Introduction
Ovarian cancer is one of the most common malignant
tumors in women, ranking third in incidence after cervical and
endometrial cancers. Epithelial ovarian cancer is the most common
type of ovarian cancer (1,2). Clinical treatment of ovarian cancer
mainly consists of surgery combined with chemotherapy. However,
over time, cancer cells can develop resistance to chemotherapy,
leading to a failure in the efficacy of therapy. At present, the
5-year survival rate of ovarian cancer is low and poses a serious
health threat to women (3,4).
Multiple tumor suppressor 1, also known as p16 gene,
was found and isolated in 1993 and is regarded as a tumor
suppressor gene (5,6). p16 gene is the inhibiting factor of
cyclin-dependent kinase 4, which is involved in the regulation of
normal cell growth. The deficiency or mutation of p16 gene is
closely related to the formation of tumors and poor prognosis of
the patient (7–9).
This study compared the expression of p16 protein in
ovarian cancer tissue and normal ovarian tissue, and tested the
influence of p16 protein expression on the invasion of ovarian
cancer cells. In addition, combined with clinical research data,
our investigation assessed the correlation between the p16 gene and
the lymph node metastasis in human ovarian cancer cases and the
patient prognosis.
Materials and methods
Materials
SKOV-3 (ovarian cancer cells) and IOSE80 (normal
ovarian cells) were sourced from Cell Bank of the Chinese Academy
of Sciences (Beijing, China). TRIzol, the RT-PCR kit and
Lipofectamine 2000 were from Invitrogen (Thermo Fisher Scientific,
Waltham, MA, USA). Fetal bovine serum (FBS) was from GE Healthcare
Life Sciences HyClone Laboratories, Inc. (Logan, UT, USA). p16,
rabbit monoclonal GAPDH primary antibody (dilution, 1:500; cat. no.
10494-1-AP) and goat anti-rabbit HRP-labeled secondary antibody
(dilution, 1:2,000; cat. no. SA00001-2) were from Wuhan Sanying
Biological Technology Co., Ltd. (Wuhan, china). RPMI-1640 medium
and a Transwell chamber were from Corning Inc. (New York, NY, USA).
Immunohistochemical staining kits (SP-9001) were from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
pEGFP-N1 was from Clontech Laboratories, Inc. (Mountain View, CA,
USA). The primer was synthesized by GenePharma (Shanghai,
China).
All patients, as well as tissue samples, in clinical
research were selected from the Qilu Hospital of Shandong
University. This study was approved by the Ethics Committee of Qilu
Hospital. Signed written informed consents were obtained from all
participants before the study. There were 20 cases with normal
ovarian tissue and 64 cases with ovarian cancer tissue, including
38 cases with lymph node metastasis and 26 cases without lymph node
metastasis.
Cell culture
IOSE80 and SKOV-3 cells were cultured at 5%
CO2 and 37°C in an incubator, using RPMI-1640 medium
(containing 10% FBS). The medium was changed every day, followed by
subculture after cell overgrowth.
Detection of p16 protein expression in
cells using western blot analysis
IOSE80 and SKOV-3 cells were each cultured and cell
lysis buffer was added to extract the total protein. Protein
concentration was determined by BCA method, followed by
electrophoresis and membrane transfer. It was then sealed by 5%
skimmed milk powder, p16 and GAPDH antibody were added, and then it
was incubated overnight at 4°C. The following day, it was incubated
with HRP-labeled secondary antibody at room temperature for 2 h,
followed by photography and film development.
Detection of p16 protein expression in
tissues using immunohistochemical techniques
Detection of p16 was carried out according to a
protocol, as follows: a paraffin section was dewaxed and 3%
H2O2 solution and used to block the
endogenous peroxidase, followed by high-pressure antigen repair. It
was sealed by 10% goat serum and the primary antibody was added
(diluted in the ratio of 1:100), then incubated at 4°C overnight
and washed three times using PBS. The biotin-labeled secondary
antibody was added and incubated for 30 min, washed three times
using PBS, developed using DAB and redyed using hematoxylin. It was
then sealed by neutral gum and photographed under a microscope
(BX-42; Olympus, Tokyo, Japan).
According to the following scoring standard, the
number of positive stained cells was calculated and divided into
the p16 low-expression group (the number of positive cells <10%)
or the p16 high-expression group (the number of positive cells
>10%). The scoring results were then summarized and
analyzed.
Construction and transfection of p16
gene high-expression vector
SKOV-3 cells in logarithmic phase were centrifuged
after digestion, and collected. The total RNA was extracted using
TRIzol, followed by reverse transcription using a reverse
transcription kit. Then, the p16 primer was added (Table I) for gene amplification. The reaction
conditions were as follows: 94°C for 5 min, degeneration at 94°C
for 30 sec, annealing at 63°C for 30 sec, extension at 72°C for 30
sec, amplification for 30 cycles, extension at 72°C for 5 min and
termination of the reaction at 4°C. The amplified fragment was then
inserted between HindIII and KpnI of pEGFP-N1,
constituting the recombinant plasmid, pEGFPN1-p16.
 | Table I.p16 gene primer sequence. |
Table I.
p16 gene primer sequence.
| Gene | Sequence |
|---|
| p16 | U:
5′-GCCGGAAGCTTATGGTGCGCAGGTTCTTGGT-3′ |
|
| D:
5′-CTAATGGTACCCAGCCAGGTCCACGGGCAGA-3′ |
The cells were divided into the normal group, the
empty plasmid group and the high-expression group in the
transfection experiment. SKOV-3 cells in logarithmic phase were
prepared into a single-cell suspension liquid using trypsin
digestion. The cells were cultured using 6-well plates. After
adherence, a p16 gene high-expression vector was used by
Lipofectamine 2000 to transfect the SKOV-3 cells in strict
accordance with the protocol.
Influence of the transfection of p16
gene on the expression and cell invasion ability of p16
protein
At 48 h after transfection, according to the above
method, the expression of p53 protein in the cells of each group
was detected using the method as identified earlier. A Transwell
chamber was used to prepare the cells at 48 h after transfection
into a single-cell suspension liquid, according to the protocol. A
total of 100 µl single-cell suspension liquid at the concentration
of 4×105/ml and 100 µl serum-free medium were added to
the upper chamber and 500 µl medium containing 30% FBS was added to
the lower chamber. After 48 h, it was fixed, dyed and analyzed.
Correlation between p16 expression in
ovarian cancer and prognosis
The patients were divided into the p16
low-expression group and the p16 high-expression group. There were
no statistically significant differences in the gender, age,
smoking history, drinking history and family history between the
two groups (P>0.05). The 5-year survival rate was then
calculated. Based on the results of the follow-up visit, the
influence of p16 expression on the prognosis of patients with
ovarian cancer was determined.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for all analyses in the study. One-way ANOVA and
Kaplan-Meier survival analysis were used for the clinical prognosis
results. P<0.05 suggested that the difference was statistically
significant.
Results
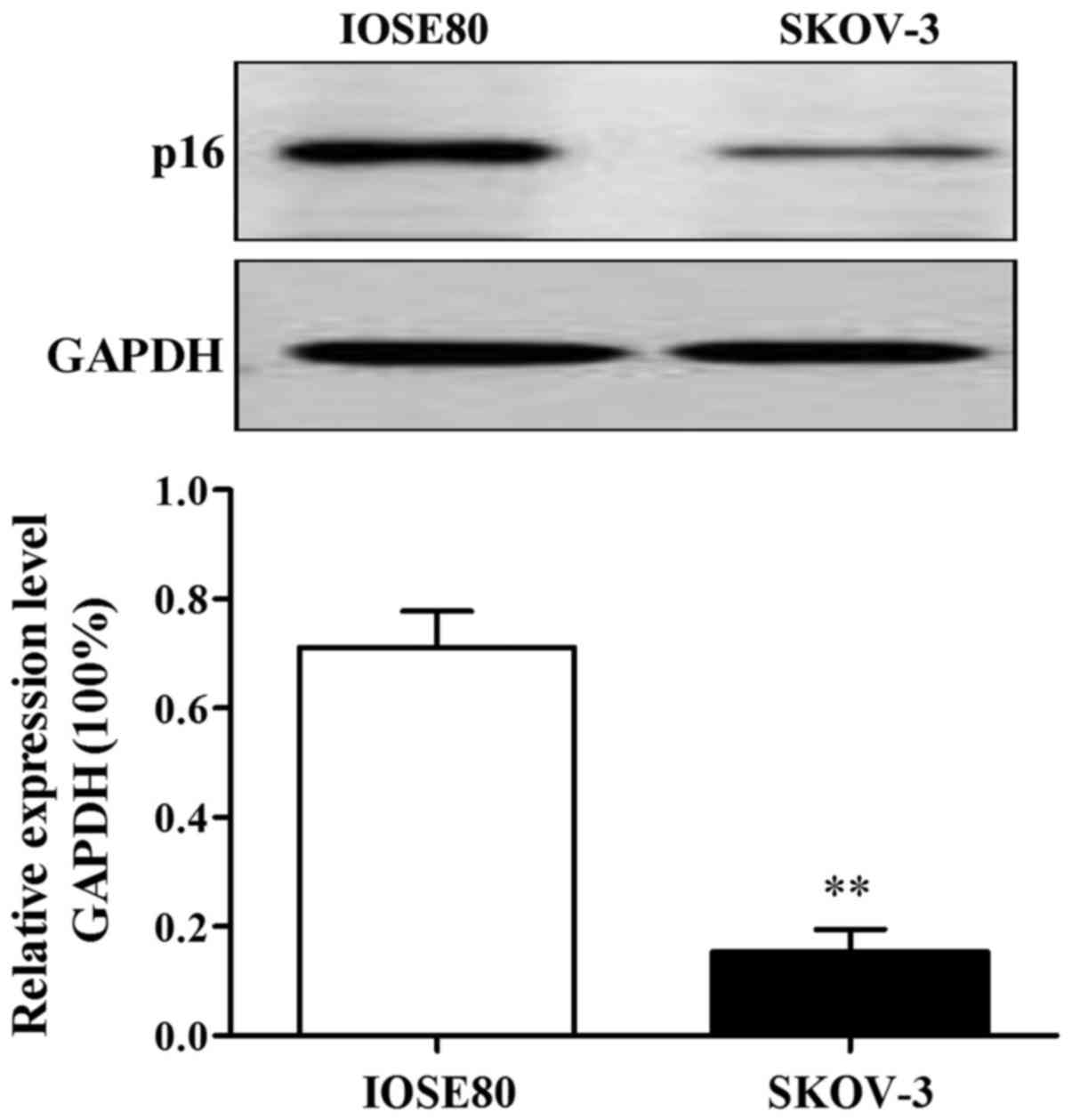
p16 protein expression in IOSE80 and
SKOV-3
The results of western blot analysis showed that p16
protein expression in SKOV-3 was lower than that in IOSE80 and the
difference was statistically significant (P<0.01) (Fig. 1).
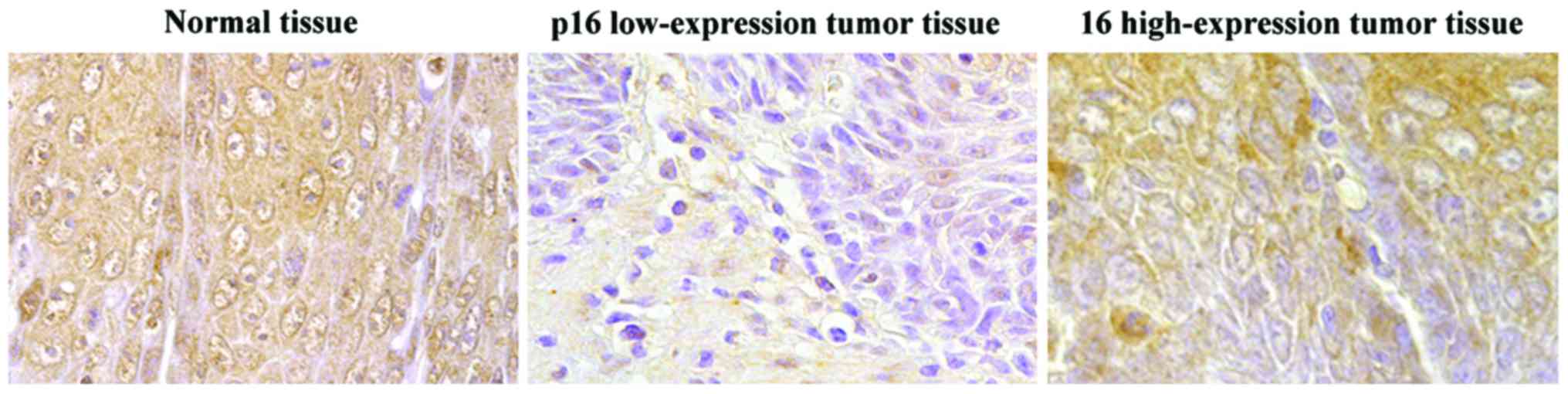
p16 protein expression in ovarian
cancer tissue
Immunohistochemical results showed that p16 protein
was stained brownish, with the normal ovarian tissue and p16
high-expression tumor tissue stained with deep brown color. The
positive cells occupied a larger proportion than the normal cells,
as shown in Fig. 2. There were 25
cases with p16 high expression (positive) (39.06%) among the 64
cases with ovarian cancer, and 14 cases with p16 high expression
(positive) (70.00%) among the normal tissue, and the differences
between the two groups were statistically significant (P<0.01)
(Table II). The expression of p16
protein was associated with lymph node metastasis in ovarian
cancer, and the positive expression rate of p16 protein in the
tissue of ovarian cancer patients with lymph node metastasis was
significantly lower than that without lymph node metastasis
(P<0.01) (Table III).
 | Table II.p16 protein expression in normal
tissue and ovarian cancer tissue. |
Table II.
p16 protein expression in normal
tissue and ovarian cancer tissue.
|
|
| p16 expression |
|
|---|
|
|
|
|
|
|---|
| Group | Cases | Positive | Negative | Positive rate
(%) |
|---|
| Normal tissue | 20 | 14 | 6 | 70.00 |
| Ovarian cancer
tissue | 64 | 25 | 39 | 39.06a |
 | Table III.Correlation between p16 protein
expression and lymph node metastasis. |
Table III.
Correlation between p16 protein
expression and lymph node metastasis.
|
|
| p16 expression |
|
|---|
|
|
|
|
|
|---|
| Group | Cases | Positive | Negative | Positive rate
(%) |
|---|
| Lymph node
metastasis | 38 | 11 | 27 | 28.95 |
| No lymph node
metastasis | 26 | 14 | 12 | 53.85a |
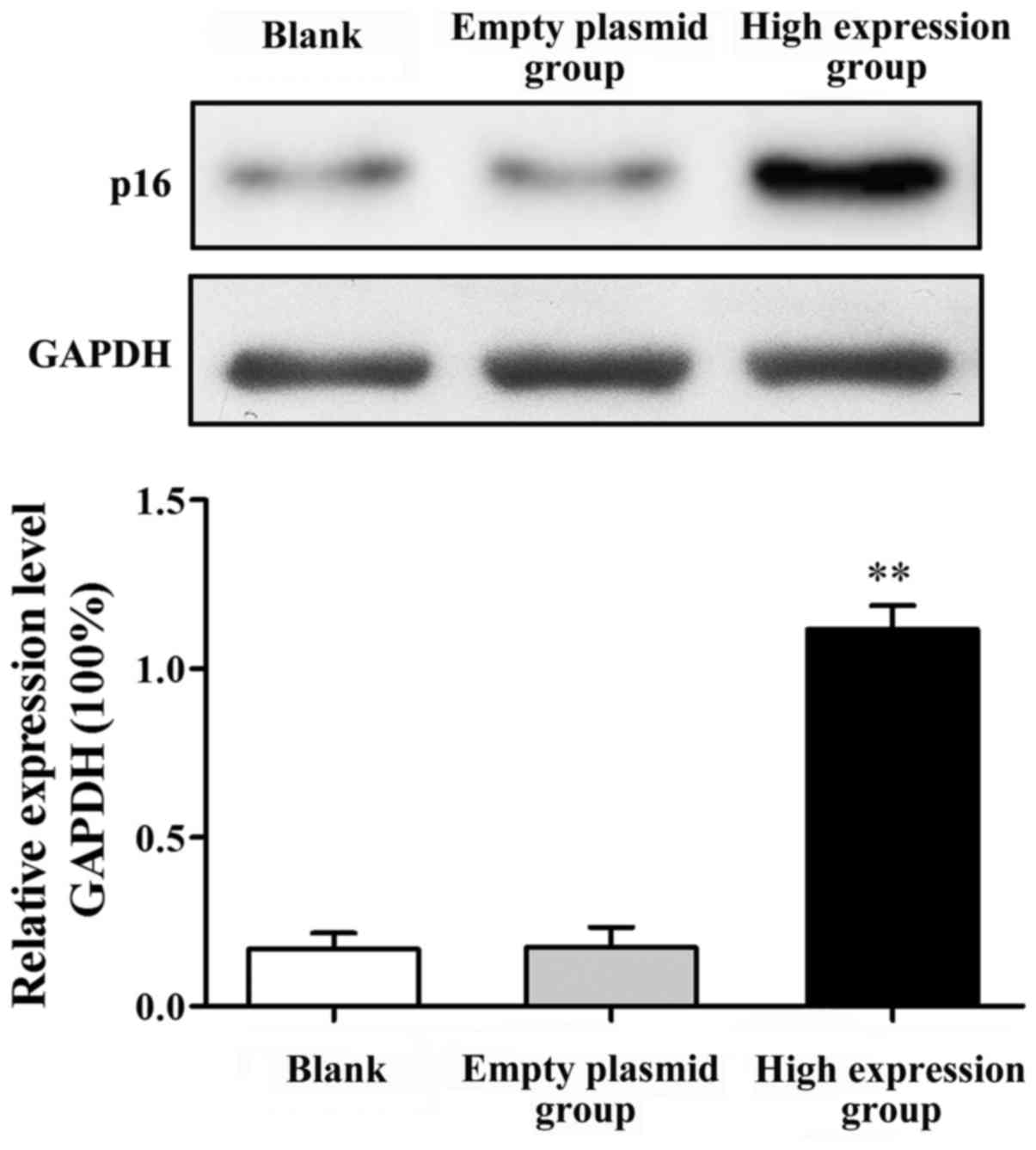
Influence of p16 gene transfection on
p16 protein expression
After SKOV-3 cell transfection, western blot
analysis was used to demonstrate p16 protein expression (Fig. 3). The p16 protein expression in the
high-expression group was significantly increased compared with
that in the blank group (P<0.01). p16 protein expression had no
significant difference between the blank group and the empty
plasmid group (P>0.05).
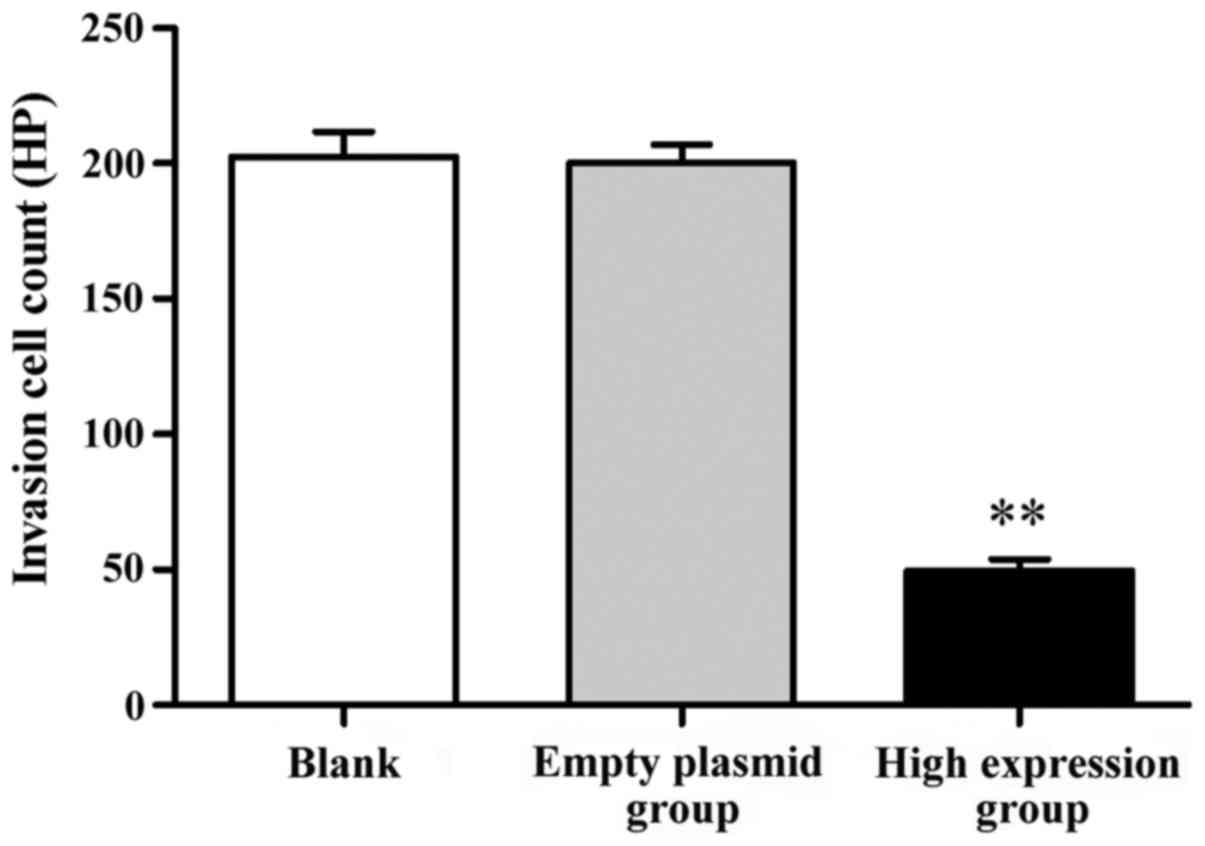
Influence of p16 gene transfection on
SKOV-3 cell invasion ability
The results showed that, after the transfection of
SKOV-3 cells by the p16 gene, the number of crossing cells was
significantly increased in the high-expression group compared with
that in the blank group, and the difference was statistically
significant (P<0.01). There was no statistically significant
difference between the blank group and the empty plasmid group,
indicating that p16 high expression could restrain the SKOV-3 cell
invasion ability (Fig. 4).
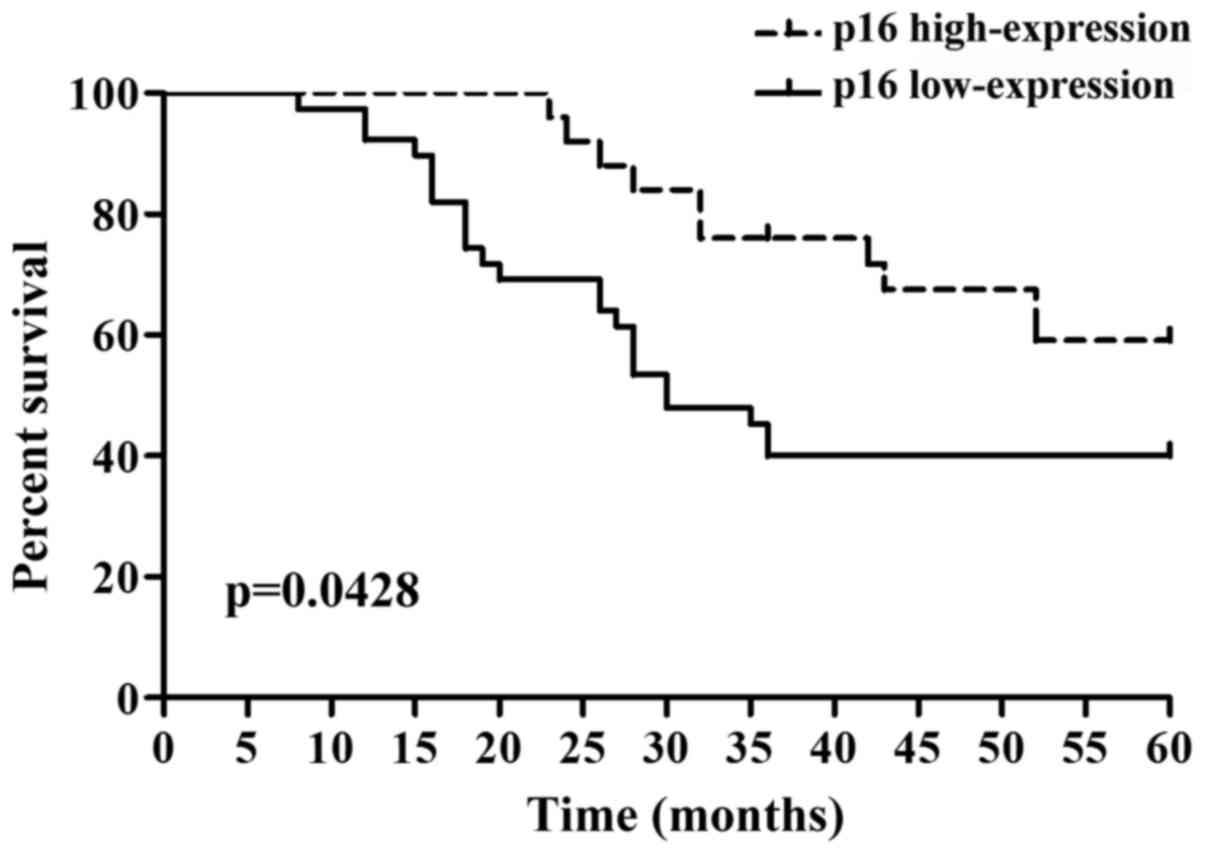
p16 expression and prognosis in
ovarian cancer
There were 39 cases of p16 low-expression with 23
cases of death and 25 cases of p16 high-expression with 10 cases of
death among a total of 64 patients with ovarian cancer in the
follow-up visit (Table IV).
Kaplan-Meier single-factor analysis showed that the expression of
p16 had a significant effect on the prognosis of patients
(P<0.05) (Fig. 5).
 | Table IV.Basic information of patients in the
follow-up visit. |
Table IV.
Basic information of patients in the
follow-up visit.
|
|
|
| Survival |
|---|
|
|
|
|
|
|---|
| p16 | Cases | of death | Cases | Ratio (%) |
|---|
| Low expression | 39 | 23 | 16 | 41.02 |
| High expression | 25 | 10 | 15 | 60.00 |
Discussion
Ovarian cancer is one of the three most common
malignant tumors of the female reproductive system. Despite
advancements in clinical treatment options for ovarian cancer, the
persisting poor prognosis of patients underscores the need for
further improvements (10).
p16 is one of the members of the INK family, which
plays a role in regulating the cell cycle, inhibiting tumor
proliferation and promoting apoptosis in cells (11). Research has shown that the mutation
rate of p16 gene is 29% in ovarian cancer cells and 15–30% in
ovarian cancer tissue (12,13). p16 is known to undergo mutation, gene
deletion and methylation in many tumors, thereby losing its normal
function (14–17). It has been found that p16 can inhibit
the expression of VEGF, thereby inhibiting tumor cell metastasis
(18,19). Other studies have confirmed that low
p16 protein expression is associated with a negative prognosis for
cancer cases (20).
The results of this study demonstrated that the
levels of p16 protein expression were decreased in SKOV-3 cells,
ovarian cancer tissues and ovarian cancer tissue with lymph node
metastasis. In order to further study the role of p16 protein, gene
transfection technology was used to increase the expression of p16
protein in SKOV-3 cells, and the results showed that the high p16
protein expression could significantly inhibit the SKOV-3 cells
invasion ability. Correlation analysis of survival prognosis showed
that lower p16 expression was negatively correlated with the
prognosis of patients with ovarian cancer. These results suggest
that p16 protein can be used as a new therapeutic target and
predictor of prognosis of patients with ovarian cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81502238) and the Key
Research and Development Projects in Shandong Province, China (no.
2016GSF201150).
References
|
1
|
Poveda Velasco A, Casado Herráez A,
Ruipérez Cervantes A, Rincón Gallardo D, García García E, Martín
González A, García López G, Fernández Mendiola C and González Ojeda
B: GEICO Group: Treatment guidelines in ovarian cancer. Clin Transl
Oncol. 9:308–316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haupt Y, Bath ML, Harris AW and Adams JM:
bmi-1 transgene induces lymphomas and collaborates with myc in
tumorigenesis. Oncogene. 8:3161–3164. 1993.PubMed/NCBI
|
|
4
|
Bhattacharyya J, Mihara K, Ohtsubo M,
Yasunaga S, Takei Y, Yanagihara K, Sakai A, Hoshi M, Takihara Y and
Kimura A: Overexpression of BMI-1 correlates with drug resistance
in B-cell lymphoma cells through the stabilization of survivin
expression. Cancer Sci. 103:34–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serrano M, Hannon GJ and Beach D: A new
regulatory motif in cell-cycle control causing specific inhibition
of cyclin D/CDK4. Nature. 366:704–707. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Khalaf HH and Aboussekhra A:
P16INK4A positively regulates p21WAF1
expression by suppressing AUF1-dependent mRNA decay. PLoS One.
8:e701332013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marx J: New tumor suppressor may rival
p53. Science. 264:344–345. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamb A, Gruis NA, Weaver-Feldhaus J, Liu
Q, Harshman K, Tavtigian SV, Stockert E, Day RS 3rd, Johnson BE and
Skolnick MH: A cell cycle regulator potentially involved in genesis
of many tumor types. Science. 264:436–440. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang Z, Ju H, Ling J, Zhuang Z, Li Z,
Wang H, Fleming JB, Freeman JW, Yu D, Huang P, et al: Cooperativity
of oncogenic K-ras and downregulated p16/INK4A in human pancreatic
tumorigenesis. PLoS One. 9:e1014522014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katsumata N: Dose-dense approaches to
ovarian cancer treatment. Curr Treat Options Oncol. 16:212015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naqshe Zahra S, Khattak NA and Mir A:
Comparative modeling and docking studies of p16ink4/Cyclin D1/Rb
pathway genes in lung cancer revealed functionally interactive
residue of RB1 and its functional partner E2F1. Theor Biol Med
Model. 10:12013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanuma T, Nishida J, Gima T, Barrett JC
and Wake N: Alterations of the p16INK4A gene in human
ovarian cancers. Mol Carcinog. 18:134–141. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schuyer M, van Staveren IL, Klijn JG, vd
Burg ME, Stoter G, Henzen-Logmans SC, Foekens JA and Berns EM:
Sporadic CDKN2 (MTS1/p16ink4) gene alterations in human ovarian
tumours. Br J Cancer. 74:1069–1073. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jha AK, Nikbakht M, Jain V, Capalash N and
Kaur J: p16INK4a and p15INK4b
gene promoter methylation in cervical cancer patients. Oncol Lett.
3:1331–1335. 2012.PubMed/NCBI
|
|
15
|
Ko E, Kim Y, Kim SJ, Joh JW, Song S, Park
CK, Park J and Kim DH: Promoter hypermethylation of the p16
gene is associated with poor prognosis in recurrent early-stage
hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev.
17:2260–2267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li G, Ji Y, Liu C, Li J and Zhou Y:
Reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 in
pancreatic carcinoma. Mol Med Rep. 5:1106–1110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai P, Xiao X, Zou J, Cui L, Bui Nguyen
TM, Liu J, Xiao J, Chang B, Wu J and Wang H: Expression of
p14ARF, p15INK4b, p16INK4a and
skp2 increases during esophageal squamous cell cancer progression.
Exp Ther Med. 3:1026–1032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeuchi H, Ozawa S, Shih CH, Ando N,
Kitagawa Y, Ueda M and Kitajima M: Loss of
p16INK4a expression is associated with vascular
endothelial growth factor expression in squamous cell carcinoma of
the esophagus. Int J Cancer. 109:483–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Ansari MM, Hendrayani SF, Tulbah A,
Al-Tweigeri T, Shehata AI and Aboussekhra A: p16INK4A
represses breast stromal fibroblasts migration/invasion and their
VEGF-A-dependent promotion of angiogenesis through Akt inhibition.
Neoplasia. 14:1269–1277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Surowiak P, Materna V, Maciejczyk A,
Pudelko M, Suchocki S, Kedzia W, Nowak-Markwitz E, Dumanska M,
Spaczynski M, Zabel M, et al: Decreased expression of p16 in
ovarian cancers represents an unfavourable prognostic factor.
Histol Histopathol. 23:531–538. 2008.PubMed/NCBI
|