an BIO-RAD gel imaging system (Bio-Rad
Technologies, Inc., Hercules, CA, USA). A gel band image analysis
system was used to analyze the electrophoresis band density with
Quantity One version 4.6.6 software (Bio-Rad Technologies, Inc.),
and the ratio of target protein band density and internal reference
band optical density was used as the relative expression for each
group of proteins.
Statistical analysis
All statistical analyses were performed using an
unpaired Student's t-test or an analysis of variance followed by
the Student-Newman-Keuls test in SPSS 19.0 (IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
SHK and AD induce cytotoxicity in A549
cells
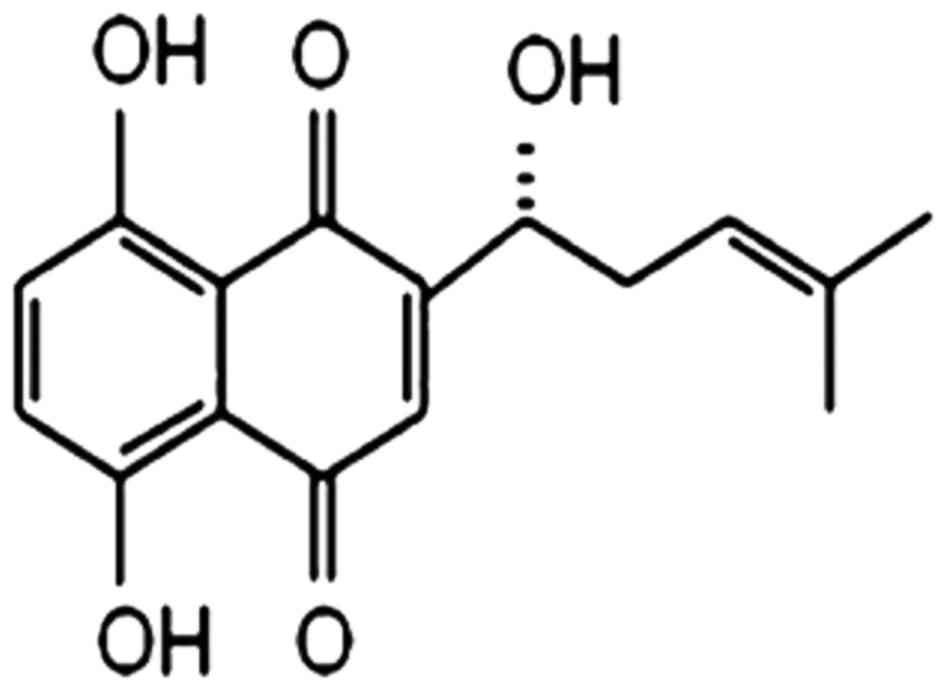
The chemical structure of SHK is illustrated in
Fig. 1. To investigate the
cytotoxicity of SHK and AD, A549 cells were treated with various
concentrations of the agents for 24 and 48 h, respectively. The MTT
assay results revealed that SHK and AD each significantly decreased
the viability of A549 cells in a dose-dependent manner
compared with the control group (Fig. 2A
and B).
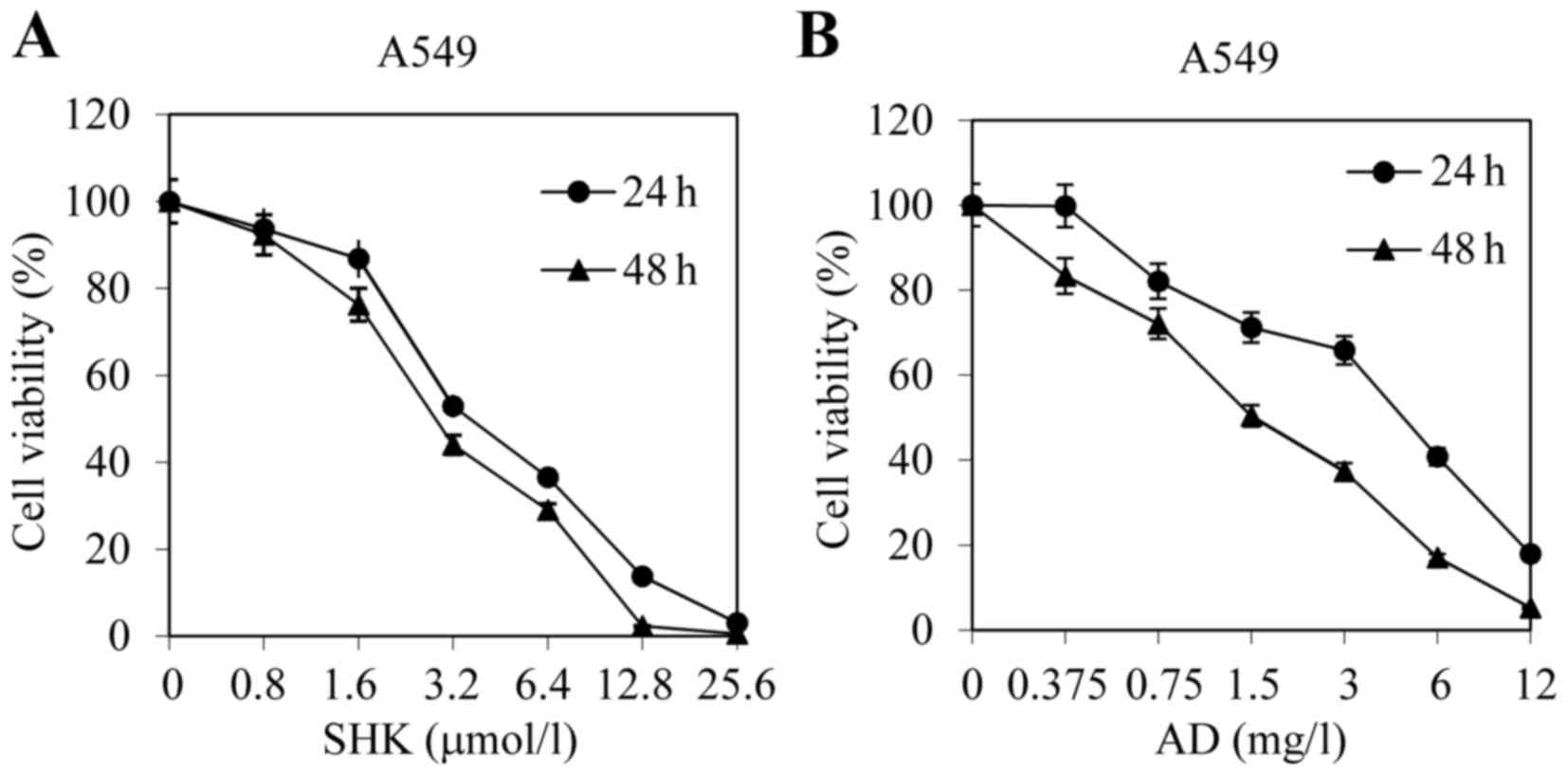 | Figure 2.SHK and AD exhibit cytotoxic effects
in A549 cells. A549 cells were treated with (A) SHK (0.8, 1.6, 3.2,
6.4,12.8 or 25.6 µmol/l) or (B) AD (0.375, 0.75, 1.5, 3, 6 or 12
mg/l) for 24 and 48 h, respectively. Cell viability was analyzed
using an MTT assay. Data are presented as the mean ± standard error
of the mean of 4 independent experiments. SHK, shikonin; AD,
Adriamycin. |
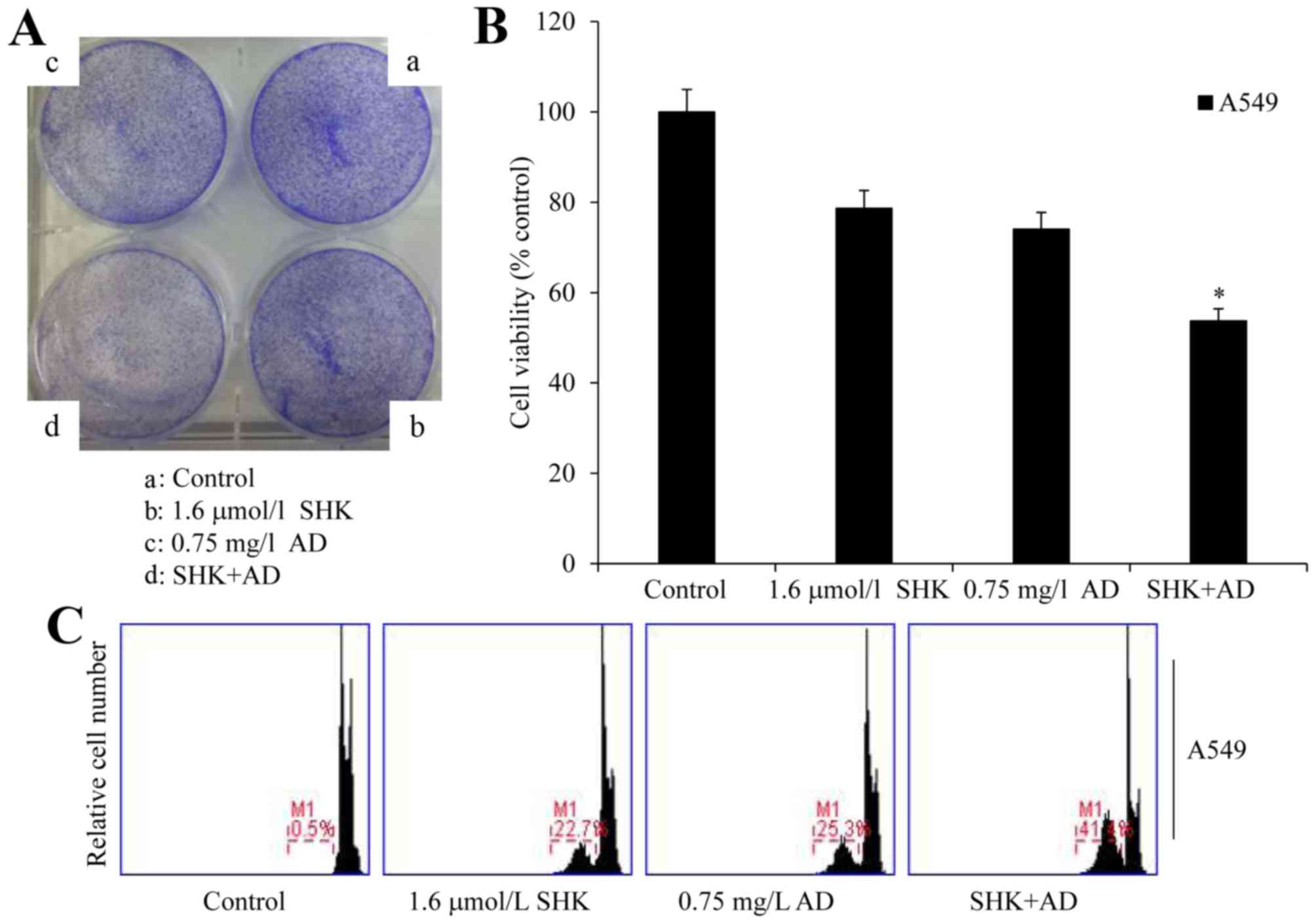
SHK sensitizes A549 cells to
AD-induced cell growth inhibition and apoptosis
To determine whether SHK sensitized A549
cells to chemotherapeutic agents, cells were co-treated with AD and
SHK. Colony formation, MTT and PI staining assays demonstrated that
SHK administered in combination with AD significantly decreased
cell viability when compared with the control group and with the
cells treated with SHK or AD alone (P<0.01), and also potently
induced the apoptosis of A549 cells (Fig.
3A-C). The survival rate of the cells treated with SHK and AD
was 53.77%, which was statistically different from that of the
single treatment groups, at 78.68% for SHK and 74.07% for AD
(P<0.01). These results suggest that SHK sensitizes A549 cells
to AD-induced cell growth inhibition and apoptosis.
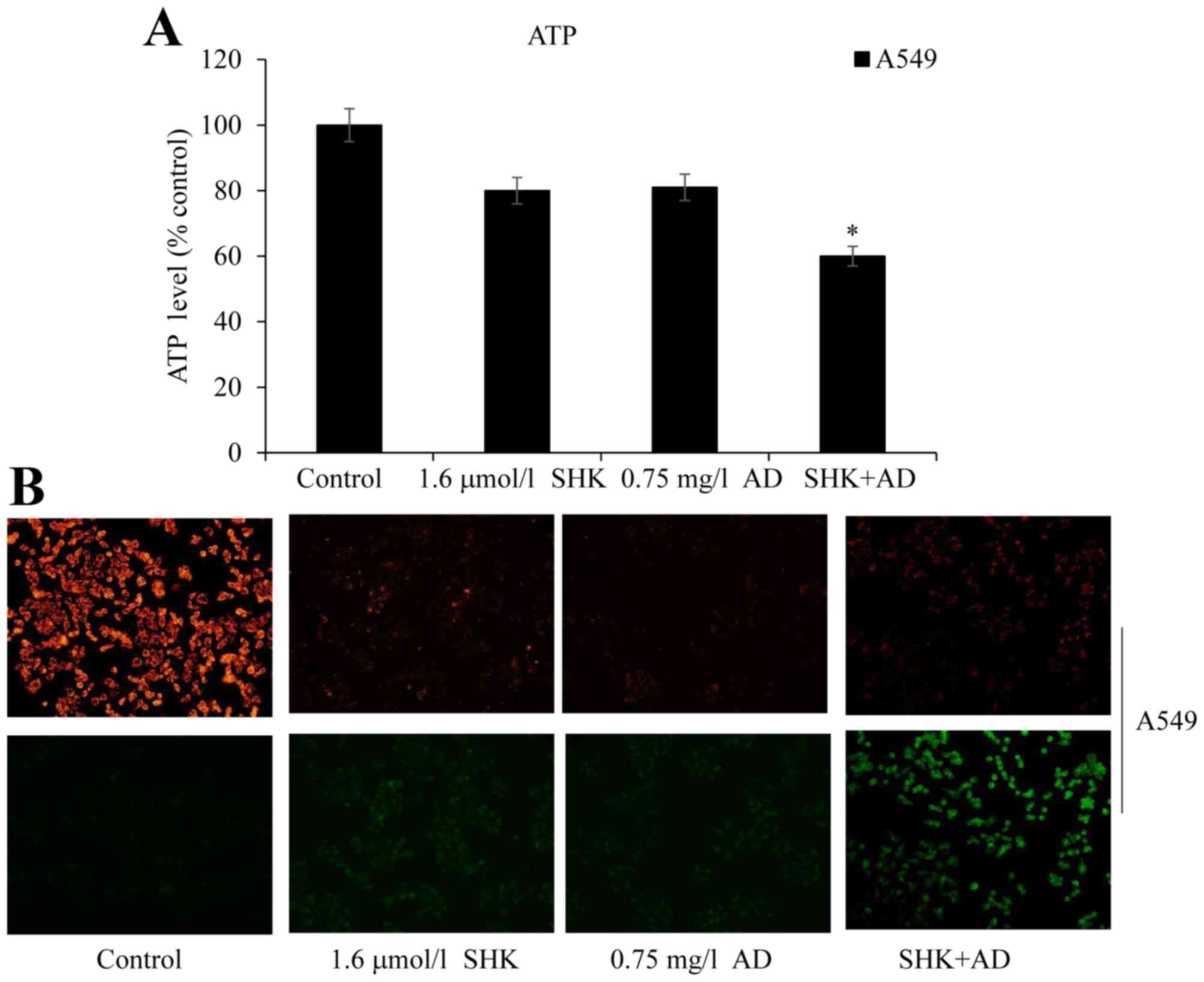
SHK damages the mitochondrial membrane
potential and inhibits ATP generation in A549 cells
Mitochondrial membrane potential loss and the
consequent production of reactive oxygen species (ROS) are the
common landmark events of early apoptosis (27). ROS interact with mitochondrial
antioxidants and induce apoptosis by releasing cytochrome c from
the mitochondria. Furthermore, ROS inhibit the production of ATP,
which in turn further increases apoptosis and ROS generation
(28,27). Treatment of A549 cells with SHK or AD
alone did not significantly decrease ATP levels compared with the
control group; however, co-treatment with SHK and AD significantly
decreased ATP levels in the A549 cells when compared with the
control (P<0.05; Fig. 4A). To
further assess changes in mitochondrial membrane potential, JC-1
was used as a fluorescent marker. When the mitochondrial membrane
potential is intact the cells fluoresce red, whereas cell
dysfunction induces a green fluorescence (29). SHK-treated and SHK/AD co-treated A549
cells exhibited clear mitochondrial membrane potential damage with
green fluorescence following JC-1 staining; the co-treated cells
exhibited a bright green fluorescence and the control cells
exhibited a bright red fluorescence (Fig.
4B). Taken together, these results suggest that SHK adversely
affects the mitochondrial membrane potential and decreases ATP
generation in A549 cells.
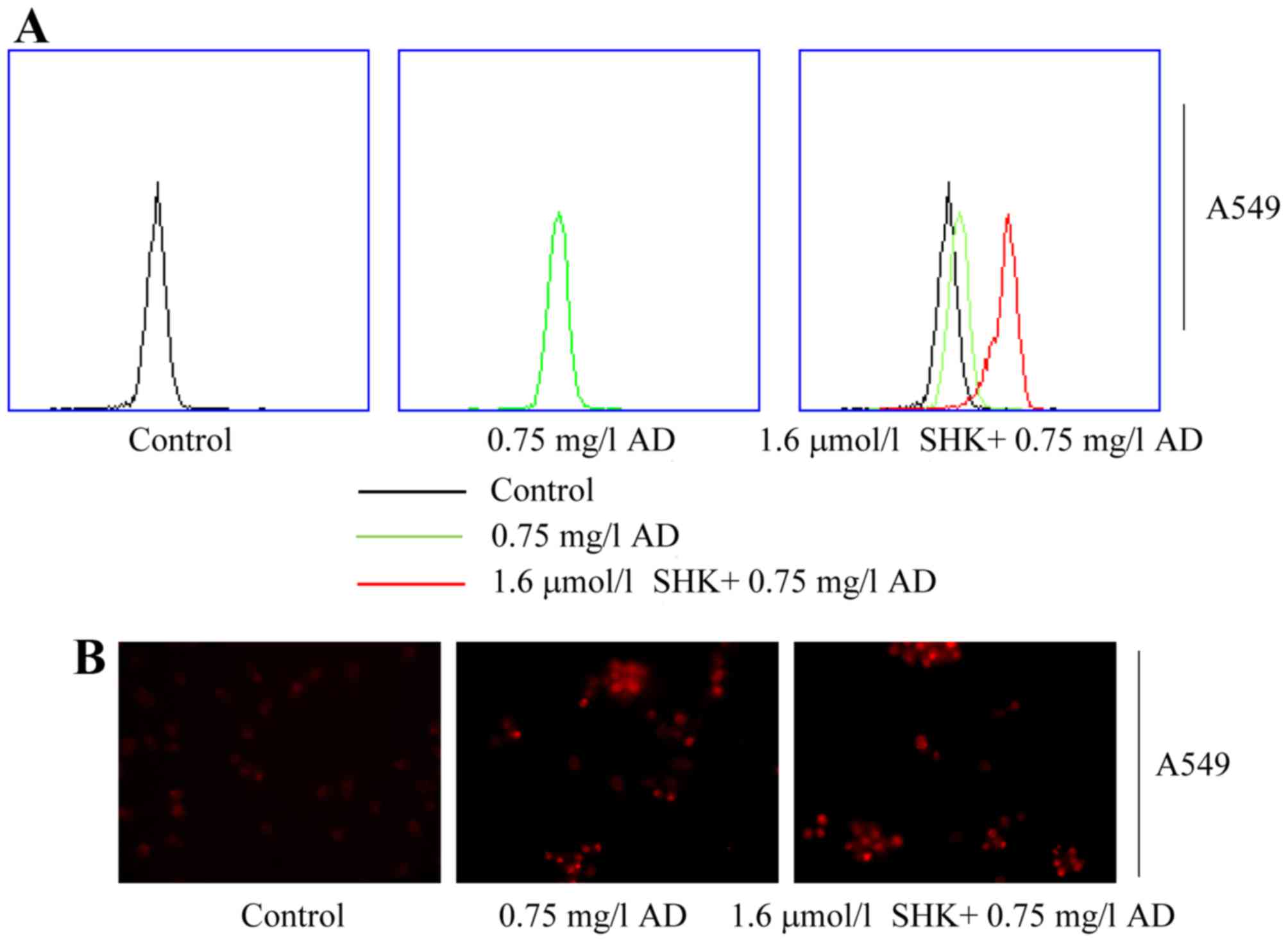
SHK enhances AD accumulation and
reduces efflux in A549 cells
The anticancer activity of SHK, as well as
inhibition of the cellular efflux of AD, requires depletion of the
cellular ATP pool as the ATP-binding cassette (ABC) transporters
are ATP-dependent (30). To assess
whether SHK enhanced AD accumulation through reduced ATP
production, the effects of glycolysis inhibition on AD accumulation
and efflux were evaluated in A549 cells. Flow cytometry analysis
revealed that SHK markedly increased intracellular AD levels in
A549 cells (Fig. 5A); a similar
result was obtained using a live imaging system (Fig. 5B). These results collectively suggest
that SHK enhances AD accumulation and reduces efflux partly by
reducing ATP levels in A549 cells.
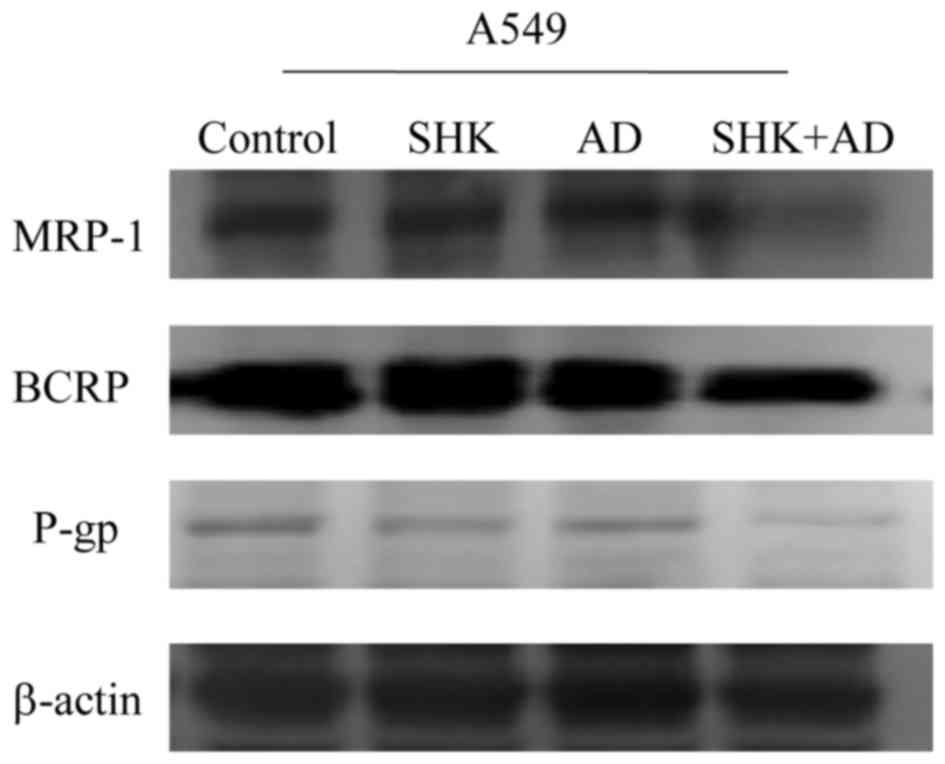
SHK enhances the antitumor effects of
AD by inhibiting ABC transporter expression
To confirm the effect of ATP reduction in A549
cells, the expression of MRP1, BCRP and P-gp was analyzed in A549
cells. Co-treatment with SHK and AD resulted in a marked decrease
in MRP1, BCRP and P-gp expression when compared with the control
and either treatment administered alone (Fig. 6).
Discussion
Multi-drug resistance (MDR) constitutes a unique and
critical spectrum of drug resistance (21,31) with
serious therapeutic consequences. MDR development is typically
associated with the development of a series of
structurally-associated compounds for cancer treatment, leading to
the development of structural and functional cross-resistance
(31). Despite the continuous
introduction of novel chemotherapeutic agents, overcoming MDR
remains a challenge in cancer chemotherapy (32,33).
Targeting cancer cell metabolism for cancer prevention and therapy
is an emerging topic of research; compared with the majority of
normal differentiated cells, cancer cells possess distinct
metabolic requirements, including the production of energy
primarily via glycolysis even in the presence of oxygen (34,35). This
phenomenon is known as the ‘Warburg effect’ and has received
increasing attention since 2011 (36). Tumor cell metabolism results in the
glycolysis-dependent production of cellular ATP due to
mitochondrial dysfunction, hypoxia, tumor cell signal transduction
or metabolic enzyme expression (37).
ABC transporters mediate cytotoxic drug active
efflux and, following repeated chemotherapy cycles, cancer cells
can develop MDR (38). Tumor cells
proliferate rapidly and require numerous proteins, nucleic acids,
lipids and ATP for their survival (39). Cancer cells are more dependent on the
glycolytic pathway for ATP generation, compared with normal cells,
and they eventually acquire drug resistance, typically due to the
aberrant expression of drug-expelling ABC transporters (12). The ATP-dependence of drug transporters
for activity (40,41) suggests that glycolysis inhibition may
increase the concentration of chemotherapeutic agents in cancer
cells (42,43). The most widely studied transporters,
including MRP1, BCRP and P-gp, are able to transport a variety of
structurally-unassociated chemotherapeutic compounds from cancer
cells, thereby inducing MDR (44).
Previous studies have suggested that certain
chemicals isolated from Chinese medicinal herbs may exhibit
antitumor activity by inducing the apoptosis of cancer cells
(16,17,45).
Results from the present study demonstrated that SHK exerts
antitumor effects by decreasing cell viability, inducing cell
apoptosis and inhibiting ATP generation in A549 cells. The
half-maximal inhibitory concentration of SHK in A549 cells, as
determined using an MTT assay, has previously been determined to be
3.52±0.17 µg/ml (46). To confirm the
effect of ATP reduction in A549 cells, the expression of MRP1, P-gp
and BCRP was analyzed in the present study following combination
treatment with SHK and AD. The results indicated that combination
treatment with SHK and AD markedly decreased the expression of
MRP1, P-gp and BCRP. Furthermore, SHK efficiently enhanced the
cytotoxicity of AD against A549 cells by decreasing the levels of
ATP, potentially leading to a decrease in activity of the
ATP-dependent efflux pumps. The data from the present study suggest
that SHK enhances the antitumor effect of AD through inhibiting ATP
generation in A549 cells. These results indicate that the
inhibition of glycolysis may be an effective therapeutic approach
for the treatment of lung cancer.
In conclusion, the results of the present study
collectively suggest that SHK may be a novel and attractive
therapeutic candidate for tumor treatment in clinical practice.
However, further studies are required in order to identify the
precise molecular mechanisms underlying the effects of SHK in A549
cells.
Acknowledgements
The present study was funded by the National Key
Clinical Specialist Construction Programs of China (grant no.
3101005005025).
References
|
1
|
Alberg AJ, Ford JG and Samet JM: American
College of Chest Physicians: Epidemiology of lung cancer: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132:(3 Suppl). 29S–55S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baltayiannis N, Chandrinos M,
Anagnostopoulos D, Zarogoulidis P, Tsakiridis K, Mpakas A,
Machairiotis N, Katsikogiannis N, Kougioumtzi I, Courcoutsakis N
and Zarogoulidis K: Lung cancer surgery: An up to date. J Thorac
Dis. 5:(Suppl 4). S425–S439. 2013.PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wingo PA, Cardinez CJ, Landis SH, Greenlee
RT, Ries LA, Anderson RN and Thun MJ: Long-term trends in cancer
mortality in the United States, 1930–1998. Cancer. 97:(12 Suppl).
S3133–S3275. 2003. View Article : Google Scholar
|
|
5
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davies J, Patel M, Gridelli C, de Marinis
F, Waterkamp D and McCusker ME: Real-world treatment patterns for
patients receiving second-line and third-line treatment for
advanced non-small cell lung cancer: A systematic review of
recently published studies. PLoS One. 12:e01756792017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon SM, Shaikh T and Hallman M:
Therapeutic management options for stage III non-small cell lung
cancer. World J Clin Oncol. 8:1–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galvani E, Peters GJ and Giovannetti E:
EGF receptor-targeted therapy in non-small-cell lung cancer: Role
of germline polymorphisms in outcome and toxicity. Future Oncol.
8:1015–1029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bach DH, Hong JY, Park HJ and Lee SK: The
role of exosomes and miRNAs in drug-resistance of cancer cells. Int
J Cancer. 141:220–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borst P, Evers R, Kool M and Wijnholds J:
A family of drug transporters: The multidrug resistance-associated
proteins. J Natl Cancer Inst. 92:1295–1302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allen JD and Schinkel AH: Multidrug
resistance and pharmacological protection mediated by the breast
cancer resistance protein (BCRP/ABCG2). Mol Cancer Ther. 1:427–434.
2002.PubMed/NCBI
|
|
12
|
Oshikata A, Matsushita T and Ueoka R:
Enhancement of drug efflux activity via MDR1 protein by spheroid
culture of human hepatic cancer cells. J Biosci Bioeng.
111:590–593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joyce H, McCann A, Clynes M and Larkin A:
Influence of multidrug resistance and drug transport proteins on
chemotherapy drug metabolism. Exp Opin Drug Metab Toxicol.
11:795–809. 2015. View Article : Google Scholar
|
|
14
|
El-Sheikh AA, Morsy MA, Mahmoud MM and
Rifaai RA: Protective mechanisms of coenzyme-Q10 may involve
up-regulation of testicular P-glycoprotein in doxorubicin-induced
toxicity. Environ Toxicol Pharmacol. 37:772–781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponnusamy L, Mahalingaiah PKS and Singh
KP: Treatment schedule and estrogen receptor-status influence
acquisition of doxorubicin resistance in breast cancer cells. Eur J
Pharm Sci. 104:424–433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang CY, Bai XY and Wang CH: Traditional
chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Chen S, Cai J, Zhang E, Lan L,
Zheng J, Liao L, Yang X, Zhou C and Du J: Traditional Chinese
medicine syndrome-related herbal prescriptions in treatment of
malignant tumors. J Tradit Chin Med. 33:19–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y, Luo
J and Hu X: Shikonin circumvents cancer drug resistance by
induction of a necroptotic death. Mol Cancer Ther. 6:1641–1649.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang H, Zhou P, Huang H, Chen D, Ma N, Cui
QC, Shen S, Dong W, Zhang X, Lian W, et al: Shikonin exerts
antitumor activity via proteasome inhibition and cell death
induction in vitro and in vivo. Int J Cancer. 124:2450–2459. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xuan Y and Hu X: Naturally-occurring
shikonin analogues-a class of necroptotic inducers that circumvent
cancer drug resistance. Cancer Lett. 274:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bailly C: Topoisomerase I poisons and
suppressors as anticancer drugs. Curr Med Chem. 7:39–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakaya K and Miyasaka T: A shikonin
derivative, beta-hydroxyisovalerylshikonin, is an
ATP-non-competitive inhibitor of protein tyrosine kinases.
Anticancer Drugs. 14:683–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SH, Kang IC, Yoon TJ, Park YM, Kang
KS, Song GY and Ahn BZ: Antitumor activities of a newly synthesized
shikonin derivative, 2-hyim-DMNQ-S-33. Cancer Lett. 172:171–175.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang IC, Huang YJ, Chiang TI, Yeh CW and
Hsu LS: Shikonin induces apoptosis through reactive oxygen
species/extracellular signal-regulated kinase pathway in
osteosarcoma cells. Biol Pharm Bull. 33:816–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long S, GuangZhi Y, BaoJie G, Wei X,
YanYong H, YingLi W, Yang Z and LiHua L: Shikonin derivatives
protect immune organs from damage and promote immune responses in
vivo in tumour-bearing mice. Phytother Res. 26:26–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu H, Xie J, Pan Q, Wang B, Hu D and Hu X:
Anticancer agent shikonin is an incompetent inducer of cancer drug
resistance. PLoS One. 8:e527062013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
EI Sayed SM, Mahmoud AA, EI Sawy SA,
Abdelaal EA, Fouad AM, Yousif RS, Hashim MS, Hemdan SB, Kadry ZM,
Abdelmoaty MA, et al: Warburg effect increases steady-state ROS
condition in cancer cells through decreasing their antioxidant
capacities (anticancer effects of 3-bromopyruvate through
antagonizing Warburg effect). Med Hypotheses. 81:866–870. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carraro M and Bernardi P: Calcium and
reactive oxygen species in regulation of the mitochondrial
permeability transition and of programmed cell death in yeast. Cell
Calcium. 60:102–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Padmapriya R, Gayathri L, Ronsard L,
Akbarsha MA and Raveendran R: In vitro anti-proliferative effect of
tephrosia purpurea on human hepatocellular carcinoma cells.
Pharmacogn Mag. 13:(Suppl 1). S16–S21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Falasca M and Linton KJ: Investigational
ABC transporter inhibitors. Expert Opin Invest Drugs. 21:657–666.
2012. View Article : Google Scholar
|
|
31
|
Joyce H, McCann A, Clynes M and Larkin A:
Influence of multidrug resistance and drug transport proteins on
chemotherapy drug metabolism. Expert Opin Drug Metab Toxicol.
11:795–809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kathawala RJ, Gupta P, Ashby CR Jr and
Chen ZS: The modulation of ABC transporter-mediated multidrug
resistance in cancer: A review of the past decade. Drug Resist
Updat. 18:1–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pavan B, Paganetto G, Rossi D and Dalpiaz
A: Multidrug resistance in cancer or inefficacy of neuroactive
agents: Innovative strategies to inhibit or circumvent the active
efflux transporters selectively. Drug Discov Today. 19:1563–1571.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ponisovskiy MR: Warburg effect mechanism
as the target for theoretical substantiation of a new potential
cancer treatment. Crit Rev Eukaryot Gene Expr. 21:13–28. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bayley JP and Devilee P: The Warburg
effect in 2012. Curr Opin Oncol. 24:62–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg's contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sotgia F, Martinez-Outschoorn UE and
Lisanti MP: Genetic induction of the Warburg effect inhibits tumor
growth. Oncotarget. 3:1266–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Granchi C and Minutolo F: Anticancer
agents that counteract tumor glycolysis. ChemMedChem. 7:1318–1350.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park S, Shimizu C, Shimoyama T, Takeda M,
Ando M, Kohno T, Katsumata N, Kang YK, Nishio K and Fujiwara Y:
Gene expression profiling of ATP-binding cassette (ABC)
transporters as a predictor of the pathologic response to
neoadjuvant chemotherapy in breast cancer patients. Breast Cancer
Res Treat. 99:9–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kovalev AA, Tsvetaeva DA and Grudinskaja
TV: Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the
development of primary and acquired multiple drug resistance in
patients with early and metastatic breast cancer. Exp Oncol.
35:287–290. 2013.PubMed/NCBI
|
|
42
|
Hou X, Huang F, Carboni JM, Flatten K,
Asmann YW, Ten Eyck C, Nakanishi T, Tibodeau JD, Ross DD, Gottardis
MM, et al: Drug efflux by breast cancer resistance protein is a
mechanism of resistance to the benzimidazole insulin-like growth
factor receptor/insulin receptor inhibitor, BMS-536924. Mol Cancer
Ther. 10:117–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nambaru PK, Hübner T, Köck K, Mews S,
Grube M, Payen L, Guitton J, Sendler M, Jedlitschky G, Rimmbach C,
et al: Drug efflux transporter multidrug resistance-associated
protein 5 affects sensitivity of pancreatic cancer cell lines to
the nucleoside anticancer drug 5-fluorouracil. Drug Metab Dispos.
39:132–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schinkel AH and Jonker JW: Mammalian drug
efflux transporters of the ATP binding cassette (ABC) family: An
overview. Adv Drug Deliv Rev. 55:3–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao H, Lamusta J, Zhang WF, Salmonsen R,
Liu Y, O'Connell E, Evans JE, Burstein S and Chen JJ: Tumor cell
selective cytotoxicity and apoptosis induction by an herbal
preparation from Brucea javanica. N Am J Med Sci (Boston). 4:62–66.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yeh YC, Liu TJ and Lai HC: Shikonin
induces apoptosis, necrosis, and premature senescence of human A549
lung cancer cells through upregulation of p53 expression. Evid
Based Complement Alternat Med. 2015:6203832015. View Article : Google Scholar : PubMed/NCBI
|