Introduction
Primary hepatocarcinoma, which arises from liver
cells or intrahepatic bile duct epithelial cells, is one of the
most fatal types of malignant tumors worldwide (1), causing 250,000 to 1,000,000 mortalities
per year, and has become the fifth and seventh most common
malignant tumor in males and females, respectively (2). The majority of hepatocarcinoma cases
occur in a number of developing countries (3). China has the highest morbidity and
mortality associated with hepatocarcinoma; the number of patients
with hepatocarcinoma in China accounts for 54% of the cases
worldwide (4). The preferred
treatment for hepatocarcinoma is liver resection, but this is
restricted to early stages of hepatocarcinoma (5). Due to the difficulty of early detection,
the rapid progression of the disease and the fact that the majority
of patients exhibit liver cirrhosis, only a limited number of
patients are able to undergo surgery, resulting in the majority of
patients exhibiting a poor prognosis (6). Therefore, the identification of a
specific tumor marker, for early diagnosis and targeted therapy, is
required.
Proton-coupled oligopeptide transporter 1 (PEPT1) is
one of the four members of the peptide transporter superfamily in
mammalian cells (7). PEPT1 is
expressed predominantly in the intestine and mediates the
absorption of dietary nutrients (di/tripeptide) or peptidomimetic
drugs (8). As PEPT1 is able to
transport a broad spectrum of substrates, it is an attractive
target for drug delivery (9).
Previous studies on the presence and function of PEPT1 in
carcinomas was limited in a number of carcinoma cell lines,
including colon carcinoma Caco-2 cells (10), pancreatic carcinoma cell lines AsPc-1
and Capan-29 (11), gastric cancer
(12), prostate cancer (13,14) and
fibroblast-derived tumor cells (15),
which indicates that PEPT1 may be expressed in other types of
cancer cells. The overexpression of PEPT1 in cancer cells may
enable the identification of a specific pathway for therapeutic
agents to exploit. Therefore, clarifying the expression patterns of
PEPT1 in other types of cancer cell may expand the value of peptide
transporters in cancer therapy.
In the present study, the expression profile and
function of PEPT1 in hepatocarcinoma were investigated in
hepatocarcinoma cells and tissues. The results of the present study
may provide novel information on the expression and function of
PEPT1 in human hepatocarcinoma, and expand the values for
hepatocarcinoma early diagnosis and tumor specific drug
delivery.
Materials and methods
Materials
D-Ala-Lys-AMCA (2.5 mg/ml) was purchased from
BIOTREND Chemikalien GmbH (Köln, Germany), and glycine
(Gly)-sarcosine (Sar), Gly-glutamine (Gln) and Gly-Gly-Gly were
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
PCR primers used in the present study were synthesized by
Integrated DNA Technologies, Inc. (Coralville, IA, USA). A rabbit
polyclonal antibody against PEPT1 (anti-PEPT1 antibody; catalog no.
78020; dilution, 1:50) was provided by Abcam (Cambridge, UK).
Additionally, the human liver cancer Bel-7402, SMMC7721, Hep3B,
HepG2, and SK-HEP-1cell lines, the human gastric cancer BGC-823
cell line and the human colon cancer Caco-2 cell line were obtained
from the Academia Sinica Cell Repository (Shanghai, China).
Cell culture
Bel-7402 cells were grown in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), whereas SK-HEP-1 cells were cultured in
Dulbecco's modified Eagle medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) medium containing 10% FBS, 100 U/ml penicillin
and 100 µg/l streptomycin. Concurrently, SMMC7721, Hep3B, HepG2 and
Caco-2 cell lines were maintained in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 1% 100 µg/ml penicillin (Sigma-Aldrich; Merck
KGaA) and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA). All
of the cells were maintained at 37°C in 5% CO2. The
culture media were changed every other day, and cells were passaged
once they reached 80–90% confluence, at which point the cells were
collected for protein or RNA extraction.
Immunofluorescence of cell lines
Subsequent to attachment onto a plate,
1×105 cells/ml (Bel-7402, SK-HEP-1, SMMC7721, Hep3B,
HepG2 and Caco-2) were washed with medium, fixed with 4%
paraformaldehyde for 5 min at room temperature, washed three times
with PBS, treated with bovine serum albumin, (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 1 h, and
subsequently incubated with the rabbit anti-humananti-PEPT1 primary
antibody (1:50 dilution) overnight at 4°C. Following three washes
with PBS, the cells were incubated with a fluorescent goat
anti-rabbit IgG secondary antibody (dilution, 1:100; catalog no.
ZF036; Beijing ZSGB-BIO Co. Ltd., China) for 1 h at 37°C, washed
with PBS, stained with DAPI (5 µg/ml) for 5 min at room
temperature, fixed, mounted, and observed using a fluorescent
microscope (magnification, ×20) to determine blue (nucleus) and red
(PEPT1) fluorescence. Each experiment was repeated three times and
Caco-2 cell lines was used as the positive control.
Western blotting
All the cell types (Bel-7402, SK-HEP-1, SMMC7721,
BGC-823, Hep3B, HepG2 and Caco-2) were seeded (1×107
cells/ml) in 100-mm cell culture dishes. Total protein was then
extracted from the cells using 1% Nonidet P-40 lysis buffer
(Sigma-Aldrich; Merck KGaA). The protein concentration was measured
using the Lowry method. The protein lysates (30 µg protein/lane)
were subsequently separated using 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes (PVDF; EMD Millipore, Billerica,
MA, USA). The blocking reagent used was 5% bicinchoninic acid, and
the PVDF membranes were blocked at 37°C for 1 h. These membranes
were incubated with the primary antibody, as aforementioned
(dilution, 1:100), followed by incubation with a horseradish
peroxidase-conjugated secondary antibody (dilution, 1:100; catalog
no. ZB-2301; Beijing ZSGB-BIO Co. Ltd) at room temperature. Protein
expression was visualized using a Super Signal Protein Detection
kit (Pierce; Thermo Fisher Scientific, Inc.). The membranes were
then stripped and re-probed with anti-PEPT1 (dilution, 1:1,000),
and an anti-β-actin primary antibody (dilution, 1:1,000; catalog
no. sc-47778; Santa Cruz Biotechnology, Inc.) served as a loading
control. Each experiment was repeated three times.
Reverse
transcription-quantitative-polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from all of the cell types
(Bel-7402, SK-HEP-1, SMMC7721, BGC-823, Hep3B, HepG2 and Caco-2)
using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and then used for cDNA synthesis by RT with M-MLV Reverse
Transcriptase (Promega Corporation, Madison, WI, USA) in a total
volume of 10 µl according to the manufacturer's protocol. The
reverse transcription RT-PCR products were separated by
electrophoresis on a 2% agarose gel. The gel was then stained with
ethidium bromide, digitally photographed, and scanned with a UVI
gel analysis system (UVItec, Cambridge, UK). Using a 7500 ABI
RT-PCR machine (Applied Biosystems; Thermo Fisher Scientific,
Inc.), expression levels were quantitatively analyzed. The TaqMan
assay kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) was
used to detect gene expression. Quantification was also performed
using amplification efficiencies derived from cDNA standard curves
to obtain the relative gene expression. The data are presented as
fold changes (2−ΔΔCq) (16) and were initially analyzed using the
Opticon Monitor V2.02 analysis software (MJ Research; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Specific RT-PCR primers
were obtained from Fulen Gene BioEngineering Inc. (Guangdong,
China). The primers for PEPT1 were 5′-GCTCGGTTCTATACTTACATC-3′
(forward) and 5′-TCCATCCTCCACTTGCCT-3′ (reverse) (10). The primers for GAPDH as the reference
gene were: 5′-AGGTCGGTGTGACGTTACGG-3′ (forward) and
5′-GGGGTCGTTGATGGCAACAA-3′ (reverse). Each was performed in
triplicate. The thermocycling conditions were as follows: 95°C for
3 min; 45 cycles of 95°C for 15 sec, 58°C for 30 sec and 72°C for
30 sec; and 72°C for 2 min.
Verification of D-Ala-Lys-AMCA
D-Ala-Lys-AMCA is a known PEPT1 substrate that emits
blue fluorescence. All cell types were cultured in 24-well plates
for 1 day, and the culture medium was then aspirated. Following one
wash with PBS, D-Ala-Lys-AMCA (1 mM in PBS) was added for 2 h at
37°C. D-Ala-Lys-AMCA fluorescence was then observed through a
fluorescence microscope (magnification, ×20).
The uptake of D-Ala-Lys-AMCA was detected under
different conditions. First, the uptake of D-Ala-Lys-AMCA at
different times (0, 15, 30, 60, 120 and 180 min) but at the same pH
value (6.0) and concentration (25 µmol/l) was detected. Second,
D-Ala-Lys-AMCA uptake at different pH levels (5.4, 6, 7.4 and 8.4)
but at the same time (2 h) and concentrations (25 µmol/l) was
detected. Third, the effects of different initial concentrations of
D-Ala-Lys-AMCA (25, 50 and 150 µmol/l) were analyzed, and these
tests were performed at a pH of 6.0 for 2 h. Inhibition tests were
conducted by pre-incubating the cells with competitive compounds
(Gly-Sar, Gly-Gln, Gly-Gly-Gly) for 30 min and then with
D-Ala-Lys-AMCA (25 µmol/l) for 1 h at 37°C prior to the detection
of fluorescence. Subsequent to removing the buffer and rapidly
washing 3 times with ice-cold PBS, the cellular uptake of
D-Ala-Lys-AMCA was examined with a BioTek Synergy 2 Multi-Mode
reader (BioTek Instruments, Inc., Winooski, VT, USA) with
excitation at 350 nm, and emission at 460 nm. All analyses were
performed in triplicate.
Immunohistochemistry and
immunofluorescence staining
A total of 82 human hepatocarcinoma tissue chips
were purchased from Xi'an Alenabio Technology Co., Ltd. (Xi'an,
China), which included 50 cases of hepatocarcinoma tissues
(pathological grade 1, 4 cases; grade 2, 20 cases; and grade 3, 26
cases), 13 cases of adjacent cancer tissues and 19 cases of normal
liver tissues. Pathological grades 1, 2 and 3 were equivalent to
well-, moderately- and poorly-differentiated, respectively
(17). The expression of PEPT1 in
these tissues was detected by immunohistochemistry. For
immunohistochemical staining, formalin-fixed tissue samples were
prepared as paraffin-embedded sections (thickness, 3 µm), and
immunostaining of the sections was performed using the
avidin-biotin-complex method: Primary antibody directed against
PEPT1 (1:100 dilution) was diluted in PBS with 0.1% Tween and
incubated with the sections overnight at 4°C. The sections were
then incubated with biotinylated secondary antibodies (dilution,
1:100; catalog no. ZB-2010; Beijing ZSGB-BIO Co. Ltd.) for 1 h at
37°C, followed by the avidin-biotin complex for an additional 1 h
at 37°C. Protein expression was detected via coloration with
3,3′-diaminobenzidine in buffer, and the sections were
counterstained with hematoxylin (2 mg/ml) at room temperature for 5
min. Using a microcamera computational image analysis system
(Cellsens standard; version 1.6; Olympus Corporation, Tokyo,
Japan), a nucleus or cytoplasm containing brown-colored particles
was considered positive. A total of five high-power fields were
randomly selected for each group, and a total of 250 cells were
counted. Sections with no labeling, or with <5% labeled cells,
were scored as 0. Sections with 5–30% positive cells were scored as
1, 31–70% positive cells as 2, and ≥71% positive cells as 3.
Staining intensity was scored similarly, with 0 for negative
staining, 1 for weakly positive, 2 for moderately positive, and 3
for strongly positive. Scores for the percentage of positive tumor
cells and staining intensity were used to generate an
immunoreactive score for each specimen. The quantity and intensity
scores were calculated such that a final score of 0–1 indicated
negative expression (−), 2–3 indicated weak expression (+), 4–5
indicated moderate expression (++), and 6 indicated strong
expression (+++) (11).
Statistical analysis
Significance of Kaplan-Meier statistics was tested
by calculating the log-rank. Data are expressed as the mean ±
standard deviation. SPSS software (version 16.0; SPSS, Inc.,
Chicago, IL, USA) was used for all calculations. P<0.05 was
considered to indicate a statistically significant difference.
Results
Immunofluorescence
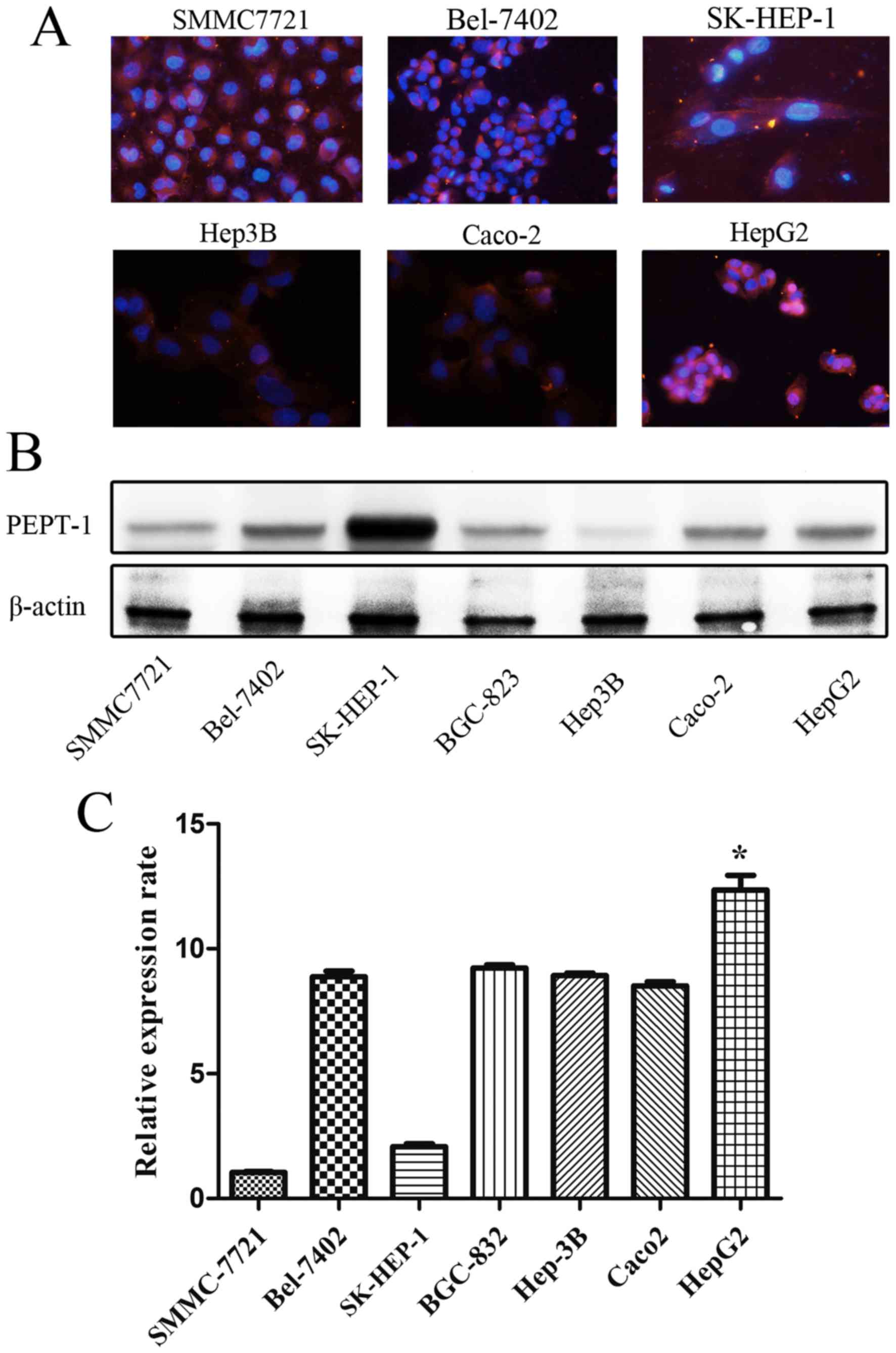
Cell nuclei were labeled with blue fluorescence and
red fluorescence indicated the presence of PEPT1 using the image
system Cellsens standard (version 1.6; Olympus Corporation). All
types of liver cancer cells emitted red fluorescence, as determined
using microscopy (Fig. 1).
Western blotting
The results demonstrated that PEPT1 was expressed in
the five liver cancer cell types studied (Bel-7402, SMMC7721,
Hep3B, HepG2 and SK-HEP-1) to different degrees with SK-HEP-1 cells
expressing the highest levels of PEPT1 (Fig. 1).
RT-qPCR
The results revealed that increased PEPT1 mRNA
expression was present in the majority of liver cancer cells
compared with Caco2 cell lines. Furthermore, a markedly increased
expression of the PEPT1 protein was observed in HepG2 cells
compared with the other cell types, as determined by RT-qPCR
(Fig. 1).
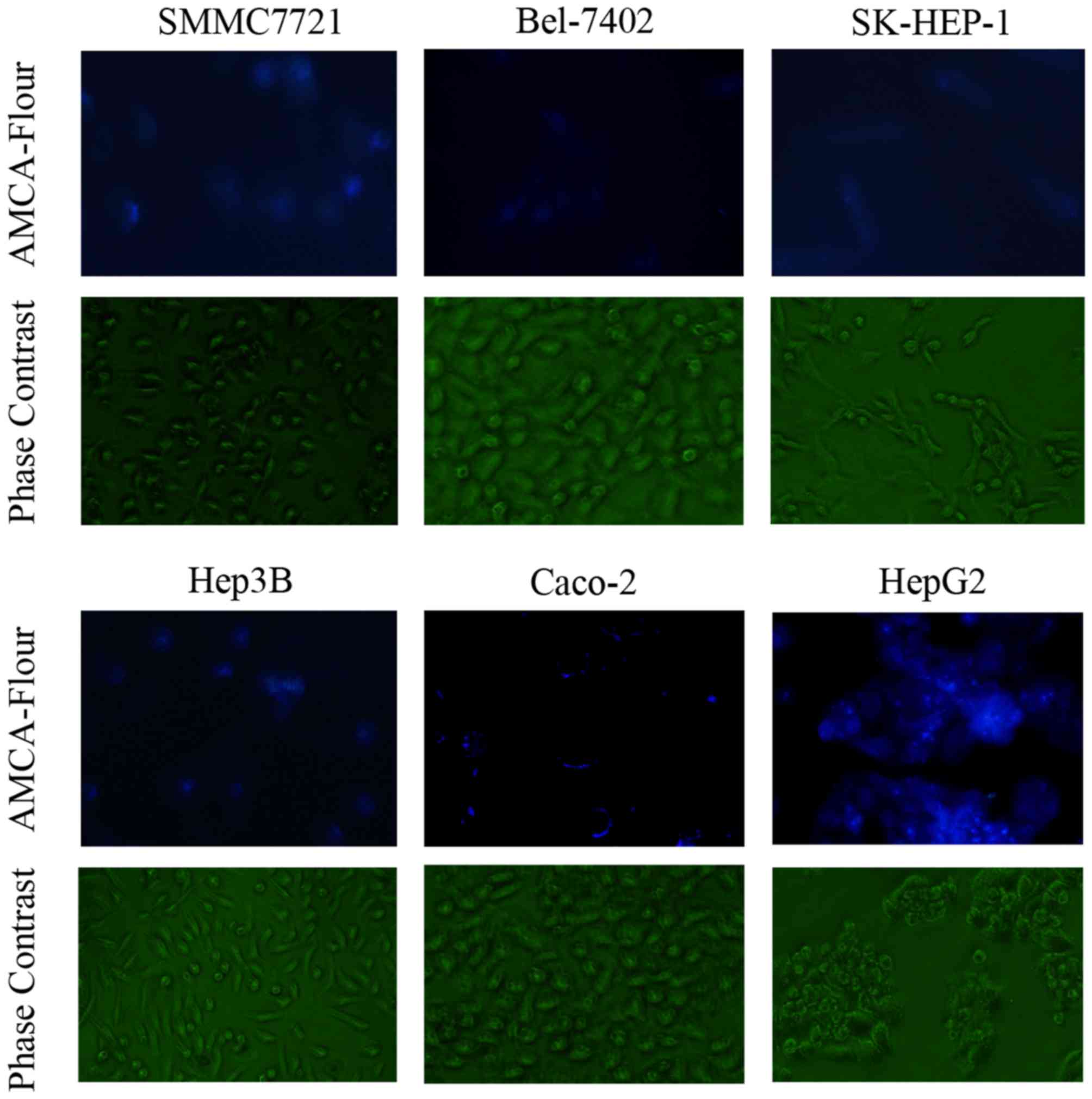
Verification of D-Ala-Lys-AMCA
Fluorescence of D-Ala-Lys-AMCA
D-Ala-Lys-AMCA is a well-known PEPT1 substrate that
emits blue fluorescence. The results of the present study validated
that D-Ala-Lys-AMCA may be transported into liver cancer and Caco-2
cells, on the basis of the emission of blue fluorescence (Fig. 2).
Uptake of D-Ala-Lys-AMCA
The uptake of Ala-Lys-AMCA was time-dependent and
also concentration-dependent, but not pH-dependent. The maximum
uptake occurred at a pH value of 7.4. Additionally, uptake was
significantly decreased by the presence of Gly-Sar, Gly-Gln or
Gly-Gly-Gly inhibitors (Fig. 3).
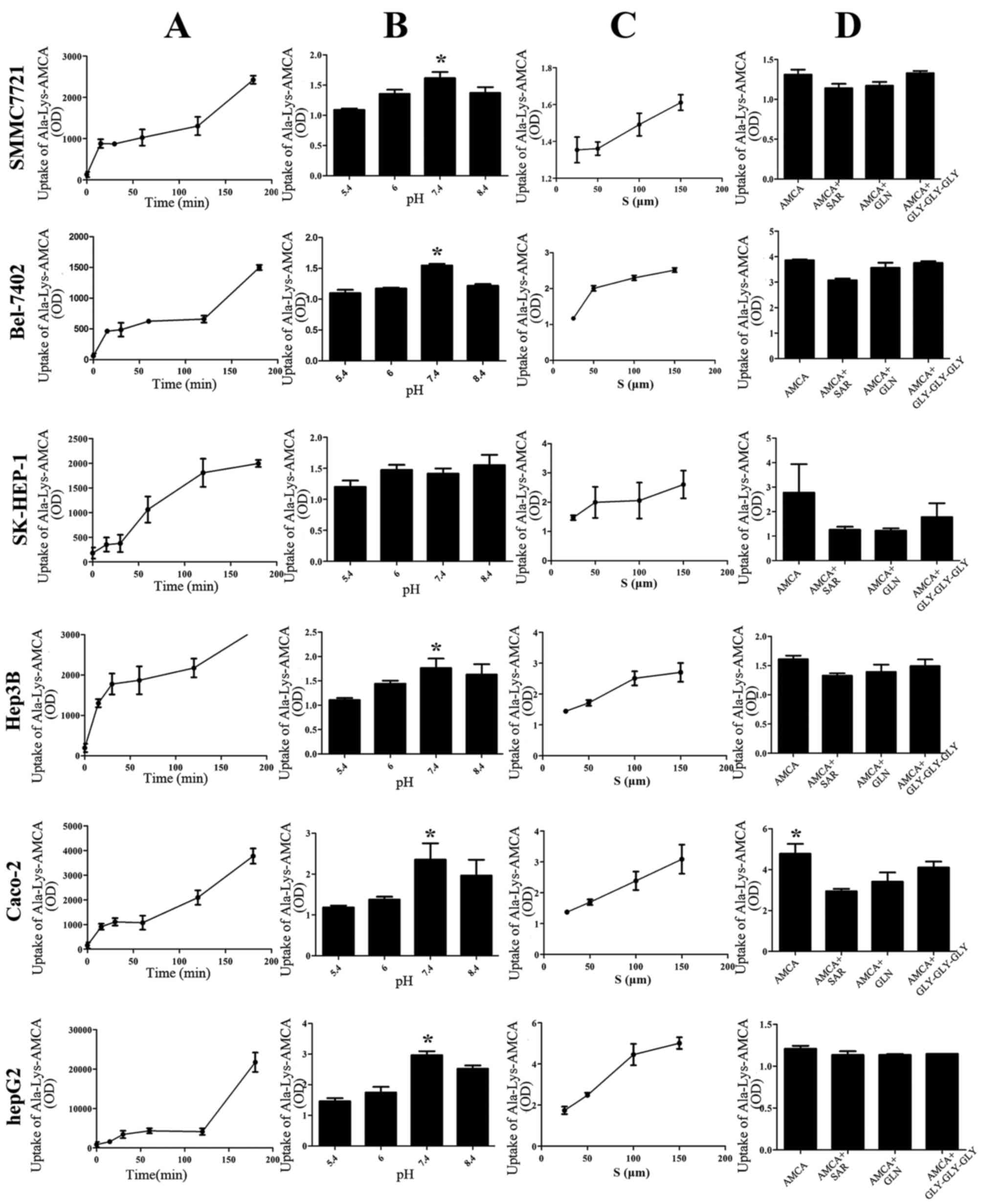 | Figure 3.Uptake of Ala-Lys-AMCA under
different conditions in five liver cancer cell types. (A) The
uptake of D-Ala-Lys-AMCA at different times (0,15,30,60,120 and 180
min), at pH 6.0 and a concentration of 25 µmol/l. (B) The uptake of
D-Ala-Lys-AMCA at different pH values (5.4, 6.0, 7.4, 8.4) for 120
min at a concentration of 25 µmol/l. (C) The uptake of
D-Ala-Lys-AMCA at different concentrations (25, 50 and 150 µmol/l),
at pH 6.0 for 120 min. (D) Inhibition tests with different
competitive compounds (Gly-Sar, Gly-Gln, Gly-Gly-Gly). OD, optical
density; S, substrates.*P<0.05. |
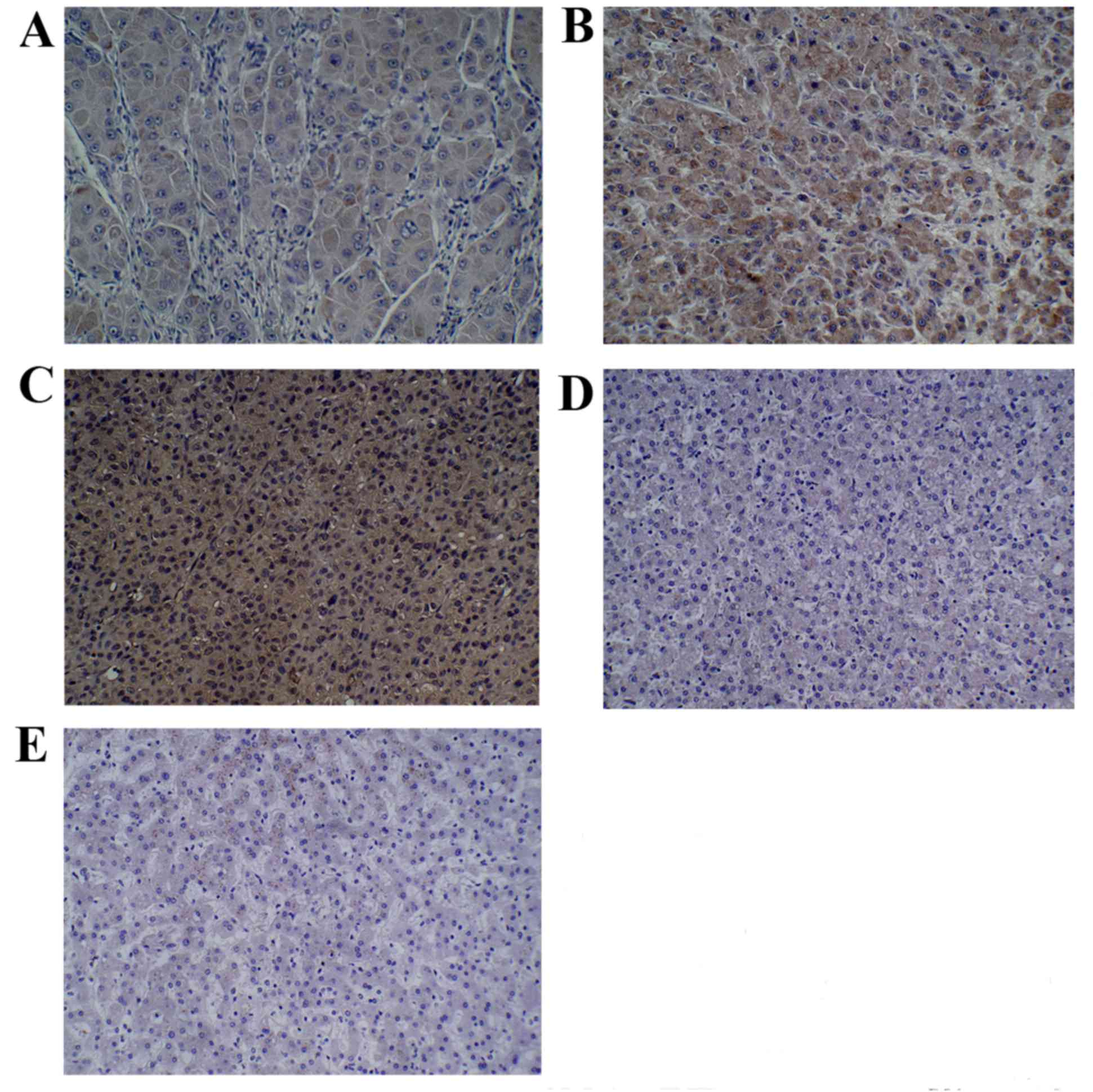
Immunohistochemistry
The results suggested that each group exhibited
PEPT1 expression to different degrees (Fig. 4). Specifically, the expression of
PEPT1 in hepatocarcinoma tissue was significantly higher compared
with the expression observed in adjacent and normal liver tissue
samples (P=0.0193 and P=0.0057, respectively, Table I). Significant differences in the
expression of PEPT1 between three different pathological grades of
liver cancer were observed, with tissues with a higher pathological
grade demonstrating greater expression of PEPT1 (P=0.0093; Table II).
 | Table I.Expression of PEPT1 in different
liver tissues. |
Table I.
Expression of PEPT1 in different
liver tissues.
|
|
| PEPT1
expression |
|
|---|
|
|
|
|
|
|---|
| Tissue type | n | − | + | ++ | +++ | Positive rate,
% |
|---|
|
Hepatocarcinoma | 50 | 7 | 6 | 12 | 25 | 86.00 |
| Adjacent
cancer | 13 | 6 | 4 | 2 | 1 | 53.85a |
| Normal liver | 19 | 9 | 5 | 4 | 1 | 52.63b |
 | Table II.Expression of PEPT1 in different
pathological hepatocarcinoma grades. |
Table II.
Expression of PEPT1 in different
pathological hepatocarcinoma grades.
|
|
| PEPT1
expression |
|
|---|
|
|
|
|
|
|---|
| Pathological
grade | n | − | + | ++ | +++ | Positive rate,
% |
|---|
| 1 | 4 | 3 | 1 | 0 | 0 | 25.00 |
| 2 | 20 | 2 | 2 | 6 | 10 | 90.00 |
| 3 | 26 | 2 | 3 | 6 | 15 | 92.31a |
Discussion
Hepatocarcinoma is one of the most common malignant
tumors worldwide (17). Each year,
>700,000 incident patients are diagnosed, and ~250,000 people
succumb to liver cancer (18). In
China, the hepatocarcinoma incidence is expected to markedly
increase over the prospective decades, due to the increasing
incidence of viral hepatitis infection, which is one of the most
important pathogenic factors for the development of hepatocarcinoma
(19). The preferred treatment for
hepatocarcinoma is liver resection, but this treatment is
restricted to patients with the very early stages of
hepatocarcinoma (5). Due to the
difficulty associated with achieving early diagnosis, the rapid
progression of the disease and the fact that the majority of
patients exhibit liver cirrhosis, few patients are able to undergo
the operation, resulting in the majority of patients having a poor
prognosis. Thus, non-operative therapy is an important treatment
strategy for advanced hepatocarcinoma (19). At present, chemotherapy drugs commonly
used in the clinical treatment of primary hepatocarcinoma include
5-fluorouracil, mitomycin, doxorubicin and epirubicin, among others
(20). However, as chemotherapy is
not specific to tumor cells, it may also affect normal cells and
result in serious adverse effects. Transcatheter arterial
chemoembolization and other local treatments may transport drugs
directly to lesions, but the effects of the dissemination of
satellite foci and portal vein tumor thrombi are limited, and these
therapies rarely control metastases that are distant from the
lesions (21). Sorafenib is one
targeted agent that serves an essential role in the treatment of
advanced hepatocarcinoma, but high concentrations are required
(22). Other novel targeted agents
remain in the trial stage and require additional investigation
(23). Therefore, the currently
available therapies offer limited benefits to patients. As a
result, there is a need for the development of novel and improved
therapeutic strategies.
A targeted drug delivery system that may be used as
an effective specific treatment may have good application
prospects. In particular, targeted drug delivery is a system that
uses a drug carrier that transports chemotherapy drugs to the
specific location of a tumor, and may achieve directional and focal
inhibition of the tumor cells, thus causing less injury to normal
cells. This system has numerous advantages. For example, the drug
maybe specifically transported to the target area, reach the
maximum drug concentration in the target area, and react directly
at the lesion site. Thus, this may promote the highest treatment
effects with minimal toxic effects on normal cells, resulting in
increased overall efficacy, safety and patient compliance with
chemotherapy (24). The
identification of a safe and effective targeted drug carrier is a
focus of ongoing study.
Previous studies have suggested that
peptide-targeted gold nanoparticles may serve as drug carriers for
the treatment of brain cancer (25)
and that biodegradable nanoparticles may deliver docetaxel to
airway cancer cells in a targeted manner (26). In addition, a study of drug carriers
for prostate cancer revealed that a peptide-drug conjugate
exhibited markedly higher uptake by prostate cancer cells in
comparison with the parent drug (27). Thus, the selection of an effective
targeted carrier based on its specific oligopeptide transport
activity is essential (28). If PEPT1
is specifically expressed in liver cancer cells and tissues, it may
be a promising carrier for the delivery of chemotherapy drugs to a
targeted region, with increased efficacy and decreased adverse
effects on healthy tissues.
Peptides and peptide analogs may enter the cells of
the body via peptide transporters in the membrane. The most widely
studied peptide transporters are PEPT1 and PEPT2, which are members
of the POT family (29). In mammals,
the POT family comprises the following 4members, which are encoded
by Solute carrier family 15 (SLC15) genes: PEPT1
(SLC15A1), PEPT2 (SLC15A2), peptide/histidine
transporter 1 (PHT1; SLC15A4), and PHT2 (SLC15A3)
(30). The functional expression of
PEPT1 and PEPT2 has been identified: These peptide transporters are
primarily expressed in the digestive tract and kidney, respectively
(31,32). The former demonstrates low affinity
and high capacity, such that it may absorb a wide range of
di/tripeptides (33,34).
PEPT1, which is phylogenetically conserved, serves
as an integral membrane protein in the cellular uptake of
di/tripeptides and certain pharmacologically active drugs (35), and mediates the uptake of peptides and
peptide-like molecules using the inwardly directed H+
gradient across the membrane (36).
PEPT1 is specifically a type of active transport protein with low
affinity and high transport capacity that is almost exclusively
expressed in humans, and several other mammalian species, including
rats and mice (37,38). This protein was first identified in
the small intestine of a rabbit during cloning (39,40). The
PEPT1 molecule recognizes a wide range of oligopeptides and other
compounds with similar structures, and its range of drug substrates
is extensive, including β-lactam antibiotic drugs,
angiotensin-converting enzyme inhibitors, antitumor and antiviral
agents, thrombin inhibitors, dopamine receptor antagonists, and
renin inhibitors (41,42). The cellular uptake of these types of
small peptides is an important physiological process mediated by
proton-coupled peptide transporters (43). PEPT1 in particular may be involved in
the transport of endogenous molecules and affects drug absorption,
distribution, metabolism, and excretion, ultimately affecting the
efficacy and toxicity of drugs. A loss of PEPT1 activity may
therefore lead to a decrease in the intestinal absorption of
di/tripeptides, peptidomimetics and peptide-like drugs (44). As PEPT1 is a proton-coupled carrier,
it has a close association with the proton concentration. To
summarize, oligopeptide transporters maybe regarded as putative
therapeutic targets in cancer cells (45).
The results of the present study demonstrated that
PEPT1 has relatively limited expression in normal tissues, but is
highly expressed in various types of tumor cells (46,47). PEPT1
was already known to exhibit high expression in the pancreatic
cancer cell lines AsPC-1 and Capan-2, and low expression in
adjacent tissues (47,48). Nakanishi et al (15) first revealed the expression of PEPT1
in the human fibro sarcoma HT1080 cell line. In addition, the
expression of PEPT1 in the gastric cancer MKN45 cell line was
previously suggested (49), and an
additional study identified high expression of PEPT1 in prostate
cancer cells (13). However, at
present, little is known about the expression of PEPT1 in primary
hepatocarcinoma or its significance for targeted drug delivery.
Caco-2 is a human colon cancer cell line that was
used as a positive control in the present study as it is generally
considered o exhibit high expression of PEPT1 (50,51). In
the immunofluorescence analysis, it was observed that the PEPT1
protein (red fluorescence) was localized to the plasma membrane of
the liver cancer cells Bel-7402, SMMC7721, Hep3B, HepG2 and
SK-HEP-1, similar to what was observed for Caco-2 cells. These
results directly demonstrated the expression of PEPT1 in liver
cancer.
A previous study identified the expression of PEPT1
in gastric cancer cells (12). In the
present study, the expression of PEPT1 in the gastric cancer cell
line BGC-823 was examined, and the results were consistent with a
previous study (12). In the case of
BGC-823 and Caco-2 cells, which were used as a positive control,
universal expression of PEPT1 was observed, similar to data
previously demonstrated for PEPT1 in other cancer cells (11–15).
Although PEPT1 demonstrated different functional activities in the
liver cell lines, the expression of PEPT1 in SK-HEP-1 cells was
highest, as determined by western blotting. In contrast, the
highest expression detected by RT-qPCR was observed in HepG2 cells.
The potential for experimental error was eliminated by repeating
experiments three times. The reasons underlying this discrepancy
may be associated with differences in cellular status, the presence
of protein isoforms and the regulation of protein transcription or
translation.
To study the functional activity of PEPT1 in liver
cancer cells and to determine the role of PEPT1 in the uptake of
PEPT1 substrates, the specific fluorescence substrate Ala-Lys-AMCA,
a well-known PEPT1 substrate, was studied in the absence and
presence of PEPT1 (30). The
fluorescence analysis of D-Ala-Lys-AMCA confirmed that
D-Ala-Lys-AMCA may be transported into liver cancer cells and
Caco-2 cells, which indirectly demonstrated the expression of PEPT1
in hepatocarcinoma cells. A previous study investigated the
function of PEPT1 in the mouse intestine through
electrophysiological methods (52).
In the present study, the absorption of substrates at different
times, pH values and concentrations were determined. It was also
identified that the uptake of Ala-Lys-AMCA was time- and
concentration-dependent. All of these data confirm that the
transport of PEPT1 may be affected by time, pH and substrate
inhibitors, as observed in previous studies (53,54).
Gly-Sar is a small peptide that also serves as a substrate for
PEPT1, which specifically recognizes and transports it (47). A study conducted by Berthelsen et
al (55) demonstrated that
basolateral Gly-Sar transport in the intestinal cell line Caco-2 is
specifically proton-coupled via PEPT1. The dipeptide Gly-Gln is
also known as a high-affinity substrate for PEPT1, which transports
it into the cell in an inward direction (13). In the present study, Gly-Sar, Gly-Gln
and Gly-Gly-Gly were all used as competitive substrates in a
competition inhibition test (56,57), which
demonstrated that the uptake of D-Ala-Lys-AMCA was significantly
decreased by all three inhibitors. Thus, this suggests that the
liver cancer cells examined expressed functionally active PEPT1 in
the plasma membrane, and that PEPT1 serves an important role in the
transport of the substrate Ala-Lys-AMCA.
A tissue microarray analysis was performed in the
present study to provide a preliminary investigation of the
expression of PEPT1 in normal liver tissues, liver cancer tissues
with different pathological grades and tissues adjacent to liver
cancer. The analysis demonstrated that the expression of PEPT1 in
cancer tissues was higher compared with that in normal tissues
(P<0.05), whereas a low expression of PEPT1 was observed in
adjacent tissues. In addition, the expression levels were
associated with the pathological grade of the liver cancer tissues.
In summary, it was demonstrated that PEPT1 is expressed in liver
cancer tissues, and that PEPT1 overexpression is associated with
more aggressive tumor malignancy and a poor prognosis. Therefore,
PEPT1 may serve as an indicator of the nature of liver cancer
(benign or malignant) and of the differentiation degree of liver
cancer cells, making it an attractive target for cancer
therapy.
In the present study, initial exploration of the
specific overexpression of PEPT1 in primary hepatocarcinoma cells
and liver cancer tissues was performed using various approaches,
and different conditions. The correlation between tumor tissues and
PEPT1 indicates that PEPT1 represents a promising molecular target
for targeted drug delivery.
The selection of a drug carrier must meet the
following criteria: First, the carrier should be able to transport
the drug into the body and to avoid attack by the immune systems of
the body. Secondly, the carrier should deliver the drug
specifically to a particular location and cell type, and should
guarantee drug release (58).
Furthermore, the targeted drug should exhibit the highest
bioavailability possible, and reach its appropriate site of action
through efficient transport by drug carriers (59).
Current tumor therapy primarily relies on highly
toxic chemical drugs, which lead to numerous serious side effects.
For example, paclitaxel and doxorubicin may induce neurotoxicity,
cardiac toxicity and bone marrow suppression during treatment of a
tumor. An effective approach to reduce the side effects of
chemotherapy would be the selective delivery of anticancer drugs to
tumor tissues (60).
As its substrate-binding site may accommodate a wide
range of molecules of different sizes, hydrophobicities and
charges, PEPT1is regarded as an excellent target for the delivery
of pharmacologically-active compounds (61).
Due to its high expression in hepatocarcinoma, PEPT1
may serve as a good transporter-mediated drug delivery target, to
improve the treatment of primary hepatocarcinoma. Generally, the
design of a drug carrier that may specifically deliver drugs to a
tumor site is of importance. Therefore, the present study
hypothesized that a chemotherapy drug may be modified to obtain a
structure similar to that of di/tripeptides, such that it may be
identified and transported into hepatocarcinoma cells by PEPT1 in a
targeted manner. This approach may constitute a potential
therapeutic strategy for cancer treatment and may lead to the
development of a chemotherapy drug with increased efficacy, and
reduced toxic effects on the heart and bone marrow. Therefore,
PEPT1 represents a novel direction for future study, and as the
next stage, cell and animal experiments should be performed to
additionally examine the aforementioned theory. In addition, all of
the hypotheses discussed in the present study require additional
verification.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81302081).
References
|
1
|
Zong G, Xu Z, Zhang S, Shen Y, Qiu H, Zhu
G, He S, Tao T and Chen X: CD109 mediates cell survival in
hepatocellular carcinoma cells. Dig Dis Sci. 61:2303–2314. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mansourian PG, Yoneda M, Rao Krishna M,
Martinez FJ, Thomas E and Schiff ER: Effects of statins on the risk
of hepatocellular carcinoma. Gastroenterol Hepatol (N Y).
10:417–426. 2014.PubMed/NCBI
|
|
3
|
Kew MC: Hepatocellular carcinoma in
developing countries: Prevention, diagnosis and treatment. World J
Hepatol. 4:99–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Yue M, Shu R, Cheng H and Hu P:
Recent advances in the management of hepatocellular carcinoma. J
BUON. 21:307–311. 2016.PubMed/NCBI
|
|
5
|
Harlan LC, Parsons HM, Wiggins CL, Stevens
JL and Patt YZ: Treatment of hepatocellular carcinoma in the
community: Disparities in standard therapy. Liver Cancer. 4:70–83.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuen MF, Ahn SH, Chen DS, Chen PJ,
Dusheiko GM, Hou JL, Maddrey WC, Mizokami M, Seto WK, Zoulim F and
Lai CL: Chronic Hepatitis B virus infection: Disease revisit and
management recommendations. J Clin Gastroenterol. 50:286–294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parker JL, Mindell JA and Newstead S:
Thermodynamic evidence for a dual transport mechanism in a POT
peptide transporter. Elife. 3:2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jensen JM, Simonsen FC, Mastali A, Hald H,
Lillebro I, Diness F, Olsen L and Mirza O: Biophysical
characterization of the proton-coupled oligopeptide transporter
YjdL. Peptides. 38:89–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong Y, Wu X, Wang T, Zhao J, Liu X, Yao
Z, Zhang Q and Jian X: Targeting PEPT1: A novel strategy to improve
the antitumor efficacy of Doxorubicin in human hepatocellular
carcinoma therapy. Oncotarget. 15:40454–40468. 2017.
|
|
10
|
Kumar KK, Karnati S, Reddy MB and
Chandramouli R: Caco-2 cell lines in drug discovery-an updated
perspective. J Basic Clin Pharm. 1:63–69. 2010.PubMed/NCBI
|
|
11
|
Gonzalez DE, Covitz KM, Sadée W and Mrsny
RJ: An oligopeptide transporter is expressed at high levels in the
pancreatic carcinoma cell lines AsPc-1 and Capan-2. Cancer Res.
58:519–525. 1998.PubMed/NCBI
|
|
12
|
Namikawa T, Yatabe T, Inoue K, Shuin T and
Hanazaki K: Clinical applications of 5-aminolevulinic acid-mediated
fluorescence for gastric cancer. World J Gastroenterol.
21:8769–8775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tai W, Chen Z and Cheng K: Expression
profile and functional activity of peptide transporters in prostate
cancer cells. Mol Pharm. 10:477–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun D, Tan F, Fang D, Wang Y, Zeng S and
Jiang H: Expression of proton-coupled oligopeptide transporter
(POTs) in prostate of mice and patients with benign prostatic
hyperplasia (BPH) and prostate cancer (PCa). Prostate. 73:287–295.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakanishi T, Tamai I, Sai Y, Sasaki T and
Tsuji A: Carrier-mediated transport of oligopeptides in the human
fibrosarcoma cell line HT1080. Cancer Res. 57:4118–4122.
1997.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong XL and Qin SK: Progress in systemic
therapy of advanced hepatocellular carcinoma. World J
Gastroenterol. 22:6582–6594. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Slotta JE, Kollmar O, Ellenrieder V,
Ghadimi BM and Homayounfar K: Hepatocellular carcinoma: Surgeon's
view on latest findings and future perspectives. World J Hepatol.
7:1168–1183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang CH, Wey KC, Mo LR, Chang KK, Lin RC
and Kuo JJ: Current trends and recent advances in diagnosis,
therapy and prevention of hepatocellular carcinoma. Asian Pac J
Cancer Prev. 16:3595–3604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sahara S, Kawai N, Sato M, Tanaka T, Ikoma
A, Nakata K, Sanda H, Minamiguchi H, Nakai M, Shirai S and Sonomura
T: Prospective evaluation of transcatheter arterial
chemoembolization (TACE) with multiple anti-cancer drugs
(epirubicin, cisplatin, mitomycin c, 5-fluorouracil) compared with
TACE with epirubicin for treatment of hepatocellular carcinoma.
Cardiovasc Intervent Radiol. 35:1363–1371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paul SB and Sharma H: Role of
transcatheter intra-arterial therapies for hepatocellular
carcinoma. J Clin Exp Hepatol. 4:(Suppl 3). S112–S121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keating GM: Sorafenib: A Review in
hepatocellular carcinoma. Target Oncol. 12:243–253. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng GL, Zeng S and Shen H: Chemotherapy
and target therapy for hepatocellular carcinoma: New advances and
challenges. World J Hepatol. 7:787–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng C, Ma C, Bai E, Yang K and Xu R:
Transferrin and cell-penetrating peptide dual-functioned liposome
for targeted drug delivery to glioma. Int J Clin Exp Med.
8:1658–1668. 2015.PubMed/NCBI
|
|
25
|
Meyers JD, Cheng Y, Broome AM, Agnes RS,
Schluchter MD, Margevicius S, Wang X, Kenney ME, Burda C and
Basilion JP: Peptide-targeted gold nanoparticles for photodynamic
therapy of brain cancer. Part Part Syst Charact. 32:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maiolino S, Russo A, Pagliara V, Conte C,
Ungaro F, Russo G and Quaglia F: Biodegradable nanoparticles
sequentially decorated with Polyethyleneimine and Hyaluronan for
the targeted delivery of docetaxel to airway cancer cells. J
Nanobiotechnology. 13:292015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tai W, Shukla RS, Qin B, Li B and Cheng K:
Development of a peptide-drug conjugate for prostate cancer
therapy. Mol Pharm. 8:901–912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakanishi T, Tamai I, Takaki A and Tsuji
A: Cancer cell-targeted drug delivery utilizing oligopeptide
transport activity. Int J Cancer. 88:274–280. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nielsen CU and Brodin B: Di/tri-peptide
transporters as drug delivery targets: Regulation of transport
under physiological and patho-physiological conditions. Curr Drug
Targets. 4:373–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun D, Wang Y, Tan F, Fang D, Hu Y, Smith
DE and Jiang H: Functional and molecular expression of the
proton-coupled oligopeptide transporters in spleen and macrophages
from mouse and human. Mol Pharm. 10:1409–1416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Daniel H and Kottra G: The proton
oligopeptide cotransporter family SLC15 in physiology and
pharmacology. Pflugers Arch. 447:610–618. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ganapathy ME, Huang W, Wang H, Ganapathy V
and Leibach FH: Valacyclovir: A substrate for the intestinal and
renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res
Commun. 246:470–475. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sugawara M, Huang W, Fei YJ, Leibach FH,
Ganapathy V and Ganapathy ME: Transport of valganciclovir, a
ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J
Pharm Sci. 89:781–789. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rubio-Aliaga I and Daniel H: Peptide
transporters and their roles in physiological processes and drug
disposition. Xenobiotica. 38:1022–1042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu Y, Xie Y, Keep RF and Smith DE:
Divergent developmental expression and function of the
proton-coupled oligopeptide transporters PepT2 and PhT1 in regional
brain slices of mouse and rat. J Neurochem. 129:955–965. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Doki S, Kato HE, Solcan N, Iwaki M, Koyama
M, Hattori M, Iwase N, Tsukazaki T, Sugita Y, Kandori H, et al:
Structural basis for dynamic mechanism of proton-coupled symport by
the peptide transporter POT. Proc Natl Acad Sci USA.
110:11343–11348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu Y, Xie Y, Wang Y, Chen X and Smith DE:
Development and characterization of a novel mouse line humanized
for the intestinal peptide transporter PEPT1. Mol Pharm.
11:3737–3746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang B, Hu Y and Smith DE: Impact of
peptide transporter 1 on the intestinal absorption and
pharmacokinetics of valacyclovir after oral dose escalation in
wild-type and PepT1 knockout mice. Drug Metab Dispos. 41:1867–1874.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boll M, Markovich D, Weber WM, Korte H,
Daniel H and Murer H: Expression cloning of a cDNA from rabbit
small intestine related to proton-coupled transport of peptides,
beta-lactam antibiotics and ACE-inhibitors. Pflugers Arch.
429:146–149. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsu CP, Hilfinger JM, Walter E, Merkle HP,
Roessler BJ and Amidon GL: Overexpression of human intestinal
oligopeptide transporter in mammalian cells via adenoviral
transduction. Pharm Res. 15:1376–1381. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Terada T and Inui K: Peptide transporters:
Structure, function, regulation and application for drug delivery.
Curr Drug Metab. 5:85–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luckner P and Brandsch M: Interaction of
31 beta-lactam antibiotics with the H+/peptide symporter PEPT2:
Analysis of affinity constants and comparison with PEPT1. Eur J
Pharm Biopharm. 59:17–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Newstead S: Molecular insights into proton
coupled peptide transport in the PTR family of oligopeptide
transporters. Biochim Biophys Acta. 1850:488–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jappar D, Wu SP, Hu Y and Smith DE:
Significance and regional dependency of peptide transporter (PEPT)
1 in the intestinal permeability of glycylsarcosine: In situ
single-pass perfusion studies in wild-type and Pept1 knockout mice.
Drug Metab Dispos. 38:1740–1746. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mrsny RJ: Oligopeptide transporters as
putative therapeutic targets for cancer cells. Pharm Res.
15:816–818. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Landowski CP, Vig BS, Song X and Amidon
GL: Targeted delivery to PEPT1-overexpressing cells: Acidic, basic
and secondary floxuridine amino acid ester prodrugs. Mol Cancer
Ther. 4:659–467. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mitsuoka K, Kato Y, Miyoshi S, Murakami Y,
Hiraiwa M, Kubo Y, Nishimura S and Tsuji A: Inhibition of
oligopeptide transporter suppress growth of human pancreatic cancer
cells. Eur J Pharm Sci. 40:202–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gonzalez DE, Covitz KM, Sadée W and Mrsny
RJ: An oligopeptide transporter is expressed at high levels in the
pancreatic carcinoma cell lines AsPc-1 and Capan-2. Cancer Res.
58:519–525. 1998.PubMed/NCBI
|
|
49
|
Inoue M, Terada T, Okuda M and Inui K:
Regulation of human peptide transporter 1 (PEPT1) in gastric cancer
cells by anticancer drugs. Cancer Lett. 230:72–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Buyse M, Berlioz F, Guilmeau S, Tsocas A,
Voisin T, Péranzi G, Merlin D, Laburthe M, Lewin MJ, Rozé C and
Bado A: PepT1-mediated epithelial transport of dipeptides and
cephalexin is enhanced by luminal leptin in the small intestine. J
Clin Invest. 108:1483–1494. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han HK, Oh DM and Amidon GL: Cellular
uptake mechanism of amino acid ester prodrugs in Caco-2/hPEPT1
cells overexpressing a human peptide transporter. Pharm Res.
15:1382–1386. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen M, Singh A, Xiao F, Dringenberg U,
Wang J, Engelhardt R, Yeruva S, Rubio-Aliaga I, Nässl AM, Kottra G,
et al: Gene ablation for PEPT1 in mice abolishes the effects of
dipeptides on small intestinal fluid absorption, short-circuit
current, and intracellular pH. Am J Physiol Gastrointest Liver
Physiol. 299:G265–G274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Verri T, Kottra G, Romano A, Tiso N, Peric
M, Maffia M, Boll M, Argenton F, Daniel H and Storelli C: Molecular
and functional characterisation of the zebrafish (Danio rerio)
PEPT1-type peptide transporter. FEBS Lett. 549:115–122. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Agu R, Cowley E, Shao D, Macdonald C,
Kirkpatrick D, Renton K and Massoud E: Proton-coupled oligopeptide
transporter (POT) family expression in human nasal epithelium and
their drug transport potential. Mol Pharm. 8:664–672. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Berthelsen R, Nielsen CU and Brodin B:
Basolateral glycylsarcosine (Gly-Sar) transport in Caco-2 cell
monolayers is pH dependent. J Pharm Pharmacol. 65:970–979. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dalmasso G, Nguyen HT, Charrier-Hisamuddin
L, Yan Y, Laroui H, Demoulin B, Sitaraman SV and Merlin D: PepT1
mediates transport of the proinflammatory bacterial tripeptide
L-Ala-{gamma}-D-Glu-meso-DAP in intestinal epithelial cells. Am J
Physiol Gastrointest Liver Physiol. 299:G687–G696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Terada T, Sawada K, Ito T, Saito H,
Hashimoto Y and Inui K: Functional expression of novel peptide
transporter in renal basolateral membranes. Am J Physiol Renal
Physiol. 279:F851–F857. 2000.PubMed/NCBI
|
|
58
|
Tan S, Wu T, Zhang D and Zhang Z: Cell or
cell membrane-based drug delivery systems. Theranostics. 5:863–881.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Foltz M, Meyer A, Theis S, Demuth HU and
Daniel H: A rapid in vitro screening for delivery of
peptide-derived peptidase inhibitors as potential drug candidates
via epithelial peptide transporters. J Pharmacol Exp Ther.
310:695–702. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang S, Placzek WJ, Stebbins JL, Mitra S,
Noberini R, Koolpe M, Zhang Z, Dahl R, Pasquale EB and Pellecchia
M: Novel targeted system to deliver chemotherapeutic drugs to
EphA2-expressing cancer cells. J Med Chem. 55:2427–2436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rubio-Aliaga I and Daniel H: Mammalian
peptide transporters as targets for drug delivery. Trends Pharmacol
Sci. 23:434–440. 2002. View Article : Google Scholar : PubMed/NCBI
|