Introduction
Glioblastoma is a heterogeneous and highly
aggressive brain tumor in the central nervous system. Patients with
glioblastoma often experience high relapse rates and poor prognoses
(1–3).
Although efforts have been made toward early diagnosis and targeted
therapy, there are currently no effective treatments to completely
cure patients with glioblastoma, particularly those at advanced
clinical stages (4–6). Therefore, it is important to identify
novel molecular targets that are effective regulators and
prognostic biomarkers for glioblastoma.
MicroRNAs (miRNAs/miRs) are formed from several
hundred single-stranded short RNAs that endogenously bind to the
3′-untranslated region (3′UTR) of target genes to
post-transcriptionally suppress gene production or induce protein
degradation, thus serving important roles in regulating cell and
tissue development and pathology in human and animals (7–10). In
human cancer types, miRNAs have been demonstrated to be aberrantly
upregulated or downregulated in cancerous tissues, thus serving as
either oncogenes or tumor suppressors to modulate cancer
development (10–12). In addition, cancerous miRNAs have been
identified to be effective biomarkers to predict prognosis in
patients with cancer (13–15). In human glioblastoma, various miRNAs
have been demonstrated to be either upregulated or downregulated in
glioma tumors, and played critical roles in regulating glioblastoma
proliferation, migration and chemosensitivity (16).
Amongst numerous other cancer-associated miRNAs,
miR-141 has been demonstrated to be an effective tumor regulatory
gene in various human cancer types, including ovarian cancer,
hepatocellular carcinoma, non-small cell lung cancer and renal
cancer (17–20). However, the expression pattern or
functional role of miR-141 in human glioblastoma remains
unknown.
In the present study, the expression pattern of
miR-141 was assessed in glioblastoma cell lines and clinical
samples from patients with glioblastoma. Secondly, synthetic miRNA
mimics were applied to ectopically overexpress miR-141 in
glioblastoma cell lines to examine whether miR-141 exhibited
functional mechanisms in regulating glioblastoma proliferation.
Thirdly, the correlation between cancerous miR-141 expression and
clinicopathological factors and overall survival rates (OS) of
patients with glioblastoma was analyzed. The purpose of the present
study was to investigate whether miR-141 may act as a cancer
regulator or as a prognostic biomarker for glioblastoma.
Patients and methods
Ethics, consent and permissions
In the present study, the clinical and experimental
procedures were reviewed and approved by the Ethic Committees at
Suining Central Hospital (Suining, China) and Chongqing Fourth
People's Hospital (Chongqing, China). All experiments were
conducted in accordance with the Declaration of Helsinki. Consent
forms were obtained from all patients enrolled.
Glioblastoma cell lines
Immortal glioblastoma T98G, U251, A172, LN229 and
U89 cell lines, and normal human astrocytes (NHA), were all
purchased from the Cell Bank Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). All cell lines were
maintained in RPMI-1640 medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma Aldrich; Millipore, Darmstadt, Germany) in a
tissue culturing environment with 5% CO2 at 37°C.
Clinical glioblastoma samples
Between September 2007 and September 2011, 91
patients (mean age, 56±6.3 years; 52 male and 39 female) with
glioblastoma were enrolled in the study at Suining Central Hospital
and Chongqing Fourth People's Hospital. Clinical samples of
glioblastoma tumors and adjacent non-tumorous brain tissues were
obtained during surgical resection. The pathological grade of
tumors, based on magnetic resonance imaging scans and histological
examination, were evaluated by a joint-team of histologists,
pathologists and radiologists according to the World Health
Organization (WHO) classification of brain tumors (21). All tumorous or non-tumorous brain
samples, once obtained from patients, were immediately snap-frozen
in liquid nitrogen and stored at −80°C for future RNA
extraction.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from clinical samples using a
TRIzol kit (Thermo Fisher Scientific, Inc.) and purified using a
QiaQuick PCR Purification kit (Qiagen, Inc., Valencia, CA, USA)
according to the manufacturers' protocols. The quantity of
extracted RNA was examined by a NanoDrop-3000 spectrophotometer
(Thermo Fisher Scientific, Inc.). A total of 5 µg RNA from each
sample was reverse synthesized to complementary DNA using a TaqMan
MicroRNA Reverse Transcription kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Gene expression of human
miR-141 (hsa-miR-141) was quantified through RT-qPCR using a TaqMan
MicroRNA assay kit (Thermo Fisher Scientific, Inc.) on an ABI Prism
7000 Sequence Detection system (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. The thermocycling
conditions used were as follows: 30 sec at 95°C; 30 sec at 60°C;
and 30 sec at 72°C for 35 cycles. The following primers were used:
hsa-miR-141 forward, 5′-CGCTAACACTGTCTGGTAAAG-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; U6 snRNA forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′. The relative expression of hsa-miR-141
was measured as a fold-change and was normalized to U6 small
nuclear RNA expression in control samples using the
2−ΔΔCq method (22).
miR-141 upregulation in
glioblastoma
Synthetic human miR-141 mimics, miR-141-mimic, and
its non-specific control miRNA, miR-Ctrl, were purchased from
Sunbiotech Co., Ltd. (Beijing, China). The glioblastoma LN229 and
U89 cell lines were transfected for 24 h at 37°C with 100 nM
miR-141-mimic or 100 nM miR-Ctrl using the Oligofectamine 2000
transfection reagent (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. The cultures were replenished with
fresh RPMI-1640 medium without synthetic miRNAs at 48 h
post-transfection. Subsequent experiments, such as RT-qPCR using
the same conditions as stated above and a proliferation assay were
performed.
Cancer proliferation assay
The in vitro proliferation of glioblastoma
cells was performed using an MTT assay (Applied Science, Grass
Valley, CA, USA) according to the manufacturer's protocols.
Subsequent to transfection with synthetic miRNAs, LN229 and U89
cells were maintained in 96-well plates at a density of 5,000
cells/well for 5 days. Every 24 h, 20 µl 1X MTT medium was added
into 96-well plates for 4 h, followed by 20 min application of
dimethyl sulphoxide. The 96-well plates were then examined using a
Synergy 2 multi-mode microplate reader (BioTek Instruments, Inc.,
Winooshi, VT, USA) at an absorbance of 490 nm according to the
manufacturer's protocol.
Statistical analysis
All experiments were conducted in triplicate and the
data are presented as the mean ± standard deviation. All
statistical analyses were conducted using SPSS software v11.0
(SPSS, Inc., Chicago, IL, USA). An unpaired two-tailed Student's
t-test was used to compare paired or grouped samples. The
associations between miR-141 and the clinicopathological features
of the patients were analyzed using rank sum and χ2
tests. The associations between the clinicopathological properties
of the patients with OS were analyzed using forward univariate or
multivariate Cox proportional hazards model with 95% confidence
intervals (CIs). The OS of the patients was estimated using a
Kaplan-Meier model and compared by log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-141 is aberrantly downregulated in
glioblastoma cell lines and human tumors
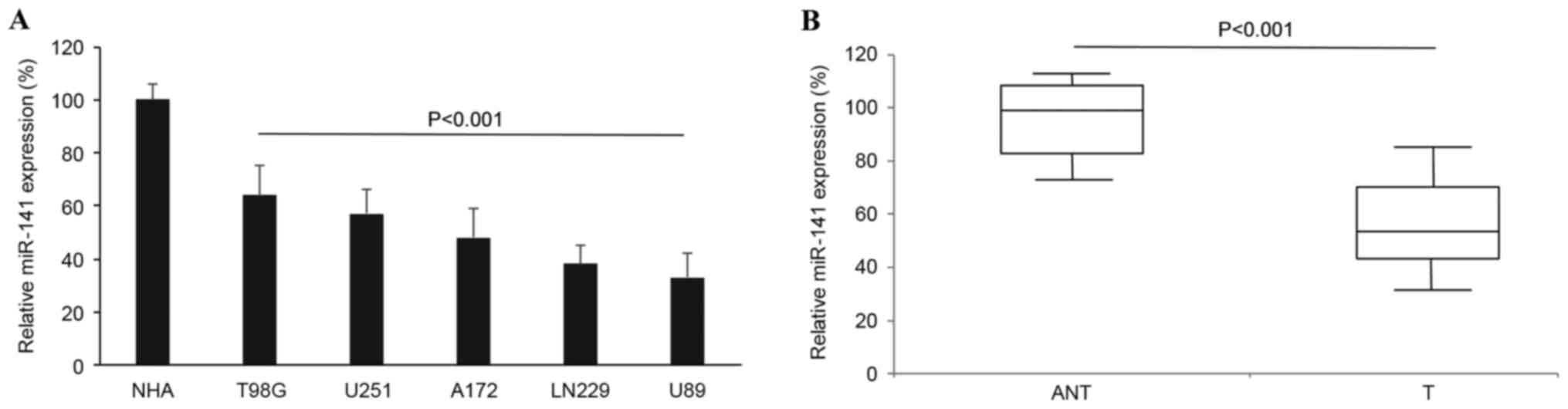
The gene expression pattern of miR-141 among
glioblastoma cell lines and human tumors was examined. In 5
glioblastoma cell lines, T98G, U251, A172, LN229 and U89, miR-141
expression was quantified by RT-qPCR and analyzed against miR-141
expression in NHAs. It was revealed that miR-141 was significantly
downregulated in the glioblastoma cell lines compared with the
level in the NHAs (Fig. 1A;
P<0.001). Secondly, miR-141 expression was examined in human
samples. In 91 patients with glioblastoma, glioblastoma tumors and
adjacent non-tumorous brain tissues were surgically sampled and
their endogenous miR-141 expression levels were compared using
RT-qPCR. Similar to the result in glioblastoma cell lines, it was
demonstrated that miR-141 was also aberrantly downregulated in
human glioblastoma tumors compared with the expression in the
non-tumorous brain tissues (Fig. 1B;
P<0.001).
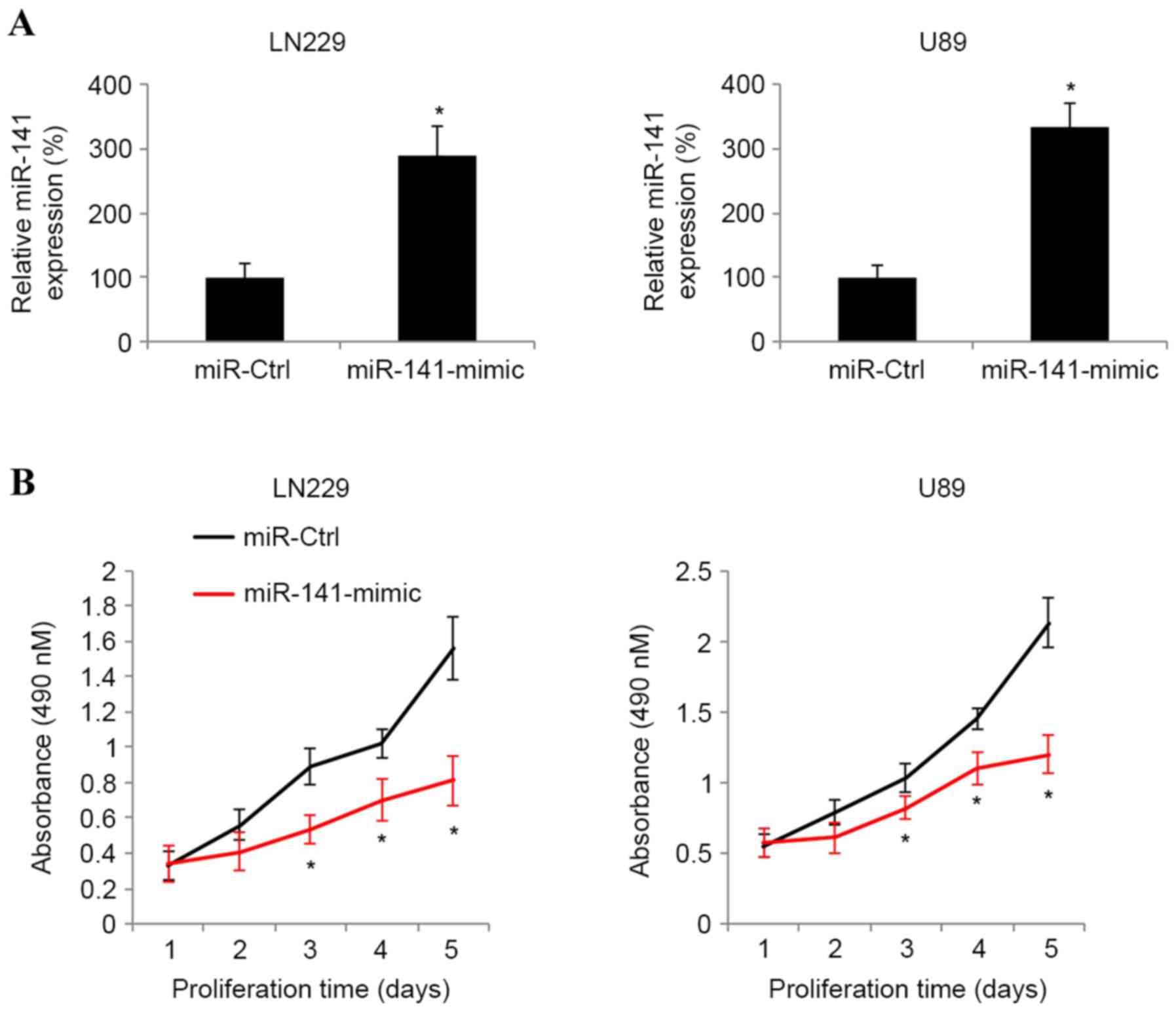
Induced miR-141 overexpression
inhibits cancer proliferation in glioblastoma
The present study next assessed whether miR-141
exhibited a functional role in glioblastoma. The glioblastoma LN229
and U89 cell lines were transfected with synthetic miR-141-mimic
miRNAs to induce endogenous overexpression of miR-141. Control
LN229 and U89 cells were transfected with a non-specific control
synthetic miRNA, miR-Ctrl. RT-qPCR analysis indicated that at 2
days post-transfection, endogenous miR-141 was significantly
upregulated in the glioblastoma cells transfected with
miR-141-mimic compared with the glioblastoma cells transfected with
miR-Ctrl (Fig. 2A; P<0.05).
The regulatory effect of miR-141 upregulation on
glioblastoma growth was then examined. Transfected LN229 and U89
cells were seeded into 96-well plates and maintained for 5 days. An
MTT assay was performed every 24 h during a 5-day period to compare
cancer proliferation between glioblastoma cells. It was revealed
that in the LN229 and U89 cells lines, miR-141 upregulation
markedly suppressed cancer cell proliferation (Fig. 2B; P<0.05).
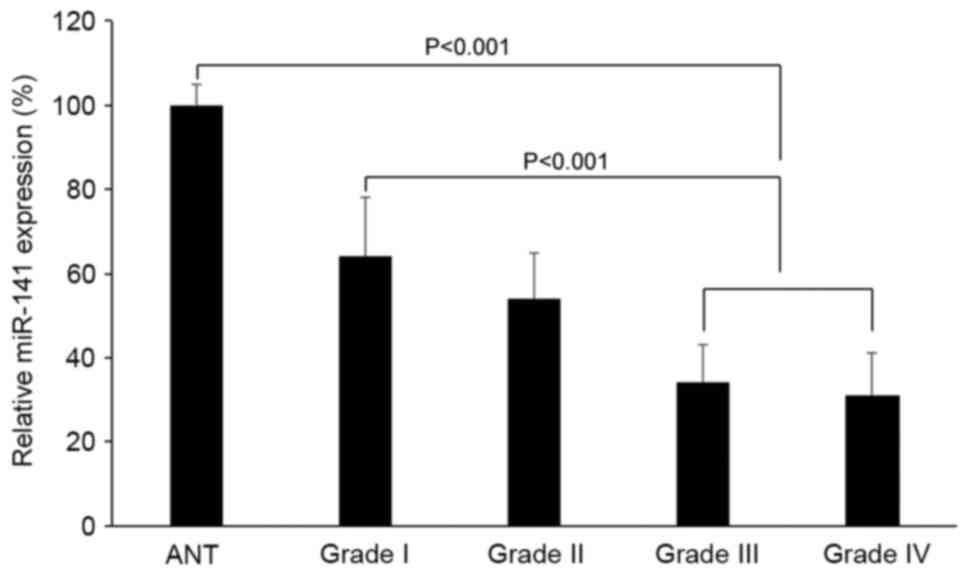
miR-141 is correlated with
clinicopathological properties of patients with glioblastoma
In 91 patients with glioblastoma, 25 exhibited WHO
grade I pilocytic astrocytomas, 23 exhibited WHO grade II diffuse
astrocytomas, 21 exhibited WHO grade III anaplasia astrocytomas and
22 exhibited WHO grade IV primary glioblastomas. The miR-141
expression levels were compared based on the tumor grades. It was
demonstrated that miR-141 expression was downregulated in grade III
or IV tumors compared with non-tumorous brain tissues (Fig. 3; P<0.05). It was also demonstrated
that miR-141 expression was significantly downregulated in grade
III or IV tumors compared with grade I or grade II tumors (Fig. 3; P<0.05).
To understand the correlation between miR-141
expression and the prognoses of patients with glioblastoma,
patients were divided into two subgroups. One group of 47 patients
was characterized to exhibit miR-141 expression levels lower than
the median value. The other group of 44 patients exhibited miR-141
expression levels higher than the median value. The
clinicopathological properties of the patients were statistically
analyzed based on miR-141 expression levels, and it was identified
that miR-141 expression demonstrated no association with patient
gender, age or tumor size (Table I).
Conversely, miR-141 expression was revealed to be significantly
correlated with the pathological grade and Karnofsky Performance
Scale (KPS) of the patients (Table I;
P<0.05).
 | Table I.Association of tumor miR-141
expression with clinicopathological properties of the patients
(n=91). |
Table I.
Association of tumor miR-141
expression with clinicopathological properties of the patients
(n=91).
|
| miR-141 expression, n
(%) |
|
|---|
|
|
|
|
|---|
|
Clinicopathol-ogicalproperty | Low (n=47) | High (n=44) | P-value |
|---|
| Gender |
|
|
|
| Male | 31 (65.96) | 30 (68.18) | N.S.S. |
|
Female | 16 (34.04) | 14 (31.82) |
|
| Age, years |
|
|
|
|
<55 | 12 (25.53) | 7 (15.91) | N.S.S. |
| ≥55 | 35 (74.47) | 37 (84.09) |
|
| WHO grade |
|
|
|
| I | 7 (14.84) | 18 (40.91) | 0.002 |
| II | 8 (17.02) | 15 (34.09) |
|
| III | 14 (29.79) | 7 (15.91) |
|
| IV | 18 (38.30) | 4 (9.09) |
|
| KPS |
|
|
|
|
<80 | 35 (74.47) | 14 (31.82) | 0.013 |
| ≥80 | 12 (25.53) | 30 (68.18) |
|
| Tumor size, cm |
|
|
|
|
<6 | 22 (46.81) | 27 (61.36) | N.S.S. |
| ≥6 | 25 (53.19) | 17 (38.64) |
|
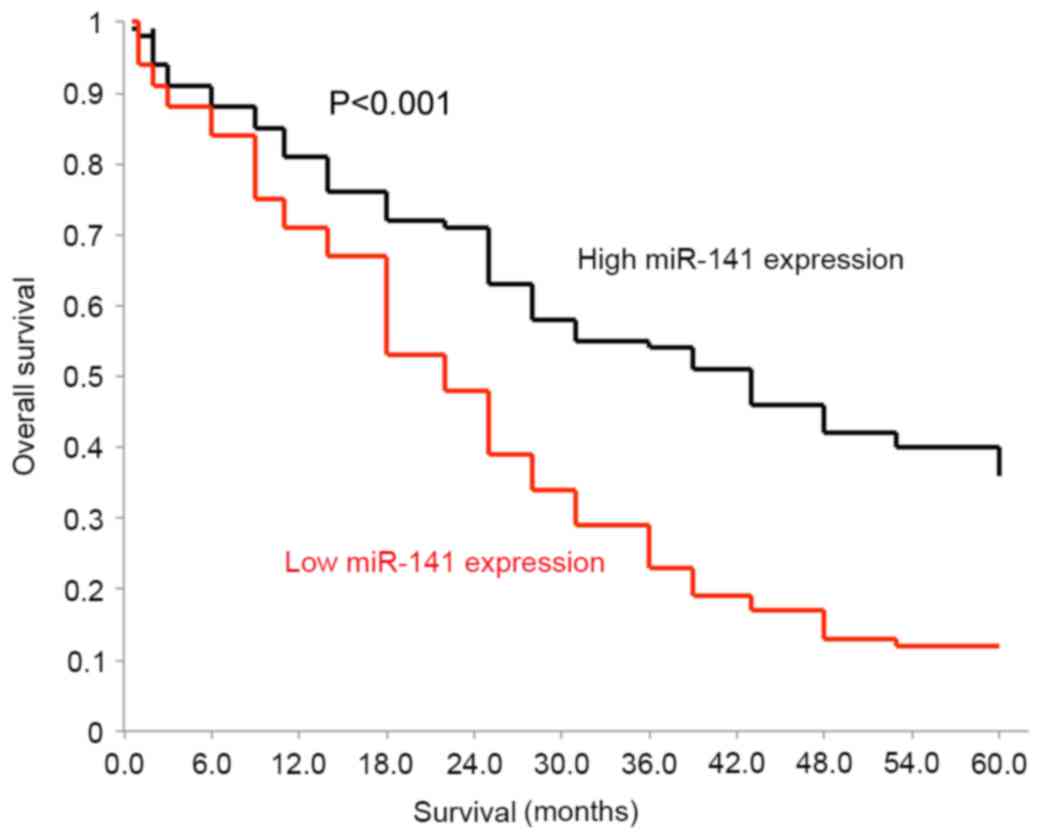
miR-141 is a prognostic biomarker for
survival of patients with glioblastoma
A Kaplan-Meier model was applied to separately
assess the OS of patients with glioblastoma and high endogenous
miR-141 expression levels, and those with low endogenous miR-141
expression levels. Using a log-rank test, it was identified that
the two OS curves were significantly different. Patients with low
endogenous miR-141 expression levels exhibited much poorer OS
compared with patients with high endogenous miR-141 expression
levels (Fig. 4; P<0.001). A Cox
regression model was applied to assess the association of the
clinicopathological properties of the patients with OS. Through the
univariate analysis, it was revealed that the WHO grade (95% CI,
3.89–5.40; P=0.020), KPS (95% CI, 1.04–4.78; P=0.241) and
endogenous miR-141 expression (95% CI, 4.83–9.63; P=0.003) of the
patients were significantly associated with OS (Table II). In addition, the multivariate
analysis demonstrated that the WHO grade (95% CI, 2.89–5.92;
P=0.107), and endogenous miR-141 expression (95% CI, 3.24–6.36;
P=0.007) of the patients were independent prognostic factors for OS
in patients with glioblastoma (Table
II).
 | Table II.Univariate and multivariate Cox
regression model of prognostic properties in patients with
glioblastoma. |
Table II.
Univariate and multivariate Cox
regression model of prognostic properties in patients with
glioblastoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Prognostic
features | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Gender | N.S.S. | 0.87 (0.63–1.41) |
|
|
| Age, years | N.S.S. | 1.21 (0.89–4.29) |
|
|
| WHO grade | 0.029 | 4.33 (3.89–5.40) | 0.107 | 3.58 (2.89–5.92) |
| KPS | 0.241 | 1.93 (1.04–4.78) | N.S.S. | 1.28
(0.67–2.71) |
| Tumor size, cm | N.S.S. | 2.04
(1.18–5.31) |
|
|
| miR-141
expression | 0.003 | 6.73
(4.83–9.63) | 0.007 | 4.12
(3.24–6.36) |
Discussion
miR-141 has been demonstrated to be an active
onco-regulator in various types of human cancer (17–20).
However, the expression pattern or mechanistic role of miR-141 in
human glioblastoma remains unknown. In the present study,
quantitative methods were used to compare miR-141 expression
between 5 in vitro glioblastoma cell lines and their
adjacent non-tumorous brain tissues. The results of the RT-qPCR
analysis clearly demonstrated that miR-141 was markedly
downregulated in in vitro glioblastoma cell lines and in
vivo glioblastoma tumors. These data are concurrent with
previous studies demonstrating decreased miR-141 expression in
hepatocellular carcinoma, and renal and gastric cancer (17,20,23),
suggesting that miR-141 downregulation or dysregulation may be the
predominant expression pattern of miR-141 within various types of
cancer.
Secondly, the functional role of miR-141 in
regulating glioblastoma was investigated in the present study. It
was revealed that induced miR-141 overexpression may effectively
inhibit glioblastoma proliferation in vitro. A tumor
suppressive role of miR-141 has also been observed in other types
of human cancer. For example, miR-141 overexpression was
demonstrated to induce cell-cycle arrest in renal cell carcinoma
(20). Notably, miR-141 may also act
as an oncogenic factor, with actions such as increasing cisplatin
chemoresistance in epithelial ovarian cancer (18). Thus, although miR-141 may be
predominantly downregulated in human cancer, the functional
mechanisms of miR-141 may be more complex, including roles as a
tumor suppressor or an oncogene, depending on the different
downstream signaling pathways associated with miR-141 regulation in
different types of cancer (24).
It was previously demonstrated that plasma miR-141
may be an effective biomarker in colon and colorectal cancer
(25,26). In addition to the RT-qPCR analysis
demonstrating miR-141 downregulation in glioblastoma cell lines and
human tumors, the present study also identified that miR-141
downregulation was associated with glioblastoma tumors of advanced
stages (Fig. 3). These data indicated
that cancerous miR-141 may be a potential cancer biomarker for
patients with glioblastoma. The clinicopathological properties and
OS of patients with glioblastoma were analyzed to evaluate the
significance of miR-141 correlation. It was revealed that low
cancerous miR-141 expression was likely to be associated with
advanced clinicopathological features in patients with
glioblastoma. Also, it was demonstrated that low cancerous miR-141
expression was significantly correlated with poor survival in
patients with glioblastoma. Additionally, multivariate Cox
regression analysis demonstrated that cancerous miR-141 expression
may be an independent predicting factor for glioblastoma. Thus, the
analysis of the clinical data of the present suggested that miR-141
may act as a tumor suppressor in regulating cancer proliferation
and may serve as a potential biomarker for glioblastoma.
Overall, the present study provided clear molecular
and clinical implications for miR-141 in glioblastoma. miR-141 is
significantly downregulated in glioblastoma cell lines and human
tumor samples. miR-141 may act as a functional tumor suppressor to
inhibit cancer proliferation in glioblastoma. Additionally, miR-141
is closely associated with the clinicopathological properties of
patients with glioblastoma, and may be a prognostic biomarker for
glioblastoma.
Acknowledgements
The present study was supported by the Health and
Family Planning Commission Research of Sichuan Province (grant no.
150245).
References
|
1
|
Alifieris C and Trafalis DT: Glioblastoma
multiforme: Pathogenesis and treatment. Pharmacol Ther. 152:63–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ellis HP, Greenslade M, Powell B, Spiteri
I, Sottoriva A and Kurian KM: Current challenges in glioblastoma:
Intratumour heterogeneity, residual disease, and models to predict
disease recurrence. Front Oncol. 5:2512015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jovčevska I, Kočevar N and Komel R: Glioma
and glioblastoma - how much do we (not) know? Mol Clin Oncol.
1:935–941. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kapoor A, Mohindra S, Singla N, Sodhi HB,
Chatterjee D and Gupta SK: Multiple glioblastoma: A diagnostic
challenge and controversies in management. Neurol India.
63:449–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Ye G, Li J and Wang Y: Recent
advance in molecular angiogenesis in glioblastoma: The challenge
and hope for anti-angiogenic therapy. Brain Tumor Pathol.
32:229–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cloughesy TF, Cavenee WK and Mischel PS:
Glioblastoma: From molecular pathology to targeted treatment. Annu
Rev Pathol. 9:1–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guarnieri DJ and DiLeone RJ: MicroRNAs: A
new class of gene regulators. Ann Med. 40:197–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Erson AE and Petty EM: MicroRNAs in
development and disease. Clin Genet. 74:296–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao S and Liu MF: Mechanisms of
microRNA-mediated gene regulation. Sci China C Life Sci.
52:1111–1116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: Past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kohlhapp FJ, Mitra AK, Lengyel E and Peter
ME: MicroRNAs as mediators and communicators between cancer cells
and the tumor microenvironment. Oncogene. 34:5857–5868. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Chen J and Sen S: MicroRNA as
biomarkers and diagnostics. J Cell Physiol. 231:25–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lan H, Lu H, Wang X and Jin H: MicroRNAs
as potential biomarkers in cancer: Opportunities and challenges.
Biomed Res Int. 2015:1250942015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Møller HG, Rasmussen AP, Andersen HH,
Johnsen KB, Henriksen M and Duroux M: A systematic review of
microRNA in glioblastoma multiforme: Micro-modulators in the
mesenchymal mode of migration and invasion. Mol Neurobiol.
47:131–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue J, Niu YF, Huang J, Peng G, Wang LX,
Yang YH and Li YQ: miR-141 suppresses the growth and metastasis of
HCC cells by targeting E2F3. Tumour Biol. 35:12103–12107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Jaarsveld MT, Helleman J, Boersma AW,
van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH,
Berns EM, Verweij J, et al: miR-141 regulates KEAP1 and modulates
cisplatin sensitivity in ovarian cancer cells. Oncogene.
32:4284–4293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tejero R, Navarro A, Campayo M, Vinolas N,
Marrades RM, Cordeiro A, Ruiz-Martinez M, Santasusagna S, Molins L,
Ramirez J and Monzó M: miR-141 and miR-200c as markers of overall
survival in early stage non-small cell lung cancer adenocarcinoma.
PLoS One. 9:e1018992014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Wang X, Ruan A, Han W, Zhao Y, Lu
X, Xiao P, Shi H, Wang R, Chen L, et al: miR-141 is a key regulator
of renal cell carcinoma proliferation and metastasis by controlling
EphA2 expression. Clin Cancer Res. 20:2617–2630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feiden S and Feiden W: WHO classification
of tumours of the CNS: Revised edition of 2007 with critical
comments on the typing und grading of common-type diffuse gliomas.
Pathologe. 29:411–421. 2008.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du Y, Xu Y, Ding L, Yao H, Yu H, Zhou T
and Si J: Down-regulation of miR-141 in gastric cancer and its
involvement in cell growth. J Gastroenterol. 44:556–561. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vrba L, Jensen TJ, Garbe JC, Heimark RL,
Cress AE, Dickinson S, Stampfer MR and Futscher BW: Role for DNA
methylation in the regulation of miR-200c and miR-141 expression in
normal and cancer cells. PLoS One. 5:e86972010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng H, Zhang L, Cogdell DE, Zheng H,
Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR and Zhang W:
Circulating plasma MiR-141 is a novel biomarker for metastatic
colon cancer and predicts poor prognosis. PLoS One. 6:e177452011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|