Introduction
The estimated occurrence of palpable thyroid nodules
in the general population ranges from 4 to 7% (1). While thyroid nodules are therefore
rather common, thyroid cancers are rare. Thyroid ultrasonography
and thyroid fine-needle aspiration (FNA) are frequently used
preoperative techniques to diagnose malignant thyroid tumors.
However, FNA leads to indeterminate biopsy results in 10–20% of all
cases, when solely based on cytopathological evaluation (2,3).
Nowadays, the preoperative characterization of
thyroid nodules remains a challenge for clinicians. To address this
issue ever increasing numbers of studies had evaluated the value of
immunohistochemical markers such as galectin (gal)-3, cytokeratin
19 (CK19), Hector Battifora Mesothelial-1 (HBME-1) and thyroid
peroxidase (TPO) (4–14). Although some are promising markers to
distinguish benign from malignant thyroid lesions, none is
individually reliable for differential diagnosis. Actually, each
marker has its limitations because of significant expression in
benign thyroid nodules to a notable extent (15–20).
Therefore, novel immunohistochemical markers or combinations are
required to define criteria for distinction between benign and
malignant thyroid lesions, especially regarding the classification
of thyroid follicular lesions.
Homodimeric gal-1 is a potential new candidate
because its expression has been shown to be upregulated in cancers
(21–24), including thyroid carcinoma (25–27).
Proteomic profiling has also suggested gal-1 to be a potential
biomarker of thyroid cancer (28,29).
Furthermore, our team has previously reported for the first time a
high serum level of both gal-1 and gal-3 in patients diagnosed with
benign thyroid lesions and well-differentiated thyroid carcinoma
compared to healthy individuals (30). In addition, having revealed functional
additivity and antagonism between members of the galectin family
(31,32), we take steps to network analysis by
co-monitoring expression of gal-7 and gal-8.
In the present study, we compared the diagnostic
value of gal-1, gal-3, CK19, HBME-1 and TPO, alone and in
combination, in benign and malignant thyroid lesions in order to
determine the usefulness of each marker or a combination of
markers, allowing the most accurate diagnosis of thyroid cancer
through preoperative assessment of nodular thyroid lesions.
Materials and methods
Clinical data
The immunohistochemical detection of gal-1, gal-3,
gal-7, and gal-8, CK19, HBME-1 and TPO was studied in two tissue
microarrays (TMA) composed of 66 follicular adenomas (FA) and 66
papillary carcinomas (PC). The available population data were
gender, age and histopathologic features. This clinical series
included 100 women and 32 men. The mean age of patients with FA was
44 years (range, 13–76 years) and 41 years for patients with PC
(range, 9–73 years) (33). The
patient samples and clinical data were retrieved from the records
of the Lille University Hospital (Lille, France) between August
2000 and September 2001, selected and analysed by two pathologists
(Professor E. Leteurtre and Dr F. Renaud). No inclusion and
exclusion criteria of the selected patients were used in the
current study. Written informed consent was obtained from all the
patients to use the surgical specimens for scientific research.
Immunohistochemistry and
histopathologic examination
Specimens were fixed in 10% buffered formalin and
paraffin-embedded. Five-micrometer sections were stained with
hematoxylin and eosin for examination by light microscopy. The
entire paraffin-embedded blocks were selected and arrayed in
triplicate 0.6 mm tissue cores for TMA construction (Beecher
Instruments, Silver Springs, MD, USA). The 5 µm-thick tissue
sections were deparaffinized and heat pretreated (citrate buffer or
EDTA; Ventana Medical Systems, Tucson, AZ, USA) before incubation
with i) specific primary antibody against gal-1 (polyclonal rabbit
anti-human galectin-1, 1:100) and gal-3 (polyclonal rabbit
anti-human galectin-3, 1:200) (34,35), gal-7
(polyclonal rabbit anti-human galectin-7, 1:50) and gal-8
(polyclonal rabbit anti-human galectin-8, 1:20) (36,37), all
anti-galectin antibodies rigorously controlled against occurrence
of cross-reactivity to human galectins and depleted by respective
cycles of affinity chromatography if positive, and CK19 (monoclonal
mouse anti-human cytokeratin-19, 1:50; M0772; Dako, Glostrup,
Denmark), HBME-1 (monoclonal mouse anti-human HBME-1, 1:100; M3505;
Dako), TPO (monoclonal mouse anti-human TPO, 1:50; TPO47; Biocytex,
Marseille, France); then ii) corresponding biotinylated secondary
antibody (760–500, ultraView Universal DAB Detection kit; Ventana
Medical Systems); and finally iii) avidin-biotin-peroxidase complex
(ABC kit; Vector Laboratories, Burlingame, CA, USA). The slides
were thoroughly washed with PBS between each incubation step.
Immunocomplexes were finally visualized by exposure to the
diaminobenzidine chromogen (DAB; BioGenex, Fremont, CA, USA) in the
presence of H2O2. After rinsing, the sections
were counterstained with luxol fast blue and mounted with a
synthetic medium. To exclude antigen-independent staining, the
incubation step with primary antibodies was omitted from the
protocol as negative controls. In all cases, these controls were
negative (data not shown). The specificity of gal-1 and gal-3
antibodies was validated by western blotting in different thyroid
cancer cell lines (B-CPAP, FTC133C and 8505C cell lines derived
from papillary, follicular and anaplastic thyroid carcinoma
respectively) reporting immunoreactive band for gal-1 at 14 kDa and
for gal-3 at 26 kDa (data not shown). The FTC133C and 8505C cell
lines were analyzed to confirm the absence of mycoplasma
contamination and the presence of characteristic markers of
follicular thyroid carcinoma and anaplastic thyroid carcinoma,
respectively. Both cell lines have been characterized at the IRIBHM
laboratory (Professor C. Maenhaut, ULB, Brussels, Belgium)
(38). A blind semi-quantitative
analysis of immunostainings was independently performed by three
pathologists (Professor E. Leteurtre, Dr F. Renaud, Professor M.
Remmelink) using a light microscope (Axiocam MRc5; Carl Zeiss,
Hallbergmoos, Germany). Each tissue specimen was scored (0–6) by
adding the percent of immunopositive cells (range, 0–3: 0=0;
1=1-33; 2=34–66 and 3=67–100%) to the staining intensity (range,
0–3: 0=none; 1=low; 2=moderate and 3=high). The overall score used
for subsequent statistical analysis was the mean of three spots of
the same tumor.
Statistical analysis
Groups of data were compared using the
non-parametric Mann-Whitney test. The diagnostic performances of
single or combined immunomarkers and the identification of the
optimal cut-off points for the diagnosis of malignancy were
evaluated using the receiver operating characteristic (ROC) curves
and the assessment of the area under the ROC curve (AUC). The
specificity, sensitivity, positive predictive value (PPV) and
negative predictive value (NPV) of markers, alone or combined, were
evaluated from crosstabs based on cut-off points and significance
were calculated using the Fisher's exact test. P-value <0.05 was
considered as significant. Statistical analyses were performed with
IBM SPSS Statistics 23 (IBM, Ehningen, Germany).
Results
Gal-1/-3/-7/-8, CK19, HBME-1 and TPO
immunostaining profiles in benign and malignant thyroid
lesions
The first aim of our study was to assess the
expression levels of seven markers (gal-1, gal-3, gal-7, gal-8,
TPO, CK19 and HBME-1) by immunohistochemistry in two series of TMAs
composed of 66 cases of FA and 66 cases of PC.
In sections of PCs, CK19 and HBME-1 staining was
either cytoplasmic or both cytoplasmic and apical, with intensity
increase at the apical membrane (Fig. 1B
and C). Signal presence for gal-1 and gal-3 was ubiquitous,
both in tumor cell (cytoplasmic and nuclear staining) and the
stroma associated to cancers and adenomas (Fig. 1A and D). The expression pattern of TPO
showed that staining was cytoplasmic, relatively intense at the
apical membrane of benign cells (Fig.
1E).
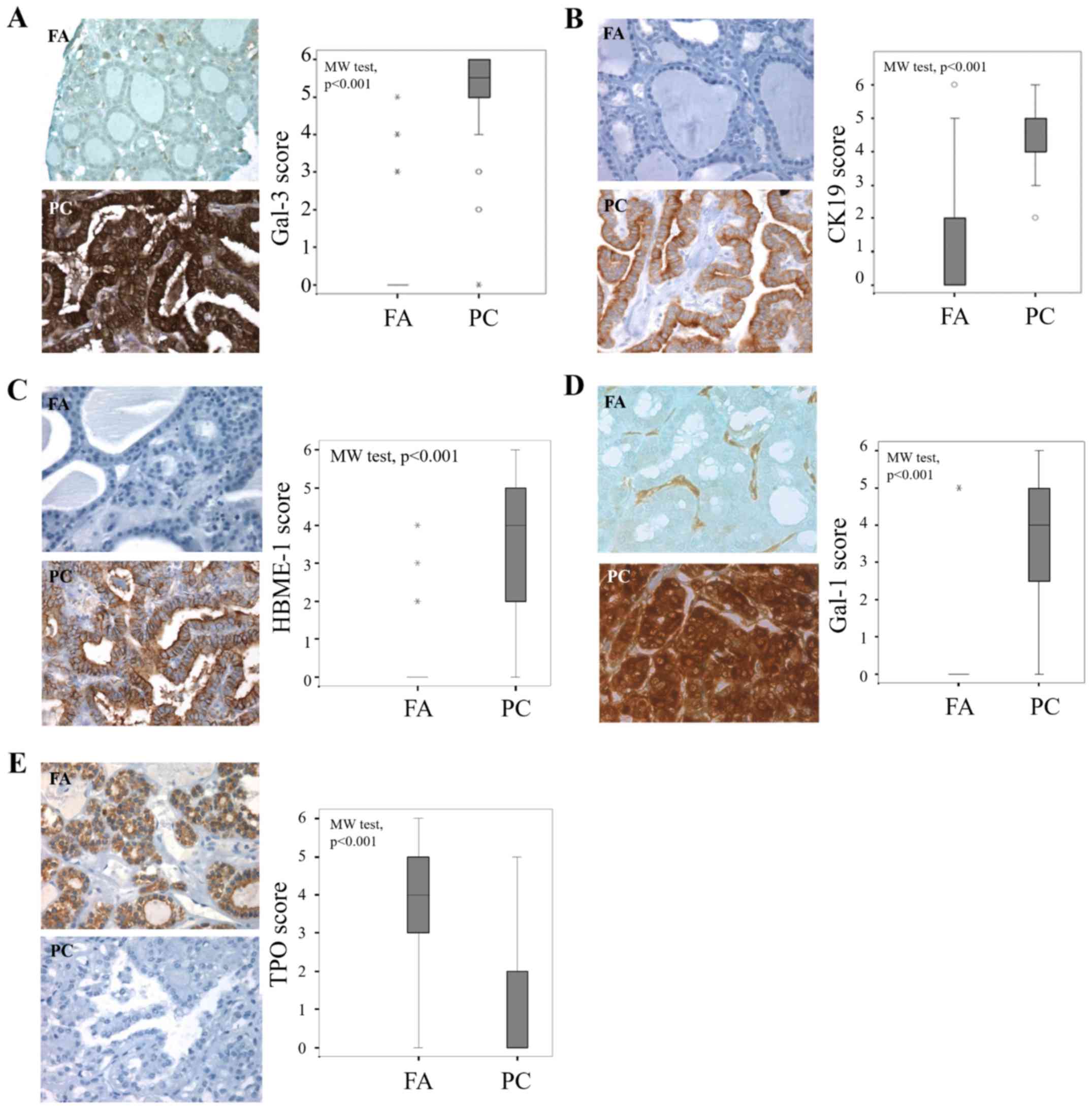 | Figure 1.Evaluation of gal-3, CK19, HBME-1,
gal-1 and TPO expression in benign and malignant thyroid lesions.
Immunohistochemical evaluation of expression and statistical
analysis of (A) gal-3, (B) CK19, (C) HBME-1, (D) gal-1 and (E) TPO
in tissue microarray composed by FA and PC (P<0.001,
Mann-Whitney test). Magnification, ×400. The immunostaining was
semi-quantitatively assessed in the cytoplasmic compartment and
scored from 0 to 6 by summing staining intensity (0–3) and
percentage of positivity (0–3). Data are presented as box plots
indicating the 1st and the 3rd quartiles centered on medians (thick
lines) with whiskers for the minimum and maximum non-outlier
values, the ‘o’ symbols are outliers and the ‘*’ symbols show the
extreme values. Gal-3, galectin-3; CK19, cytokeratin 19; HBME-1,
Hector Battifora Mesothelial Epitope-1; TPO, thyroid peroxidase;
FA, follicular adenoma; PC, papillary carcinoma. |
The statistical analysis of the results of the
cytoplasmic immunostaining revealed that the level expression of
the four markers (gal-1, gal-3, CK19 and HBME-1) was significantly
higher in cancer cells of PC compared to epithelial cells in FA
(P<0.001, Mann-Whitney test) (Fig.
1A-D). By contrast, the cytoplasmic expression of TPO is higher
in adenomas than in cancers (P<0.001, Mann-Whitney test)
(Fig. 1E).
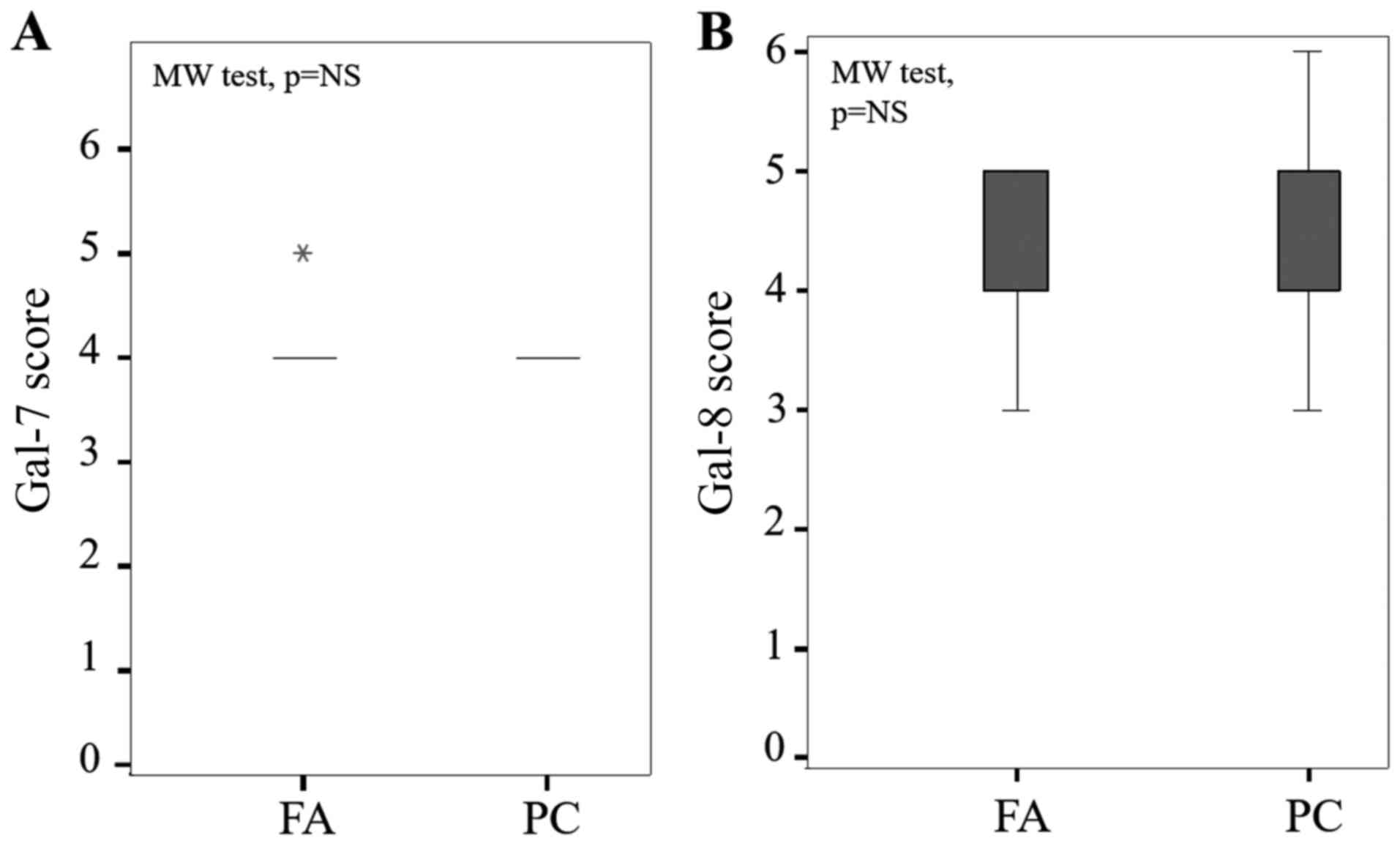
Of note, staining levels of gal-7 and gal-8 were
nuclear and cytoplasmic both in FA and PC without any statistical
difference between cytoplasmic immunostaining in each group (P=0.12
and P=0.47, Mann-Whitney test, respectively) (Fig. 2).
Gal-1 was completely absent in the epithelial
compartment on almost all benign thyroid neoplasms (Fig. 1D), whereas a weak to moderate signal
was found in benign samples for gal-3, CK19 and HBME-1 (Fig. 1A, B and C, respectively), suggesting
that monitoring gal-1 could be more reliable to distinguish
malignant from benign lesions.
Diagnostic performances of individual
or combined immunomarkers
For each individual marker, the cut-off has been
defined from the ROC curve (Fig. 3A).
The cut-offs allowing to separate negative/low vs. positive
immunostaining were >0 for gal-1, gal-3 and HBME-1, and >2
for CK19 and TPO (Table I and
Fig. 1). The diagnostic performance
of the markers was individually evaluated by comparing areas under
the ROC. As described in Table I, the
area under the ROC curve of gal-3 (AUC=0.957) is greater than the
area under the ROC curves of the CK19 (AUC=0.947), HBME-1
(AUC=0.910), gal-1 (AUC=0.883) and TPO (AUC=0.879). From crosstab
analyses, gal-3 and CK19 appear to be the most sensitive markers
(97 and 98%, respectively), while gal-1 is the most specific one
(97%) (P<0.001, Fisher's exact test). HBME-1 and TPO exhibited
good sensitivities (88 and 83%, respectively) and good
specificities (79 and 80%, respectively) (Table I).
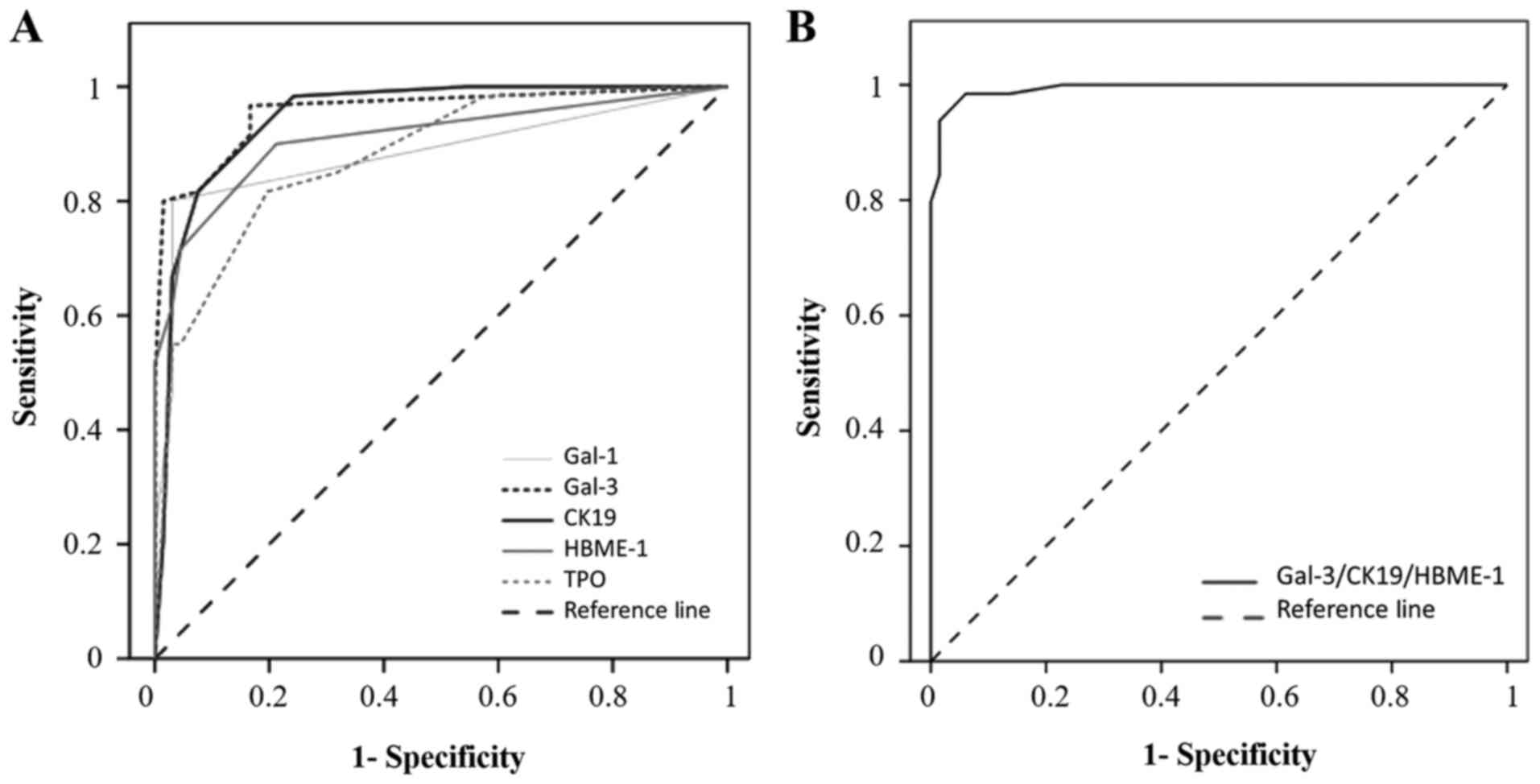 | Figure 3.Receiver operating characteristic
curves of single and combined thyroid markers. (A) ROC curve for
positive immunocytochemistry expression of gal-1, gal-3, CK19,
HBME-1 and TPO in the diagnosis of PCs. Areas under the curve:
Gal-3=0.957; CK19=0.047; HBME-1=0.910; gal-1= 0.883; TPO=0.879. (B)
ROC curve for positive immunohistochemistry expression of the
combination of three markers (gal-3/CK19/HBME-1) in the diagnosis
of PCs. Area under the curve, 0.994. ROC, receiver operating
characteristic; gal-1, galectin-1; CK19, cytokeratin 19; HBME-1,
Hector Battifora Mesothelial Epitope-1; TPO, thyroid peroxidase;
PC, papillary carcinoma. |
 | Table I.Diagnostic value of individual
markers to distinguish malignant from benign thyroid tumors. |
Table I.
Diagnostic value of individual
markers to distinguish malignant from benign thyroid tumors.
| Markers | AUC | Cut-off | Spe (%) | Sens (%) | PPV (%) | NPV (%) | Fisher's exact test
(10−15) |
|---|
| Galectin-3 | 0.957 | >0 | 83 | 97 | 85 | 96 | P=1.07 |
| CK19 | 0.947 | >2 | 76 | 98 | 80 | 98 | P=1.02 |
| HBME-1 | 0.910 | >0 | 79 | 88 | 81 | 87 | P=4.20 |
| Galectin-1 | 0.883 | >0 | 97 | 80 | 96 | 83 | P=5.16 |
| TPO | 0.879 | >2 | 80 | 83 | 80 | 83 | P=345.47 |
Statistical analysis for all possible permutations
using the five markers to discriminate between FA and PC was shown
in Table II. Of note, as TPO is a
negative diagnostic marker for cancer, we used its inverse score
(6-score) to combine it to others, and mean scores (0–6) were
calculated for all combinations. The diagnostic performance of the
combination of markers was evaluated by comparing areas under the
ROC curves. The data revealed that the combination of
gal-3/CK19/HBME-1 exhibits the highest performance to assess the
diagnosis of malignancy (AUC=0.994, cut-off >2.5) (Table II and Fig.
3B). The sensitivity, specificity, PPV and NPV were also
calculated for the panel of combined markers. Several combinations
of markers such as gal-3/CK19, gal-3/CK19/HBME-1, gal-3/CK19/gal-1,
gal-3/HBME-1/gal-1/TPO or gal-3/CK19/HBME-1/gal-1/TPO improves
specificity (>95%) and sensitivity (>90%). However, based on
these calculations, the combination of gal-3/CK19/HBME-1 is the
best one, associating high sensitivity (95%) and high specificity
(97%) for the diagnosis of PC (P<0.001, Fisher's exact test)
(Table II).
 | Table II.Diagnostic value of combined markers
in discrimination of malignant from benign thyroid tumors. |
Table II.
Diagnostic value of combined markers
in discrimination of malignant from benign thyroid tumors.
| Markers | AUC | Cut-off | Spe (%) | Sens (%) | PPV (%) | NPV (%) | Fisher's exact test
(10−15) |
|---|
|
Gal-3/CK19/HBME-1 | 0.994 | >2.5 | 97 | 95 | 97 | 96 | P=1.57 |
|
Gal-3/CK19/HBME-1/gal-1 | 0.991 | >2 | 95 | 95 | 95 | 95 | P=1.57 |
|
Gal-3/CK19/HBME-1/TPO | 0.991 | >2.5 | 97 | 90 | 97 | 91 | P=16.73 |
|
Gal-3/CK19/HBME-1/gal-1/TPO | 0.989 | >2.5 | 97 | 91 | 96 | 91 | P=4.63 |
| Gal-3/CK19 | 0.988 | >3 | 99 | 92 | 98 | 93 | P=5.48 |
|
Gal-3/HBME-1/TPO | 0.986 | >2.5 | 94 | 87 | 93 | 88 | P=16.73 |
|
Gal-3/CK19/gal-1 | 0.985 | >2 | 97 | 92 | 97 | 93 | P=8.91 |
| Gal-3/HBME-1 | 0.985 | >2 | 95 | 95 | 95 | 95 | P=8.29 |
| Gal-3/CK19/TPO | 0.985 | >2.5 | 92 | 94 | 92 | 91 | P=4.71 |
|
Gal-3/HBME-1/gal-1/TPO | 0.985 | >2 | 97 | 91 | 95 | 91 | P=1.01 |
|
Gal-3/HBME-1/gal-1 | 0.984 | >2 | 95 | 87 | 95 | 89 | P=6.18 |
|
Gal-3/CK19/gal-1/TPO | 0.984 | >2 | 94 | 95 | 94 | 95 | P=1.50 |
|
CK19/HBME-1/gal-1 | 0.982 | >2 | 94 | 91 | 94 | 91 | P=7.06 |
| Gal-3/TPO | 0.979 | >2.5 | 92 | 90 | 92 | 91 | P=4.42 |
|
Gal-3/gal-1/TPO | 0.979 | >2 | 94 | 82 | 93 | 84 | P=12.86 |
|
CK19/HBME-1/gal-1/TPO | 0.979 | >2 | 91 | 90 | 90 | 91 | P=22.82 |
| CK19/HBME-1 | 0.972 | >2 | 89 | 91 | 90 | 91 | P= 14.85 |
| CK19/gal-1 | 0.972 | >2 | 95 | 92 | 95 | 93 | P=7.06 |
| CK19/gal-1/TPO | 0.969 | ≥2.5 | 94 | 90 | 93 | 91 | P=20.51 |
|
HBME-1/gal-1/TPO | 0.966 | >2 | 95 | 85 | 95 | 87 | P=12.30 |
|
CK19/HBME-1/TPO | 0.966 | >3 | 95 | 87 | 95 | 86 | P=7.68 |
| HBME-1/gal-1 | 0.965 | >2 | 97 | 80 | 96 | 83 | P= 5.16 |
| Gal-3/gal-1 | 0.957 | >0 | 83 | 97 | 85 | 96 | P=11.13 |
| CK19/TPO | 0.949 | >3 | 84 | 86 | 84 | 85 | P=14.29 |
| Gal-1/TPO | 0.942 | >2 | 95 | 84 | 95 | 86 | P=1.91 |
| HBME-1/TPO | 0.936 | >2.5 | 92 | 86 | 92 | 97 | P=1.47 |
Discussion
Although conventional histology and FNA are
considered as gold standards, the pathologists are confronted with
difficulties in reaching an accurate differential diagnosis between
benign and malignant thyroid nodules. To improve disease
identification, immunohistochemical markers, such as gal-3, CK19,
HBME-1 and TPO, have been proposed and their efficiencies for
thyroid cancer diagnosis have been evaluated. CK19 is the smallest
member of cytokeratin family responsible for the structural
integrity of epithelial cells. Several studies reported that CK19
expression is intense and diffuse in PC and absent or low in benign
thyroid lesions (5,6,12–14). Gal-3, a structurally unique member of
galectin family with an N-terminal tail composed of nine
collagen-like repeats (39,40), is associated with the pathogenesis of
well-differentiated thyroid carcinoma (4,7–11). HBME-1 is a surface antigen localized
in the microvilli of the mesothelial cells (41). It had a wider expression in PC
compared to follicular carcinomas and FA (42,43). TPO
is a membrane enzyme involved in the synthesis of thyroid hormones
(44). By contrast, it has been shown
to be relevant in the diagnosis of FAs. Its labeling is negative in
carcinomas regardless of their histopathologic status (papillary,
follicular, medullary or anaplastic) (45,46).
However, the value of clinical use of these markers
is controversial, because positivity was also reported in benign
cases (13–20,41,42).
Mehrotra et al showed that gal-3 was expressed in a large
proportion of FAs, multinodular goiters and Hashimoto's thyroiditis
(16). In the study of Mataraci et
al, CK19 expression was found in adenomatous nodular
hyperplasia and FA (13).
Furthermore, a focal positive labeling may exist in FAs and goiter
for HBME-1 (41,42). In addition, a study by Weber et
al reported to TPO a 50% sensitivity for diagnosis of PC,
suggesting that TPO should be combined with other markers such as
gal-3 (47).
Thus, the current challenge is to find new
immunohistochemical markers that might be more helpful to refine
differential diagnosis between benign and malignant. Widely studied
in other types of cancers, gal-1 remained poorly documented in the
thyroid pathologies (26–28). Because of that, one of the main
contribution of this study to advance the status of the field was
to determine whether gal-1 may be viewed as a complementary
biomarker for diagnosis of thyroid cancer assessing nodular
lesions. In this context, we studied the diagnostic performance of
galectin-1 individually and in combination with four other thyroid
tumor markers used in clinical practice. Our data showed that the
expression of this galectin is significantly higher in PC than in
FA. As a single marker, gal-1 displayed a higher specificity (97%)
than gal-3 and CK19 which showed higher sensitivity (97 and 98%,
respectively). Of note, comparing to the serum levels of both gal-1
and gal-3 (30), our current study on
tissues revealed higher values for the sensitivity and specificity.
Gal-1, Gal-3 and CK19 can be used in association to improve
discrimination between malignant and benign thyroid neoplasms. So,
when we combined two to five markers, we significantly improved the
specificity, the sensitivity as well as the PPVs/NPVs for
malignancy. The association of positivity for gal-3, CK19 and
HBME-1 proved to be the most relevant combination in the
distinction between PCs and FAs. Hence, our data advocate the
concept of the use of combinations of immunomarkers in clinical
practice to diagnose thyroid carcinomas. Evidently, a panel of
markers might be more helpful than the use of a single one to
improve diagnostic accuracy (20,48–51). Of
relevance for the galectin network (52), our data indicate that different
members of this family have non-redundant distribution profiles,
indicating non-overlapping functional spectra. Galectins are widely
distributed in a tissue- and cell-specific manner. This is
particularly emphized for gal-7 and gal-8 which are not
differentially expressed between FA and PC, suggesting that these
galectins are not involved in thyroid carcinogenesis or that
transformation to tumor cells did not impact their expressions.
In summary, the diagnostic problems in thyroid
pathology are still present in many laboratories and this paper can
be potentially useful for improving information. In this study, we
used TMAs to test a panel of markers that also include gal-1, gal-7
and gal-8. We demonstrated that gal-1 is a useful
immunohistochemical marker to discriminate malignant tumors from
benign thyroid nodules. Our observations further validate that
gal-3 is a sensitive marker for the diagnosis of thyroid
malignancy, and we add support for its combination with CK19 and
HBME-1 with the highest performance for the diagnosis of
well-differentiated thyroid cancer. Such combination of markers
should be validated in a larger series of tissues including various
subtypes of thyroid lesions.
Acknowledgements
The study received financial support from the
University of Mons (Mons, Belgium). We thank Dr Köhrle (Institute
of Experimental Endocrinology of the Charité, Humboldt University,
Berlin, Germany) and Professor C. Maenhout (IRIBHM ULB, Brussels,
Belgium) for providing the FTC133C and 8505C cell lines (derived
from follicular and anaplastic thyroid carcinoma respectively).
References
|
1
|
Hegedüs L: Thyroid ultrasonography as a
screening tool for thyroid disease. Thyroid. 14:879–880. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gharib H and Papini E: Thyroid nodules:
Clinical importance, assessment and treatment. Endocrinol Metab
Clin North Am. 36707–735. (vi)2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams ED: Guest editorial: Two
proposals regarding the terminology of thyroid tumors. Int J Surg
Pathol. 8:181–183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Z, Li X, Shi L, Maimaiti Y, Chen T, Li
Z, Wang S, Xiong Y, Guo H, He W, et al: Cytokeratin 19,
thyroperoxidase, HBME-1 and galectin-3 in evaluation of aggressive
behavior of papillary thyroid carcinoma. Int J Clin Exp Med.
7:2304–2308. 2014.PubMed/NCBI
|
|
5
|
Flanagan JN, Pineda P, Knapp PE, De Las
Morenas A, Lee SL and Braverman LE: Expression of cytokeratin 19 in
the diagnosis of thyroid papillary carcinoma by quantitative
polymerase chain reaction. Endocr Pract. 14:168–174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krzeslak A, Gaj Z, Pomorski L and Lipinska
A: Expression of cytokeratin 19 in the cytosolic fraction of
thyroid lesions: ELISA and western blot analysis. Mol Med Rep.
1:565–569. 2008.PubMed/NCBI
|
|
7
|
Sumana BS, Shashidhar S and Shivarudrappa
AS: Galectin-3 immunohistochemical expression in thyroid neoplasms.
J Clin Diagn Res. 9:EC07–EC11. 2015.PubMed/NCBI
|
|
8
|
Bartolazzi A, Gasbarri A, Papotti M,
Bussolati G, Lucante T, Khan A, Inohara H, Marandino F, Orlandi F,
Nardi F, et al: Application of an immunodiagnostic method for
improving preoperative diagnosis of nodular thyroid lesions.
Lancet. 357:1644–1650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inohara H, Honjo Y, Yoshii T, Akahani S,
Yoshida J, Hattori K, Okamoto S, Sawada T, Raz A and Kubo T:
Expression of galectin-3 in fine-needle aspirates as a diagnostic
marker differentiating benign from malignant thyroid neoplasms.
Cancer. 85:2475–2484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gasbarri A, Martegani MP, Del Prete F,
Lucante T, Natali PG and Bartolazzi A: Galectin-3 and CD44v6
isoforms in the preoperative evaluation of thyroid nodules. J Clin
Oncol. 17:3494–3502. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carpi A, Rossi G, Coscio GD, Iervasi G,
Nicolini A, Carpi F, Mechanick JI and Bartolazzi A: Galectin-3
detection on large-needle aspiration biopsy improves preoperative
selection of thyroid nodules: A prospective cohort study. Ann Med.
42:70–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Yu P, Xiong Y, Zeng W, Li X,
Maiaiti Y, Wang S, Song H, Shi L, Liu C, et al: Significance of
CK19, TPO, and HBME-1 expression for diagnosis of papillary thyroid
carcinoma. Int J Clin Exp Med. 8:4369–4374. 2015.PubMed/NCBI
|
|
13
|
Mataraci EA, Ozgüven BY and Kabukçuoglu F:
Expression of cytokeratin 19, HBME-1 and galectin-3 in neoplastic
and nonneoplastic thyroid lesions. Pol J Pathol. 63:58–64.
2012.PubMed/NCBI
|
|
14
|
Schmitt AC, Cohen C and Siddiqui MT:
Paired box gene 8, HBME-1, and cytokeratin 19 expression in
preoperative fine-needle aspiration of papillary thyroid carcinoma:
Diagnostic utility. Cancer Cytopathol. 118:196–202. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niedziela M, Maceluch J and Korman E:
Galectin-3 is not an universal marker of malignancy in thyroid
nodular disease in children and adolescents. J Clin Endocrinol
Metab. 87:4411–4415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehrotra P, Okpokam A, Bouhaidar R,
Johnson SJ, Wilson JA, Davies BR and Lennard TW: Galectin-3 does
not reliably distinguish benign from malignant thyroid neoplasms.
Histopathology. 45:493–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mills LJ, Poller DN and Yiangou C:
Galectin-3 is not useful in thyroid FNA. Cytopathology. 16:132–138.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park YJ, Kwak SH, Kim DC, Kim H, Choe G,
Park DJ, Jang HC, Park SH, Cho BY and Park SY: Diagnostic value of
galectin-3, HBME-1, cytokeratin 19, high molecular weight
cytokeratin, cyclin D1 and p27(kip1) in the differential diagnosis
of thyroid nodules. J Korean Med Sci. 22:621–628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu X, Sun T, Lu H, Zhou X, Lu Y, Cai X
and Zhu X: Diagnostic significance of CK19, RET, galectin-3 and
HBME-1 expression for papillary thyroid carcinoma. J Clin Pathol.
63:786–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barroeta JE, Baloch ZW, Lal P, Pasha TL,
Zhang PJ and LiVolsi VA: Diagnostic value of differential
expression of CK19, Galectin-3, HBME-1, ERK, RET and p16 in benign
and malignant follicular-derived lesions of the thyroid: An
immunohistochemical tissue microarray analysis. Endocr Pathol.
17:225–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Danguy A, Camby I and Kiss R: Galectins
and cancer. Biochim Biophys Acta. 1572:285–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Demydenko D and Berest I: Expression of
galectin-1 in malignant tumors. Exp Oncol. 31:74–79.
2009.PubMed/NCBI
|
|
23
|
Balan V, Nangia-Makker P and Raz A:
Galectins as cancer biomarkers. Cancers (Basel). 2:592–610. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smetana K Jr, André S, Kaltner H, Kopitz J
and Gabius HJ: Context-dependent multifunctionality of galectin-1:
A challenge for defining the lectin as therapeutic target. Expert
Opin Ther Targets. 17:379–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiariotti L, Berlingieri MT, Battaglia C,
Benvenuto G, Martelli ML, Salvatore P, Chiappetta G, Bruni CB and
Fusco A: Expression of galectin-1 in normal human thyroid gland and
in differentiated and poorly differentiated thyroid tumors. Int J
Cancer. 64:171–175. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu XC, el-Naggar AK and Lotan R:
Differential expression of galectin-1 and galectin-3 in thyroid
tumors. Potential diagnostic implications. Am J Pathol.
147:815–822. 1995.PubMed/NCBI
|
|
27
|
Salajegheh A, Dolan-Evans E, Sullivan E,
Irani S, Rahman MA, Vosgha H, Gopalan V, Smith RA and Lam AK: The
expression profiles of the galectin gene family in primary and
metastatic papillary thyroid carcinoma with particular emphasis on
galectin-1 and galectin-3 expression. Exp Mol Pathol. 96:212–218.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Torres-Cabala C, Bibbo M, Panizo-Santos A,
Barazi H, Krutzsch H, Roberts DD and Merino MJ: Proteomic
identification of new biomarkers and application in thyroid
cytology. Acta Cytol. 50:518–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paron I, D'Ambrosio C, Scaloni A,
Berlingieri MT, Pallante PL, Fusco A, Bivi N, Tell G and Damante G:
A differential proteomic approach to identify proteins associated
with thyroid cell transformation. J Mol Endocrinol. 34:199–207.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saussez S, Glinoer D, Chantrain G, Pattou
F, Carnaille B, André S, Gabius HJ and Laurent G: Serum galectin-1
and galectin-3 levels in benign and malignant nodular thyroid
disease. Thyroid. 18:705–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanchez-Ruderisch H, Fischer C, Detjen KM,
Welzel M, Wimmel A, Manning JC, André S and Gabius HJ: Tumor
suppressor p16 INK4a: Downregulation of galectin-3, an endogenous
competitor of the pro-anoikis effector galectin-1, in a pancreatic
carcinoma model. FEBS J. 277:3552–3563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weinmann D, Schlangen K, André S, Schmidt
S, Walzer SM, Kubista B, Windhager R, Toegel S and Gabius HJ:
Galectin-3 induces a pro-degradative/inflammatory gene signature in
human chondrocytes, teaming up with galectin-1 in osteoarthritis
pathogenesis. Sci Rep. 6:391122016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verhulst P, Devos P, Aubert S, Buob D,
Cranshaw I, Do Cao C, Pattou F, Carnaille B, Wemeau JL and
Leteurtre E: A score based on microscopic criteria proposed for
analysis of papillary carcinoma of the thyroid. Virchows Arch.
452:233–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Toegel S, Bieder D, André S, Kayser K,
Walzer SM, Hobusch G, Windhager R and Gabius HJ: Human
osteoarthritic knee cartilage: Fingerprinting of
adhesion/growth-regulatory galectins in vitro and in situ indicates
differential upregulation in severe degeneration. Histochem Cell
Biol. 142:373–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaltner H, Seyrek K, Heck A, Sinowatz F
and Gabius HJ: Galectin-1 and galectin-3 in fetal development of
bovine respiratory and digestive tracts. Comparison of cell
type-specific expression profiles and subcellular localization.
Cell Tissue Res. 307:35–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Langbein S, Brade J, Badawi JK, Hatzinger
M, Kaltner H, Lensch M, Specht K, André S, Brinck U, Alken P and
Gabius HJ: Gene-expression signature of adhesion/growth-regulatory
tissue lectins (galectins) in transitional cell cancer and its
prognostic relevance. Histopathology. 51:681–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Danguy A, Rorive S, Decaestecker C,
Bronckart Y, Kaltner H, Hadari YR, Goren R, Zich Y, Petein M,
Salmon I, et al: Immunohistochemical profile of galectin-8
expression in benign and malignant tumors of epithelial,
mesenchymatous and adipous origins, and of the nervous system.
Histol Histopathol. 16:861–868. 2001.PubMed/NCBI
|
|
38
|
Saiselet M, Floor S, Tarabichi M, Dom G,
Hébrant A, van Staveren WC and Maenhaut C: Thyroid cancer cell
lines: An overview. Front Endocrinol (Lausanne).
3:1332012.PubMed/NCBI
|
|
39
|
Kopitz J, Vértesy S, André S, Fiedler S,
Schnölzer M and Gabius HJ: Human chimera-type galectin-3: Defining
the critical tail length for high-affinity glycoprotein/cell
surface binding and functional competition with galectin-1 in
neuroblastoma cell growth regulation. Biochimie. 104:90–99. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ippel H, Miller MC, Vértesy S, Zheng Y,
Cañada FJ, Suylen D, Umemoto K, Romanò C, Hackeng T, Tai G, et al:
Intra- and intermolecular interactions of human galectin-3:
Assessment by full-assignment-based NMR. Glycobiology. 26:888–903.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rigau V, Martel B, Evrard C, Rousselot P
and Galateau-Salle F: HBME-1 immunostaining in thyroid pathology.
Ann Pathol. 21:15–20. 2001.(In French). PubMed/NCBI
|
|
42
|
Prasad ML, Pellegata NS, Huang Y, Nagaraja
HN, de la Chapelle A and Kloos RT: Galectin-3, fibronectin-1,
CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful
for the differential diagnosis of thyroid tumors. Mod Pathol.
18:48–57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu YY, Morreau H, Kievit J, Romijn JA,
Carrasco N and Smit JW: Combined immunostaining with galectin-3,
fibronectin-1, CITED-1, Hector Battifora mesothelial-1,
cytokeratin-19, peroxisome proliferator-activated receptor-{gamma},
and sodium/iodide symporter antibodies for the differential
diagnosis of non-medullary thyroid carcinoma. Eur J Endocrinol.
158:375–384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Micco C, Savchenko V, Giorgi R, Sebag F
and Henry JF: Utility of malignancy markers in fine-needle
aspiration cytology of thyroid nodules: Comparison of Hector
Battifora mesothelial antigen-1, thyroid peroxidase and dipeptidyl
aminopeptidase IV. Br J Cancer. 98:818–823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
De Micco C, Ruf J, Chrestian MA, Gros N,
Henry JF and Carayon P: Immunohistochemical study of thyroid
peroxidase in normal, hyperplastic, and neoplastic human thyroid
tissues. Cancer. 67:3036–3041. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Faggiano A, Caillou B, Lacroix L, Talbot
M, Filetti S, Bidart JM and Schlumberger M: Functional
characterization of human thyroid tissue with immunohistochemistry.
Thyroid. 17:203–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weber KB, Shroyer KR, Heinz DE, Nawaz S,
Said MS and Haugen BR: The use of a combination of galectin-3 and
thyroid peroxidase for the diagnosis and prognosis of thyroid
cancer. Am J Clin Pathol. 122:524–531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
de Matos LL, Del Giglio AB, Matsubayashi
CO, de Lima Farah M, Del Giglio A and da Silva Pinhal MA:
Expression of CK-19, galectin-3 and HBME-1 in the differentiation
of thyroid lesions: Systematic review and diagnostic meta-analysis.
Diagn Pathol. 7:972012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dunđerović D, Lipkovski JM, Boričic I,
Soldatović I, Božic V, Cvejić D and Tatić S: Defining the value of
CD56, CK19, Galectin 3 and HBME-1 in diagnosis of follicular cell
derived lesions of thyroid with systematic review of literature.
Diagn Pathol. 10:1962015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
de Matos PS, Ferreira AP, de Oliveira
Facuri F, Assumpção LV, Metze K and Ward LS: Usefulness of HBME-1,
cytokeratin 19 and galectin-3 immunostaining in the diagnosis of
thyroid malignancy. Histopathology. 47:391–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cheung CC, Ezzat S, Freeman JL, Rosen IB
and Asa SL: Immunohistochemical diagnosis of papillary thyroid
carcinoma. Mod Pathol. 14:338–342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kaltner H, Toegel S, Caballero GG, Manning
JC, Ledeen RW and Gabius HJ: Galectins: Their network and roles in
immunity/tumor growth control. Histochem Cell Biol. 147:239–256.
2017. View Article : Google Scholar : PubMed/NCBI
|