Introduction
Prognosis of hepatocellular carcinoma (HCC), a
hypervascular cancer, remains poor due to early recurrence and
metastasis following treatment (1).
It has become the second leading cause of cancer-associated
mortality worldwide (2,3). Asia accounts for ~80% of newly
developing HCC cases globally (4).
The majority of patients with HCC are at the intermediate or
advanced stage when diagnosed, and are therefore not amenable to
curative resection or transplantation (4). Consequently, hepatic transcatheter
arterial chemoembolization (TACE), a minimally invasive procedure
to block the blood supply of tumors and release of cytotoxic
agents, is recommended to patients who cannot undergo surgical
resection or local ablation (5–7). Although
TACE prior to curative treatment may induce tumor necrosis in
certain patients, the long-term effects of TACE are unsatisfactory
and survival benefits from this procedure remain controversial
(8,9).
TACE induces significant tumor necrosis and severe hypoxia
(10–12). Local tissue and plasma exhibit an
increased number of angiogenic factors following TACE (13,14). Such
a microenvironment tilts the balance toward stimulatory
angiogenesis, which accelerates recurrence and metastasis of
residual tumors following TACE (13,14).
Therefore, therapeutic strategies for inhibiting local angiogenesis
may improve the outcome of patients with HCC treated with TACE.
Tumor infiltrating cells, including the majority of
macrophages, are considered to perform a pivotal role in
remodulation of tumor microenvironments (15–17).
Clinical and epidemiological evidence has indicated that the
occurrence and promotion of HCC are accompanied by these
inflammatory cells (15–17). Tumor associated macrophages (TAMs), a
primary subset of tumor infiltrating cells, may stimulate tumor
growth and angiogenesis as M2 polarization through the expression
of cytokines, chemokines, growth factors, and matrix
metalloproteases (18,19). A high density of TAMs was associated
with more microvessels and poor prognosis in the majority of types
of tumors, including HCC (20–24).
However, few studies have investigated the behavior of TAMs in HCC
following TACE. The present study focused on the changes in TAMs
following TACE, and explored the effects of combination with the
TAM inhibitor zoledronic acid (ZA) in rat HCC models.
Materials and methods
Rat HCC models
All Sprague-Dawley rats (male; age, 10–14 weeks;
body weight, 230±15 g) were obtained from the Animal Experimental
Center of Nanjing Medical University (Nanjing, China). The rats
were maintained under specific pathogen-free conditions and were
fed with sterilized rodent diet and water ad libitum. A
total of 1×106 Walker-256 cells (Shanghai Institute of
Cell Biology, Chinese Academy of Sciences, Shanghai, China) per rat
were injected into rat flanks. Rats were sacrificed when tumors
grew to 10 mm in diameter. Tumors were then harvested for
implantation into rat livers. At 12 days following implantation,
tumor xenografts grew to ~7 mm in diameter, and these orthotopic
HCC models were used for subsequent studies (25).
A total of 18 rats were randomly divided into 3
groups (n=6): A Sham TACE group [sham TACE at day 0 with a
subsequent daily intraperitoneal injection (i.p.) of PBS]; a TACE
alone group (TACE at day 0 with a subsequent daily i.p. of PBS);
and a TACE combined with ZA (Norvartis International AG, Basel,
Switzerland) treatment group [TACE at day 0 with a subsequent daily
i.p. of ZA solution (100 µg/kg body weight)]. Rats were sacrificed
with carbon dioxide asphyxiation (1 liters/min flow rate of
CO2 in a 10-liter cage, ~20% of cage volume of
CO2 and finally, mortality was ensured by lack of
cardiac pulse) at 7–14 days following TACE. Animal studies were
approved by the Institutional Animal Care and Use Committee of
Nanjing Medical University.
TACE procedure
Through a subxiphoid median incision, gastroduodenal
arteries were inserted retrogradely with 2-F silicone tubing with a
tip located at hepatic artery. Lipidol (0.3 ml; Laboratories
Gilbert, Hérouville Saint Clair, France) was then injected into the
rat liver while securing the branches of the right lobe arteries.
Following ligation of gastroduodenal artery and suture of abdomen,
rats were allowed to recover (25).
Immunohistochemical staining
Under light microscope (Olympus Corporation, Tokyo,
Japan; magnification, ×40), implanted HCC tumors obtained from
livers following animal euthanasia were examined. Tumor samples
(~5×5×5 mm3) were washed (5 min each time for 3 washes) with
pre-cooled PBS and fixed with 4% paraformaldehyde at 4°C for 24 h.
Subsequently, samples were embedded into Tissue-Tek O.C.T compound
(Sakura Finetek USA, Inc., Torrance, CA, USA) and cut into tissue
sections (10-µm thick) for histological analysis. Vessel density of
tumor tissues was assessed by immunohistochemical staining with
anti-cluster of differentiation (CD) 31 antibody (1:200 dilution;
Abcam, Cambridge, UK; cat. no., ab119339). Following overnight
incubation with CD31 antibody at 4°C, sections were incubated with
horseradish peroxidase conjugated secondary antibody (1:1,000
dilution; Abcam; cat no., ab6789) for an hour at room temperature.
Mean vessel density (MVD) was calculated as previously described
(26). Briefly, sections were scanned
using a light microscope (Olympus corporation, Tokyo, Japan) at a
magnification, ×40 and the region with the highest microvascular
density (neovascular hotspot) was identified. This region was
counted at a magnification of ×200 for microvasculature,
highlighted by CD31-positive staining. Using the same method, TAMs
in tumor tissues were detected by immunohistochemical staining with
anti-F4/80 antibody (1:100 dilution; Abcam; cat. no., ab100790)
followed by Cy3-conjugated secondary antibody (1:500 dilution;
Abcam; cat no. ab6939).
Flow cytometry analysis
Tumor tissues were harvested under a light
microscope (Olympus corporation, Tokyo, Japan; magnification, ×40)
and digested with collagenase IV solution (1 mg/ml; Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany) at 37°C for 30 min. Isolated cells
were filtered through a nylon mesh and washed with media
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Subsequently, lymphocytes in
tumor tissues were purified using Percoll (Sigma-Aldrich; Merck
KGaA) gradient centrifugation (48,000 × g, 10 min; at 4°C). These
tumor infiltrating lymphocytes were stained with
fluorescence-conjugated anti-F4/80 antibody (1:200 dilution;
eBioscience, Inc., San Diego, CA, USA; cat. no., 11-4801) in the
dark for 15 min at room temperatureFlow cytometric analysis was
performed on a FACSCalibur flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA). Data was expressed as the percentage of
F4/80-positive cells in the tumor infiltrating lymphocyte
population.
Measurement by dynamic contrast
enhancement-magnetic resonance imaging (DCE-MRI)
MRI (Magnetom avanto; Siemens AG, Munich, Germany)
and image processing (Syngo MR 2004 Version; Siemens) was used to
measure tumor volume and assess intensity-time curves with dynamic
contrast enhancement. Gadolinium-pentetic acid (Magnevist, Bayer,
Newbury, UK) was applied for enhancement with 0.1 mmol/kg body
weight. T2 weighted images (WIs) were first obtained, and then a
series of dynamic enhancements (25 series in total) were collected
using multi-section T1-weighted fast spoiled gradient-recalled
acquisition. The following parameters were applied: Repetition
time/echo time, 9.58 msec/3.76 msec; flip angle, 25°; matrix,
192×192; section thickness, 3 mm; number of signals acquired, 2;
and field of view, 75×75 mm. Tumor volume (V) was calculated based
on T2WI:V=(a × b2)/3 (a, long diameter; b, short diameter).
Contrast enhancement-time curves were constructed from perfusion
image series. Initial slope (IS; slope of the curve at the time
point of the maximal contrast agent inflow) was calculated
according to the formula IS=max[d(CTCi)/dt].
CTCi (curve at the time point i) was calculated
according to the formula
CTCi=(Ii-Io)/Io.
Ii is the signal intensity at perfusion imaging at time
point i, and Io is the signal intensity at the
baseline time point. The time following contrast agent
administration is indicated by t (27,28).
Western blot analysis
Following washing (5 min each time for 3 washes)
with cold PBS, tumor tissues were suspended with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China; cat. no., P0013) containing
proteinase inhibitors. Tissues were lysed completely for 30 min on
ice, and whole-cell protein extracts from them were prepared by
centrifugation at 12,000 × g for 20 min at 4°C. Protein
concentrations were determined using enhanced BCA Protein Assay Kit
(Beyotime Institute of Biotechnology; cat. no. P0010) according to
the manufacturer's protocol. Protein extracts (20 µg protein per
lane) were separated by 10% SDS-PAGE and then transferred to
polyvinylidene fluoride membranes (EMD Millipore; Billerica, MA,
USA). Membranes were incubated with anti-VEGF primary antibody
(1:400 dilution; Abcam; cat. no., ab53465) overnight at 4°C.
Following washing (5 min each time for 3 washes) with PBS membranes
were incubated with horseradish peroxidase conjugated secondary
antibody (1:5,000 dilution; cat. no., ab6721) for 1 h at room
temperature. Bands were detected by an enhanced chemiluminescence
system (Pierce ECL Western Blotting kit, Thermo Fisher Scientific,
Inc.) The expression level of the proteins were normalized to
ß-actin.
ELISA
Following euthanasia, rats underwent laparotomy and
blood samples were drawn from the inferior vena cave prior to
harvest of tumor tissues. Plasma were collected following
centrifugation (1,000 × g for 10 min at 4°C), and levels of VEGF in
them were determined with use of a quantitative rat VEGF ELISA kit
(Abcam; cat. no., ab100786) according to the manufacturer's
protocol. All analyses were performed in duplicate.
Statistical analysis
Data was presented as the mean ± standard deviation.
Significance between groups was evaluated using two-tailed
Student's t-tests, one-way analysis of variance with the post-hoc
test of Student Newman Keuls or Tamhane's T2, or χ2 tests as
appropriate, with the use of SPSS 11.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Combination of TACE with ZA treatment
enhances inhibition of TACE on tumor growth in rat HCC models
To reveal the effects of TACE combined with or
without ZA treatment in rat HCC models, MRI was used to evaluate
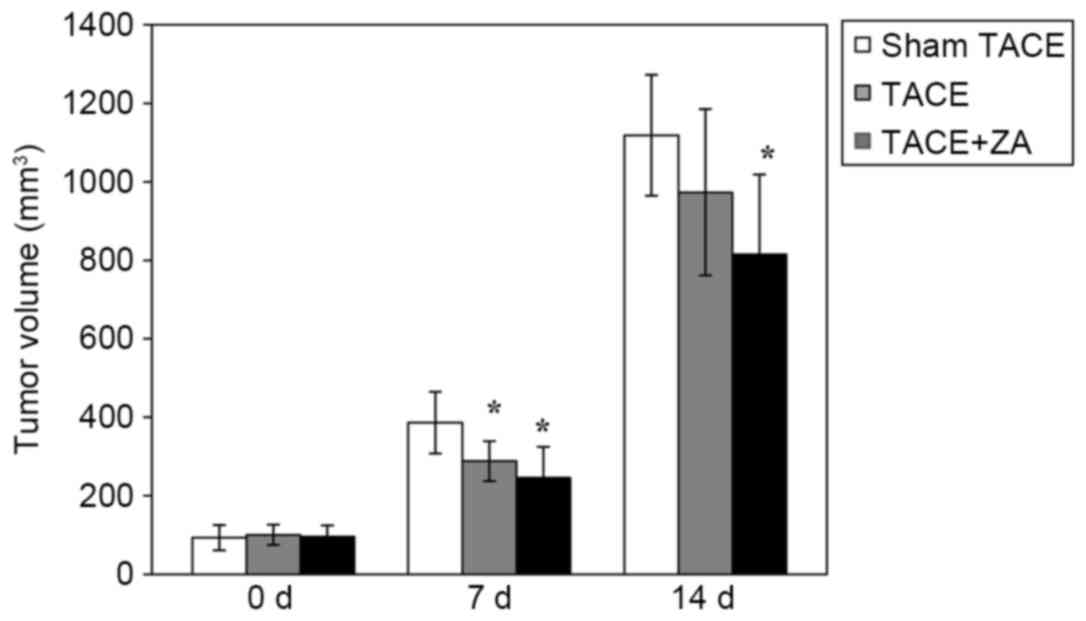
tumor growth until 14 days following TACE. As presented in Fig. 1, the TACE alone group had reduced
tumor volume compared with the sham TACE group at 7 days subsequent
to the procedure (P<0.05). After 14 days following the
procedure, inhibition of tumor growth of TACE alone group was
attenuated, yet no significant difference in tumor volume was
observed between these two groups (TACE alone vs. sham TACE,
P>0.05) If combined with ZA treatment subsequent to the
procedure, TACE displayed a stable inhibitory effect on tumor
growth until the end of observation (14 days following TACE;
P<0.05). These data indicated that combination with ZA treatment
enhanced TACE-mediated inhibition of tumor growth in rat HCC
models.
ZA inhibits TAM infiltration following
TACE
ZA has been demonstrated to be an inhibitor of TAMs
in various tumors. Therefore, in the present study, TAM
infiltration was firstly examined in the HCC models by
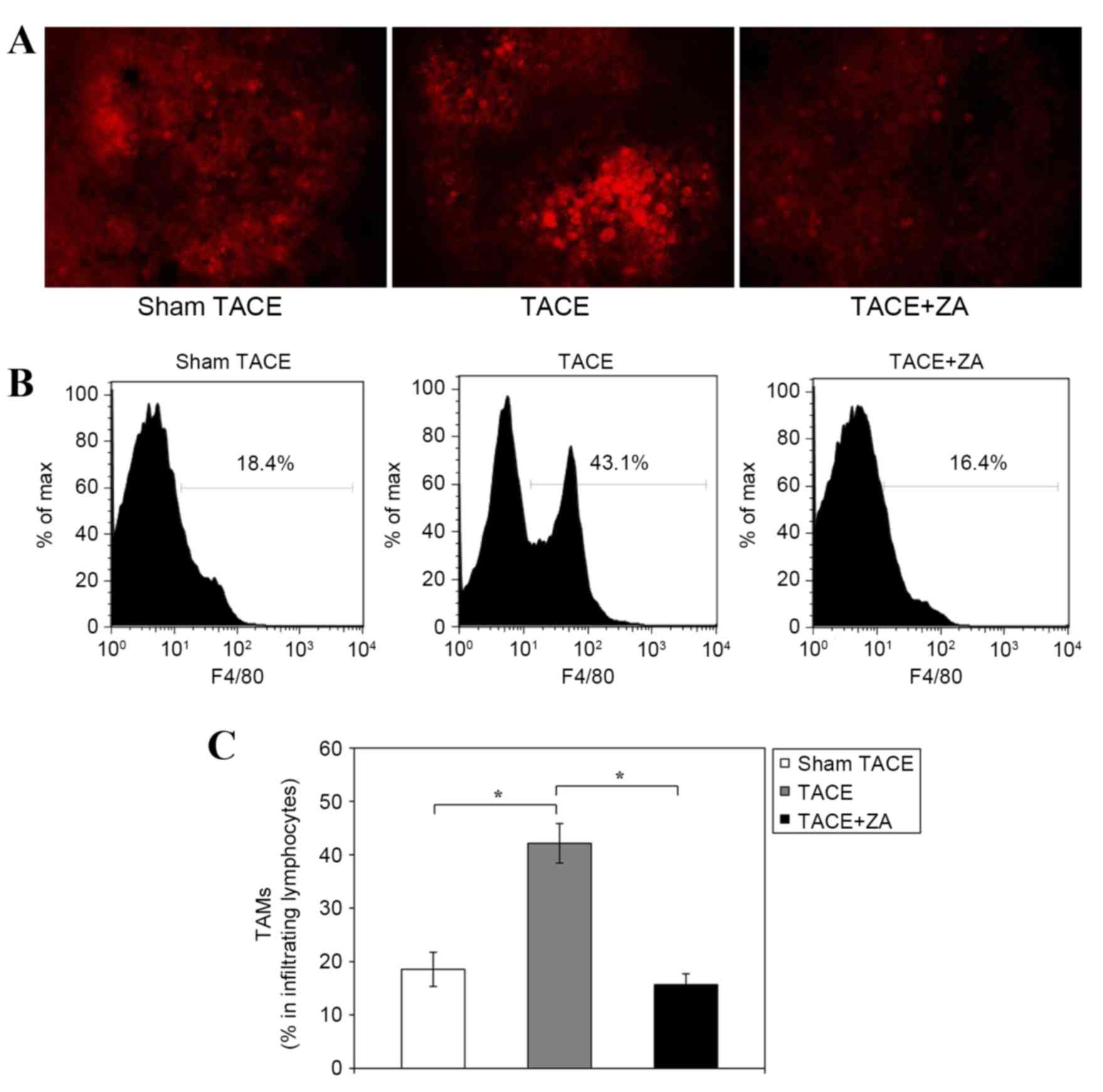
immunohistochemical staining. Histological analysis revealed that
compared with the sham control group, the TACE alone group had more
F4/80-positive TAMs infiltrating orthotopic tumors at 14 days
following the procedure. However, TAM infiltration was markedly
inhibited in the TACE combined with ZA treatment group (Fig. 2A). In addition, quantitative analysis
of isolated tumor infiltrating cells by flow cytometry validated
these findings. The TACE alone group had more TAMs with positive
F4/80 staining compared with the sham TACE group (percentage of
TAMs in tumor infiltrating lymphocytes, 42.2±3.7% vs. 18.5±3.2%;
P<0.01). Combination with ZA treatment significantly inhibited
TAM infiltration following TACE in rat HCC models. The percentage
of F4/80-positive TAMs in tumor infiltrating lymphocytes was
reduced by ~60% compared with the TACE alone group (15.7±2.0%;
P<0.01; Fig. 2B and C).
ZA inhibits tumor angiogenesis
following TACE
In the subsequent studies, MVD and enhanced DCE-MRI
were used to evaluate tumor angiogenesis following TACE in HCC
models. The data revealed that at 14 days following the procedure,
the TACE alone group had significantly increased MVD in tumors
compared with sham TACE group (46.8±5.2% vs. 34.8±3.6%; P<0.01).
However, the TACE combined with ZA treatment group had inhibited
tumor MVD compared with TACE alone group (24.6±1.8% vs. 46.8±5.2;
P<0.01; Fig. 3A and B).
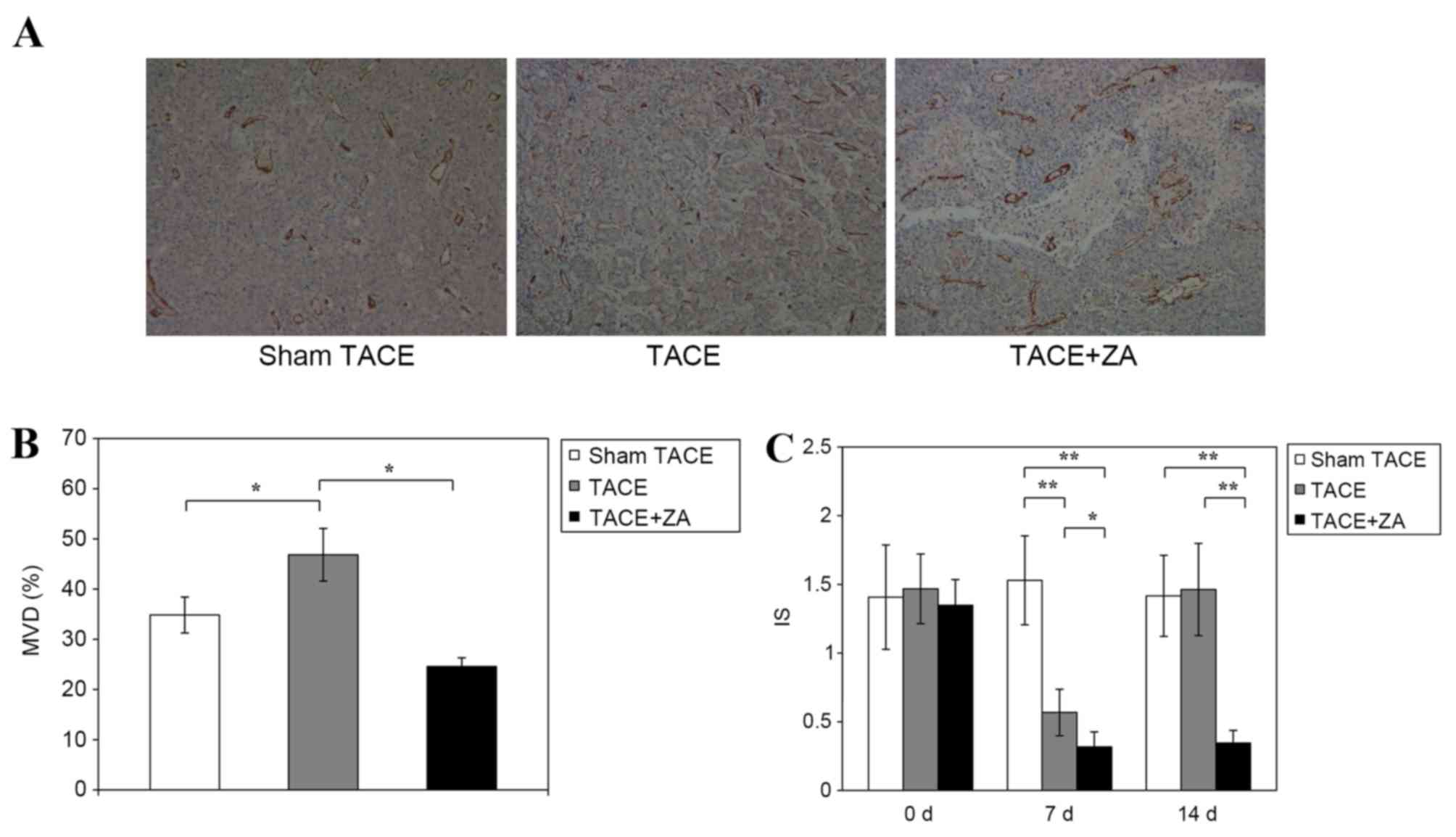 | Figure 3.Tumor angiogenesis following TACE in
hepatocellular carcinoma. (A) At 14 days subsequent to the
procedure, vessel density of tumor tissues from various groups,
including sham TACE, TACE alone and TACE combined with ZA treatment
groups, was assessed by immunohistochemical staining with anti-CD31
antibody (magnification, ×40). The figures are representative of
three separate experiments. (B) MVD of tumor tissues was calculated
by the method described in materials and methods. The data were
expressed as the mean ± standard deviation. *P<0.01. (C) At 7
and 14 days following TACE, MRI was used to measure intensity-time
curves of tumors with dynamic contrast enhancement. IS, a
proportional index of tumor angiogenesis, was calculated from MRI
images. The data are expressed as the mean ± standard deviation.
*P<0.05, **P<0.01. MRI, magnet resonance imaging; IS, initial
slope; MVD, mean vessel density; TACE, transcatheter arterial
chemoembolization; ZA, zoledronic acid. |
IS is a proportional index of tumor angiogenesis
calculated from DCE-MRI images (21).
No significant differences were observed between the rat models
prior to treatment. At 7 days following procedure, the TACE alone
group showed decreased IS compared with the sham TACE group
(0.57±0.17 vs. 1.53±0.32; P<0.01). The TACE combined with ZA
treatment group showed a more decreased IS (~40% decrease) compared
with the TACE alone group (0.32±0.11 vs. 0.57±0.17; P<0.05). At
14 days following the procedure, the TACE alone group exhibited
restored IS, which was not significantly different to the sham TACE
group (1.42±0.30 vs. 1.46±0.34; P=0.805). However, the group
combined with ZA treatment had decreased IS compared with the TACE
alone group (0.35±0.09 vs. 1.42±0.30; P<0.01), which stabilized
at a comparative level similar to early stage (7 days) following
the procedure (Fig. 3C). These data
suggested that combination with ZA treatment allowed TACE to
achieve sustained inhibition of tumor angiogenesis in rat HCC
models.
ZA inhibits secretion of VEGF
following TACE
VEGF is generally considered to be one of the most
important angiogenic factors released by macrophages. In order to
establish whether secretion of VEGF may be affected by TACE
combined with ZA treatment, local and systemic VEGF levels were
examined in the HCC models at 14 days following the procedure.
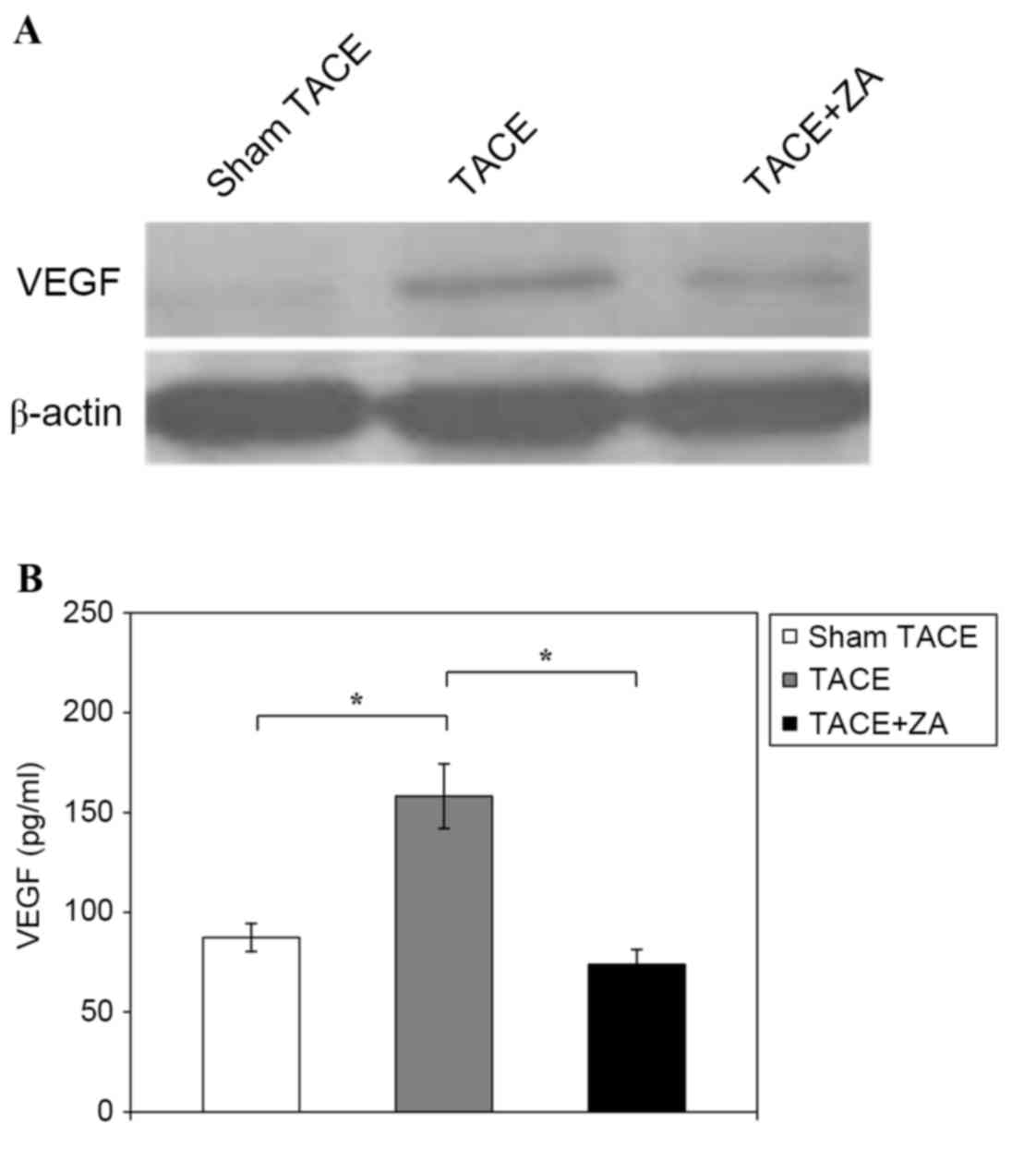
Western blot analysis of tumor tissues revealed that the TACE alone
group had notably increased VEGF expression compared with the sham
TACE group, but expression may be inhibited if combined with ZA
treatment following procedure (Fig.
4A). In addition, ELISA analysis revealed that the TACE alone
group had significantly increased concentration of VEGF in plasma
compared with the sham TACE group (158.2±16.2 pg/ml vs. 87.3±7.0
pg/ml; P<0.01). TACE combined with ZA treatment group revealed
inhibited VEGF expression in plasma compared with the TACE alone
group (73.8±7.5 pg/ml vs. 158.2±16.2 pg/ml; P<0.01; Fig. 4B). These data indicated that
combination with ZA treatment inhibited secretion of VEGF following
TACE in rat HCC models.
Discussion
In the clinic, TACE is recommended to patients with
advanced HCC, as it is able to deprive tumor blood supply that
induce severe hypoxia and tumor necrosis (5–7). However,
numerous previous studies have proposed that complete tumor
necrosis is seldom achieved in patients with HCC following TACE
(10,11,29,30). In
addition, a few studies reported biological changes in residual HCC
following TACE (13,14,31).
Significantly increased VEGF expression and more angiogenesis were
observed in plasma and residual tumors (13,14,31). Local
hypoxia occurs following establishment of a TACE-induced
pro-angiogenic microenvironment, which weakens the treatment effect
of the procedure and fertilizes residual tumors (13,14,31). This
may be one reason why complete tumor necrosis following TACE is
challenging to achieve. Such a microenvironment may accelerate
recurrence and metastasis of residual tumors, which leads to
failure of TACE treatment. Therefore, therapeutic strategies for
inhibiting tumor angiogenesis may improve outcomes of patients with
HCC receiving with TACE treatment.
Hypoxia tumor infiltrating cells were also
considered to serve an important role in modulation of
microenvironment (15–17). As a primary subset of tumor
infiltrating cells, TAMs have been implicated in tumor growth and
prognosis in patients with HCC (22,23). In
the present study, compared with the sham control, the TACE alone
group exhibited more F4/80-positive TAMs infiltrating orthotopic
tumors at 14 days following the procedure. Characteristics of the
microenvironment following TACE, including local hypoxia and
inflammation, evidently induced TAM infiltration.
ZA is established in the clinic to inhibit bone
resorption, primarily in metastatic tumors (32,33). It
may exert an indirect effect on TAMs in certain types of tumors
(34). Coscia et al (35) applied ZA with a clinically compatible
dose to treat mouse mammary tumors. The results demonstrated that
administration of ZA significantly reduced the number of TAMs in
tumors. Using ZA in liver tumors, Zhang et al (36) found that it ZA improved the antitumor
effects of sorafinib as a TAM inhibitor. The present data indicated
that combination with ZA treatment enhanced the TACE-induced
inhibition of tumor growth in rat HCC models. Mechanism
investigation demonstrated that ZA significantly inhibited TAM
infiltration following TACE.
TAMs have been reported to facilitate angiogenesis
in non-liver tumors (21,37). In accordance with these findings, the
present data identified that the combination with ZA treatment
enabled sustained inhibition of tumor angiogenesis following TACE
in rat HCC models. In addition, the present study revealed that ZA
treatment inhibits secretion of VEGF following TACE. VEGF is an
important pro-angiogenic factor released by TAMs, which is involved
in the initiation and remodeling of tumor angiogenesis (34). ZA treatment inhibited tumor
angiogenesis following TACE, which was mediated by VEGF secreted
from TAMs.
In conclusion, combination with ZA treatment
enhanced the effects of TACE through inhibiting TAM infiltration
and tumor angiogenesis following TACE in rat HCC models. It may
serve as a novel therapeutic strategy for improving the outcomes of
TACE treatment in patients with advanced HCC.
Acknowledgements
The present study was supported by grants from the
Scientific Research Foundation of Jiangsu Province (grant no.
BK20130259) and the National Natural Science Foundation of China
(grant no. 31000526).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Connell LC, Harding JJ and Abou-Alfa GK:
Advanced hepatocellular cancer: The current state of future
research. Curr Treat Options Oncol. 17:432016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M, Sherman M and Gores G: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han KH, Kudo M, Ye SL, Choi JY, Poon RT,
Seong J, Park JW, Ichida T, Chung JW, Chow P and Cheng AL: Asian
consensus workshop report: Expert consensus guideline for the
management of intermediate and advanced hepatocellular carcinoma in
Asia. Oncology. 81:(Suppl 1). S158–S164. 2011. View Article : Google Scholar
|
|
5
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benson AB III, Abrams TA, Ben-Josef E,
Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D'Angelica
MI, Davila R, et al: NCCN clinical practice guidelines in oncology:
Hepatobiliary cancers. J Natl Compr Canc Netw. 7:350–391. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM,
Poon RT, Fan ST and Wong J: Randomized controlled trial of
transarterial lipiodol chemoembolization for unresectable
hepatocellular carcinoma. Hepatology. 35:1164–1171. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobayashi T, Ishiyama K and Ohdan H:
Prevention of recurrence after curative treatment for
hepatocellular carcinoma. Surg Today. 43:1347–1354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: A
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuo N, Uchida H, Nishimine K, Soda S,
Oshima M, Nakano H, Nagano N, Nishimura Y, Yoshioka T, Guo Q, et
al: Segmental transcatheter hepatic artery chemoembolization with
iodized oil for hepatocellular carcinoma: Antitumor effect and
influence on normal tissue. J Vasc Interv Radiol. 4:543–549. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higuchi T, Kikuchi M and Okazaki M:
Hepatocellular carcinoma after transcatheter hepatic arterial
embolization. A histopathologic study of 84 resected cases. Cancer.
73:2259–2267. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weintraub JL and Salem R: Treatment of
hepatocellular carcinoma combining sorafenib and transarterial
locoregional therapy: State of the science. J Vasc Interv Radiol.
24:1123–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ and
Cao GW: Increased expression of vascular endothelial growth factor
in hepatocellular carcinoma after transcatheter arterial
chemoembolization. Acta Radiol. 49:523–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantovani A, Garlanda C and Allavena P:
Molecular pathways and targets in cancer-related inflammation. Ann
Med. 42:161–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Capece D, Fischietti M, Verzella D,
Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F and Alesse E:
The inflammatory microenvironment in hepatocellular carcinoma: A
pivotal role for tumor-associated macrophages. Biomed Res Int.
2013:1872042013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Riabov V, Gudima A, Wang N, Mickley A,
Orekhov A and Kzhyshkowska J: Role of tumor associated macrophages
in tumor angiogenesis and lymphangiogenesis. Front Physiol.
5:752014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bingle L, Brown NJ and Lewis CE: The role
of tumour-associated macrophages in tumour progression:
Implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leek RD and Harris AL: Tumor-associated
macrophages in breast cancer. J Mammary Gland Biol Neoplasia.
7:177–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding T, Xu J, Wang F, Shi M, Zhang Y, Li
SP and Zheng L: High tumor-infiltrating macrophage density predicts
poor prognosis in patients with primary hepatocellular carcinoma
after resection. Hum Pathol. 40:381–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang
W, Xiong YQ, Wu WZ, Wang L, Tang ZY and Sun HC: High expression of
macrophage colony-stimulating factor in peritumoral liver tissue is
associated with poor survival after curative resection of
hepatocellular carcinoma. J Clin Oncol. 26:2707–2716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta S, Kobayashi S, Phongkitkarun S,
Broemeling LD and Kan Z: Effect of transcatheter hepatic arterial
embolization on angiogenesis in an animal model. Invest Radiol.
41:516–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thoeny HC, De Keyzer F, Vandecaveye V,
Chen F, Sun X, Bosmans H, Hermans R, Verbeken EK, Boesch C, Marchal
G, et al: Effect of vascular targeting agent in rat tumor model:
Dynamic contrast-enhanced versus diffusion-weighted MR imaging.
Radiology. 237:492–499. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Buadu LD, Murakami J, Murayama S,
Hashiguchi N, Sakai S, Masuda K, Toyoshima S, Kuroki S and Ohno S:
Breast lesions: Correlation of contrast medium enhancement patterns
on MR images with histopathologic findings and tumor angiogenesis.
Radiology. 200:639–649. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bharat A, Brown DB, Crippin JS, Gould JE,
Lowell JA, Shenoy S, Desai NM and Chapman WC: Pre-liver
transplantation locoregional adjuvant therapy for hepatocellular
carcinoma as a strategy to improve longterm survival. J Am Coll
Surg. 203:411–420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duvoux C, Cherqui D, Van Nhieu JT,
Vavasseur D, Zafrani ES, Mathieu D, Fagniez PL and Dhumeaux D:
Chemoembolization for hepatocellular carcinoma in cirrhotic
patients: Assessment of efficacy on total hepatectomy specimens.
Transplant Proc. 26:3572–3573. 1994.PubMed/NCBI
|
|
31
|
Liao X, Yi J, Li X, Yang Z, Deng W and
Tian G: Expression of angiogenic factors in hepatocellular
carcinoma after transcatheter arterial chemoembolization. J
Huazhong Univ Sci Technolog Med Sci. 23:280–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zekri J, Mansour M and Karim SM: The
anti-tumour effects of zoledronic acid. J Bone Oncol. 3:25–35.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Green J and Lipton A: Anticancer
properties of zoledronic acid. Cancer Invest. 28:944–957. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rogers TL and Holen I: Tumour macrophages
as potential targets of bisphosphonates. J Transl Med. 9:1772011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coscia M, Quaglino E, Iezzi M, Curcio C,
Pantaleoni F, Riganti C, Holen I, Mönkkönen H, Boccadoro M, Forni
G, et al: Zoledronic acid repolarizes tumour-associated macrophages
and inhibits mammary carcinogenesis by targeting the mevalonate
pathway. J Cell Mol Med. 14:2803–2815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang
PY, Xu HX, Kong LQ, Wang L, Wu WZ and Tang ZY: Depletion of
tumor-associated macrophages enhances the effect of sorafenib in
metastatic liver cancer models by antimetastatic and antiangiogenic
effects. Clin Cancer Res. 16:3420–3430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|