Introduction
The incidence of prostate cancer occupies the first
place among male malignant tumors, and its mortality rate ranks 2nd
in Europe and the United States. In China, the disease ranks 5th
among cancers in males (1). The main
prostate cancer therapies include surgical resection combined with
radiotherapy and chemotherapy, but 20–40% of patients exhibit
resistance to the radiotherapy and/or chemotherapy (2). Approximately 10–50% of the mass of the
tumors is composed of hypoxic cells lacking oxygen-free radicals
that can induce DNA damage to destroy the tumorigenic cells
(3). The expression of
hypoxia-inducible factor-1 (HIF-1) can be induced under hypoxic
conditions in tumor cells. HIF-1α is a nuclear protein with
transcriptional activity modulating oxygen homeostasis in tissues.
After binding to its target gene, it regulates the adaptive
response of cells to hypoxia-ischemia and is involved in cell
proliferation, energy metabolism, vascular endothelial growth
factor (VEGF) production (4),
epithelial-mesenchymal transition, tumor invasion and metastasis,
and resistance to radiotherapy and chemotherapy (5). Tumor stem cells (CSCs) have been
described in lung, breast, colorectal cancer and other tumor
tissues (6) where they promote tumor
proliferation, differentiation, apoptosis and autophagy (7). CD133 is a commonly used CSC marker,
CD133+ cells express an ATP-binding drug transporter
that can affect the sensitivity of tumor cells to radiotherapy and
chemotherapy (8).
Based on this, the aim of the present study was to
analyze the relationship between the expression of
CD133+, HIF-1α, VEGF and the proliferation or apoptosis
of prostate cancer cells to expand the repertoire of possible
targets for improving the radio/chemosensitivity of tumor
cells.
Materials and methods
Materials
The human prostate LNCaP cancer cell line was
purchased from the Cell Resource Center of the Shanghai Sangon
Biological Engineering Technology and Service Co. (Shanghai,
China), RPMI-1640 medium and trypsin were both purchased from Gibco
(Carlsbad, CA, USA), neonatal calf serum was purchased from Hyclone
(Logan, UT, USA), the cell culture incubator was purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
LNCaP cells were routinely thawed, cultured and
passaged. For experiments the cells were divided into two batches,
cells in the control group were grown in a 5% CO2 + 20%
O2 environment and cells in the hypoxia group in a 5%
CO2 + 1% O2 environment. All the cells were
cultured at 37°C with saturated humidity. RPMI-1640 containing 10%
neonatal calf serum, 100 U/ml of penicillin and 100 µg/ml of
streptomycin were used as culture media. The cells grew adhering to
the flask wall and media were exchanged every two days. The cells
were digested and passaged with 0.25% trypsin, and cells in the
logarithmic growth phase were used for the subsequent
experiments.
Methods
The percentage of CD13+ cells in each
group was determined by flow cytometry, the expression of HIF-1α
and VEGF were detected by western blot analysis, the cell
proliferation rate was detected by the MTT assay and the apoptotic
rate was detected by flow cytometry at 12, 24 and 72 h
respectively.
Flow cytometric determination of the
percentage of CD133+ and apoptotic cells
Equivalent amounts of cells from each group were
resuspended at final concentration of 2×106/ml and mixed
with mouse anti-human monoclonal antibody (1:200; Sigma-Aldrich,
St. Louis, MO, USA). The mixtures were incubated for 1 h at room
temperature in the dark and then centrifuged at 2,000 × g for 10
min. The supernatants were discarded. Then, 5 ml phosphate-buffered
saline (PBS) (10 mM, pH=7.4) were added and the samples were
resuspended, after centrifugation at 2,000 × g for 10 min, the
supernatants were discarded again, 1 ml PBS was added and the
samples were resuspended again. The percentages of
CD133+ cells were determined within 1 h using a
FACSCaliber flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
To determine the apoptotic cell percentages
equivalent amounts of cells were centrifuged at 1,500 × g for 10
min, the supernatants were discarded and the samples were washed
with PBS. After centrifugation at 1,500 × g for 10 min the
precipitates were collected. Binding buffer (100 µl) and 10 µl of
FITC-conjugated Annexin V (20 µg/ml) (both from Jiangsu Biyuntian
Technology, Jiangsu, China) were added to each sample and were
placed in an incubator for 30 min at room temperature in the dark.
Subsequently, 5 µl of PI (50 µg/ml; Jiangsu Biyuntian Technology)
were added and the samples were incubated for 5 min in the dark.
Then, 400 µl binding buffer were added and the samples were
detected within 1 h by flow cytometry. The cells without Annexin
V-FITC and PI were used as negative controls.
Determination of HIF-1α and VEGF
expression levels by western blot analysis
Equivalent amounts of cell culture medium from each
batch of cells were centrifuged at 1,500 × g for 10 min and the
supernatants were discarded. The samples were washed with PBS and
then centrifuged at 1,500 × g for 10 min, 0.2 g of cell precipitate
were collected and washed with PBS. RIPA lysis buffer (500 µl;
Jiangsu Biyuntian Technology) was added, each sample was
homogenized in a vortex 10 times and then transferred to a
centrifuge tube. The centrifuge tube was placed in an ice bath and
proteins were allowed to lyse for 30 min, then each sample was
centrifuged at 4°C at 3,000 × g for 5 min and the supernatant was
transferred to 1.5 ml centrifuge tubes and stored at −20°C.
The BCA assay (Jiangsu Biyuntian Technology) was
used to measure protein concentrations and purity. Total protein
(30 µg) was mixed with 5X loading buffer. A 10% separation gel and
a 4% concentration gel were prepared, and the samples were
separated by 8% SDS-PAGE (Invitrogen, Carlsbad, CA, USA). The
electrophoresis voltage was set at 80 V for 30 min for the
concentration gel and 120 V for 3 h for the separation gel (until
the bromophenol blue reached the bottom of the gel). The separated
proteins were transferred to a PVDF membrane (Invitrogen). Mouse
monoclonal HIF-1α antibody (dilution, 1:500; cat. no. ab113642),
mouse monoclonal VEGF antibody (dilution, 1:500; cat. no. ab9530)
were added and the membranes were incubated overnight at 4°C,
β-actin was used as internal reference protein for normalizing
quantities. Secondary rabbit anti-mouse (HRP) IgG antibody
(dilution, 1:2,000; cat. no. ab6728) was added and the membranes
were incubated at room temperature for 4 h,then the membranes were
washed with PBS, ECL was added onto each membrane and blots were
developed in the dark. Images were scanned and stored. The data was
semi-quantitatively analyzed with LabWorks 4.5 Image Analysis
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Determination of cell proliferation
rate by the MTT assay
Cell samples were resuspended to a final
concentration of 2×106/ml and seeded in 96-well plates
with 100 µl/well. After 12, 24 and 72 h, 10 µl of 5 mg/ml MTT
(Bio-Rad Laboratories, Inc.) were added into each well. After 4 h,
the supernatants were discarded and 150 µl of DMSO (Bio-Rad
Laboratories, Inc.) were added into each well. The samples were
agitated for 10 min and the optical density (OD) at A490 nm was
measured in a microplate reader (Bio-Rad Laboratories, Inc.).
Samples were measured three times, and the calculated average was
used as the result in each case. The cell proliferation rates were
calculated according to the following formula: Proliferation rate
(%) = OD value of the experimental group/OD value of the control
group × 100%.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
statistical software (SPSS, Inc., Chicago, IL, USA). Measurement
data were expressed as means ± standard deviation, and the one-way
analysis of variance was used to compare the data at different
time-points. P<0.05 was considered to indicate a statistically
significant difference.
Results
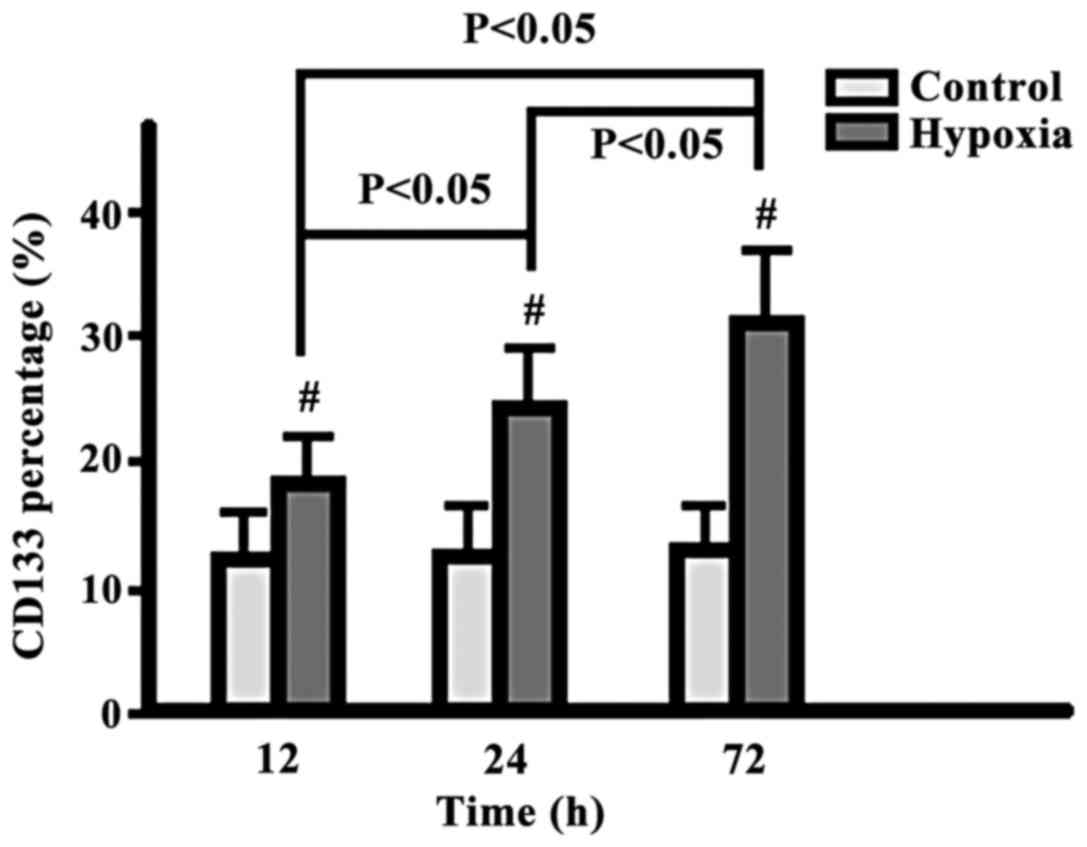
Comparison of CD133+ cell
percentages
The percentage of CD133+ cells in the
hypoxia group increased gradually from 12 to 24 to 72 h, while
there was no change in the control group. The difference at each
time-point was significantly higher in the hypoxia group
(p<0.05) (Fig. 1).
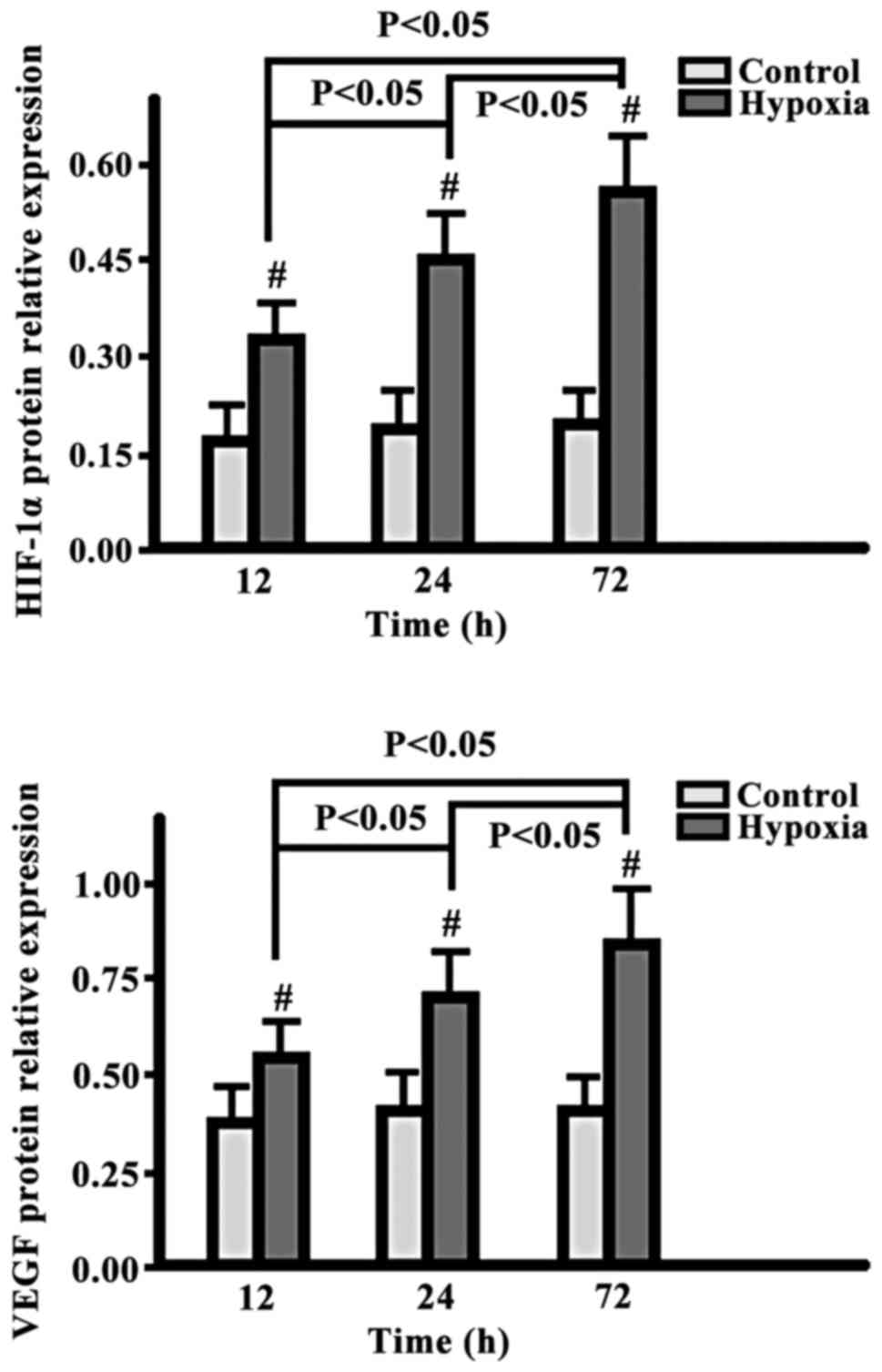
Comparison of HIF-1α and VEGF
expression levels
The expression levels of HIF-1α and VEGF in the
hypoxia group were gradually increased from 12 to 24 and to 72 h,
while the levels in the control group exhibited no changes. The
level in the hypoxia group at each time-point was significantly
higher than the corresponding level in the control group
(p<0.05) (Fig. 2).
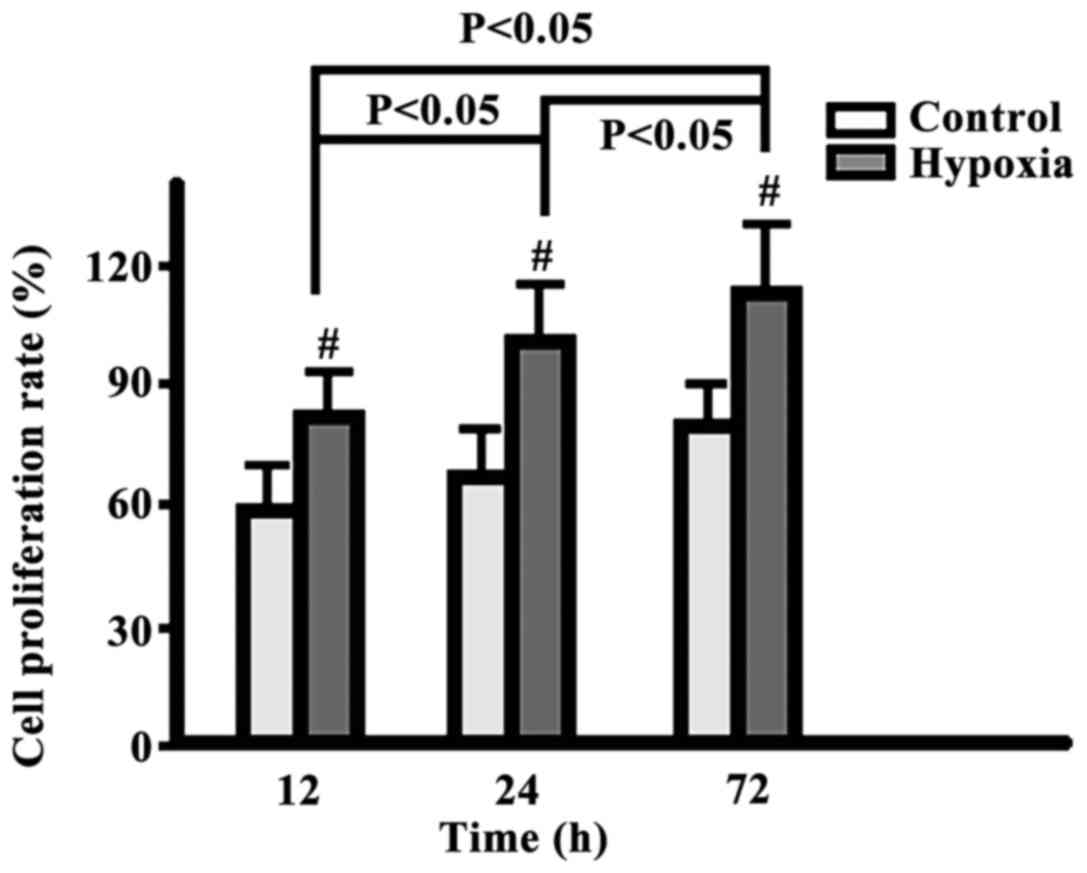
Comparison of cell proliferation
rate
The cell proliferation rates in the two groups
increased gradually from 12 to 24 and to 72 h, but the
proliferation in the hypoxia group was significantly higher than
that in the control group (p<0.05) (Fig. 3).
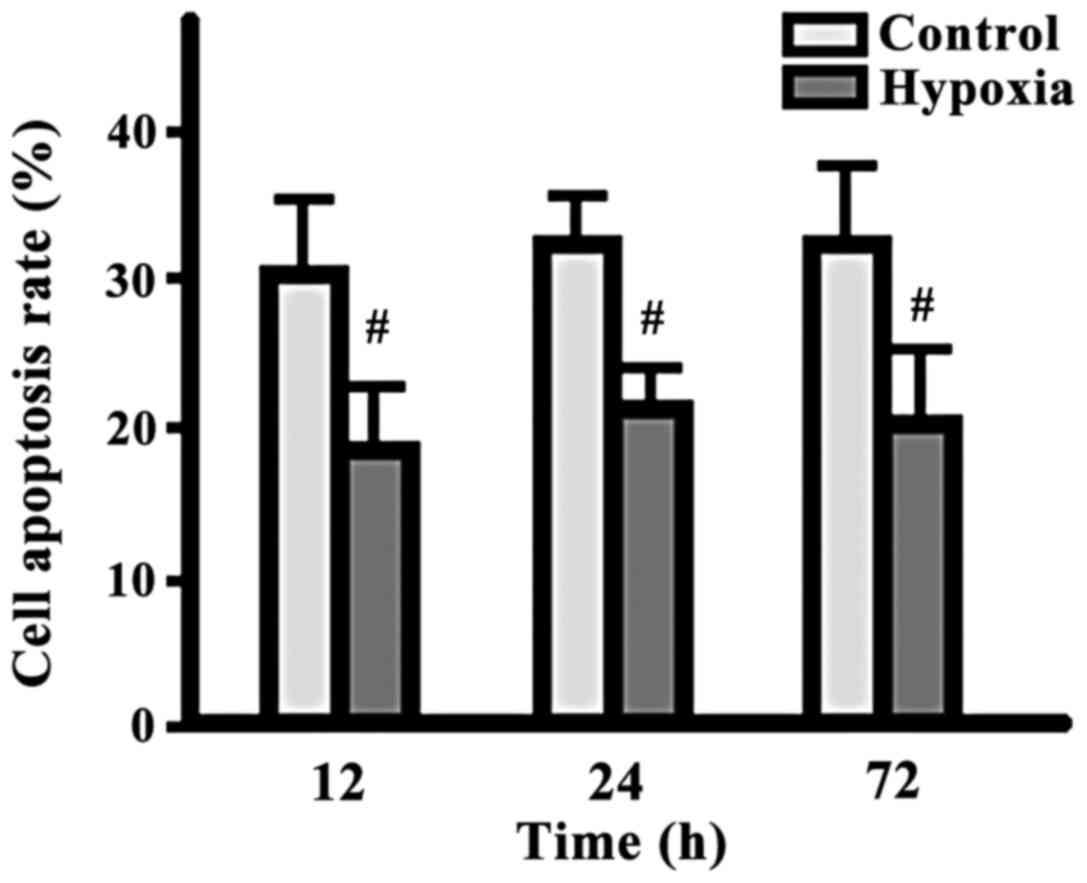
Comparison of cell apoptotic cell
percentages
There were no significant changes in terms of the
apoptotic cell percentages between the two groups at different
time-points, but the percentage in the hypoxia group was
significantly lower than that in the control group at each same
time-point (p<0.05) (Fig. 4).
Discussion
Tissue hypoxia is an important factor in the
occurrence of a variety of diseases, such as cancer, pulmonary
vascular remodeling and aging (9).
Hypoxia in tumor cells is related to energy metabolism disorders,
DNA damage repair, inflammatory response, oxidative stress and
apoptosis (10). In this study, our
results showed that the percentage of CD133+ cells, and
the expression of HIF-1α and VEGF in the hypoxia group cells
increased gradually, while the cells in the control group exhibited
no such changes. Moreover, the values for the hypoxia group cells
at each time-point were significantly higher than those in the
control group cells. The cell proliferation rate increased
gradually in the two groups, but the proliferation in the hypoxia
group was significantly higher than that in the control group at
any time-point. The percentage of apoptotic cells did not change
significantly with time in either group, whereas the hypoxia group
had less apoptotic cells than the control group at all the
time-points.
The results suggest that the expression of
CD133+, HIF-1α and VEGF in human prostate cancer cells
is associated with hypoxia. It is known that hypoxic environments
can increase the expression of HIF-1α and VEGF proteins in tumor
cells, promote cell proliferation and inhibit cell apoptosis. In
fact, the hypoxic prostate LNCaP cancer cell line was transplanted
subcutaneously to mice in another study, and the tumor growth rate
was significantly faster than that in mice transplanted with
normoxic cells (11). Furthermore,
immunohistochemical staining of human prostate cancer tissues found
that CD133+ cell, HIF-1α and VEGF expression levels were
correlated with tumor clinical stage and differentiation degree,
and patients with a high expression of these proteins often showed
poor clinical prognosis (12). By
contrast, CD133+ were notably absent from normal
prostate tissues (13).
The resistance to chemotherapy is increased in
CD133+ hepatocellular carcinoma cells, where selective
activation of the Akt/PKB and Bcl-2 signaling pathways inhibits
cell apoptosis and promotes cell survival (14). CD133+ human prostate cancer
cells exhibit a strong proliferative capacity and invasiveness, and
may promote angiogenesis by inducing the expression of matrix
metalloproteinase (MMP)-9 (15) and
VEGF (16). Furthermore, these cells
play a role escaping the immune system by association with the MHC
class I-related chain A (ADAM9/MHC class I-related chain A, MICA)
pathway (17). HIF-1α is an
oxygen-dependent transcriptional activator that induces
transcsription of a variety of downstream target genes by binding
to the hypoxia response element (HRE) (18). HIF-1α is highly expressed in a variety
of tumor tissues, and the VEGF gene promoter contains the HIF-1α
binding sequence (18). The
hypoxia-induced expression of HIF-1α is transient, and iron-ion
chelating agents binding to iron ions on HIF-1α can inhibit the
degradation of HIF-1α (19). However,
simply increasing the oxygen concentration in tissues does not lead
to inhibition of cell proliferation, cell apoptosis promotion or a
reduction in CD133+ cells, suggesting that hypoxia
stimulation is the determinant factor (20). Nevertheless, inhibiting the expression
of CD133+, HIF-1α and VEGF is a potential strategy for
interfering with tumors. As an example of this approach, a study by
Liu et al (21) confirmed that
the compound berberine inhibited HIF-1α and VEGF expression
activity in tumor cells, resulting in tumor proliferation
inhibition.
In conclusion, the present study proved that hypoxia
can induce the expression of CD133+, HIF-1α and VEGF in
prostate cancer cells and thereby regulate cell proliferation and
apoptosis. A treatment to alter the expressions of
CD133+, HIF-1α and VEGF may be a successful approach to
prevent and cure prostate cancer.
References
|
1
|
He F, Fang Z, Shen C and Li L:
Meta-analysis of the effect of postoperative radiotherapy on
prognosis of prostatic cancer following radical prostatectomy. Int
J Clin Exp Med. 8:20589–20595. 2015.PubMed/NCBI
|
|
2
|
Zhang Q, Zhang C, Yang X, Yang B, Wang J,
Kang Y, Wang Z, Li D, Huang G, Ma Z, et al: Berberine inhibits the
expression of hypoxia induction factor-1alpha and increases the
radiosensitivity of prostate cancer. Diagn Pathol. 9:982014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mimeault M and Batra SK: Hypoxia-inducing
factors as master regulators of stemness properties and altered
metabolism of cancer- and metastasis-initiating cells. J Cell Mol
Med. 17:30–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li
Y, Banerjee S, Padhye S and Sarkar FH: Hypoxia induced
aggressiveness of prostate cancer cells is linked with deregulated
expression of VEGF, IL-6 and miRNAs that are attenuated by CDF.
PLoS One. 7:e437262012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitajima Y and Miyazaki K: The critical
impact of HIF-1a on gastric cancer biology. Cancers (Basel).
5:15–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu F, Qi CL, Kong M, Liu TT, Li L and Li
BJ: Screening specific polypeptides of breast cancer stem cells
from a phage display random peptide library. Oncol Lett.
12:4727–4731. 2016.PubMed/NCBI
|
|
7
|
Jaworska D, Król W and Szliszka E:
Prostate cancer stem cells: Research advances. Int J Mol Sci.
16:27433–27449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bayin NS, Modrek AS, Dietrich A, Lebowitz
J, Abel T, Song HR, Schober M, Zagzag D, Buchholz CJ, Chao MV, et
al: Selective lentiviral gene delivery to CD133-expressing human
glioblastoma stem cells. PLoS One. 9:e1161142014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Labrecque MP, Takhar MK, Nason R,
Santacruz S, Tam KJ, Massah S, Haegert A, Bell RH, Altamirano-Dimas
M, Collins CC, et al: The retinoblastoma protein regulates
hypoxia-inducible genetic programs, tumor cell invasiveness and
neuroendocrine differentiation in prostate cancer cells.
Oncotarget. 7:24284–24302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu M, Wang X, McGregor N, Pienta KJ and
Zhang J: Dynamic regulation of Rad51 by E2F1 and p53 in prostate
cancer cells upon drug-induced DNA damage under hypoxia. Mol
Pharmacol. 85:866–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deep G and Panigrahi GK: Hypoxia-induced
signaling promotes prostate cancer progression: Exosomes role as
messenger of hypoxic response in tumor microenvironment. Crit Rev
Oncog. 20:419–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pellacani D, Packer RJ, Frame FM, Oldridge
EE, Berry PA, Labarthe MC, Stower MJ, Simms MS, Collins AT and
Maitland NJ: Regulation of the stem cell marker CD133 is
independent of promoter hypermethylation in human epithelial
differentiation and cancer. Mol Cancer. 10:942011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dey P, Velazquez-Villegas LA, Faria M,
Turner A, Jonsson P, Webb P, Williams C, Gustafsson JÅ and Ström
AM: Estrogen receptor β2 induces hypoxia signature of gene
expression by stabilizing HIF-1α in prostate cancer. PLoS One.
10:e01282392015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen H, Luo Z, Dong L, Tan Y, Yang J, Feng
G, Wu M, Li Z and Wang H: CD133/prominin-1-mediated autophagy and
glucose uptake beneficial for hepatoma cell survival. PLoS One.
8:e568782013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mak AB, Schnegg C, Lai CY, Ghosh S, Yang
MH, Moffat J and Hsu MY: CD133-targeted niche-dependent therapy in
cancer: A multipronged approach. Am J Pathol. 184:1256–1262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Wang H, Cannon V, Wolcott KM, Song
H and Yates C: Side population rather than CD133(+) cells
distinguishes enriched tumorigenicity in hTERT-immortalized primary
prostate cancer cells. Mol Cancer. 10:1122011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reyes EE, Gillard M, Duggan R, Wroblewski
K, Kregel S, Isikbay M, Kach J, Brechka H, Weele DJ, Szmulewitz RZ,
et al: Molecular analysis of CD133-positive circulating tumor cells
from patients with metastatic castration-resistant prostate cancer.
J Transl Sci. 1:2015.doi: 10.15761/JTS.1000104. PubMed/NCBI
|
|
18
|
Park JJ, Jin YB, Lee YJ, Lee JS, Lee YS,
Ko YG and Lee M: KAI1 suppresses HIF-1α and VEGF expression by
blocking CDCP1-enhanced Src activation in prostate cancer. BMC
Cancer. 12:812012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Benzonana LL, Zhao H, Watts HR,
Perry NJ, Bevan C, Brown R and Ma D: Prostate cancer cell
malignancy via modulation of HIF-1α pathway with isoflurane and
propofol alone and in combination. Br J Cancer. 111:1338–1349.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SO, Kim JS, Lee MS and Lee HJ:
Anti-cancer effect of pristimerin by inhibition of HIF-1α involves
the SPHK-1 pathway in hypoxic prostate cancer cells. BMC Cancer.
16:7012016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu CH, Tang WC, Sia P, Huang CC, Yang PM,
Wu MH, Lai IL and Lee KH: Berberine inhibits the metastatic ability
of prostate cancer cells by suppressing epithelial-to-mesenchymal
transition (EMT)-associated genes with predictive and prognostic
relevance. Int J Med Sci. 12:63–71. 2015. View Article : Google Scholar : PubMed/NCBI
|