Introduction
Breast cancer is the most common malignancy in women
worldwide. Various histological types of breast cancer have been
reported, with invasive ductal carcinoma (invasive carcinoma of no
special type) being the most frequently occurring type (1,2).
Therefore, considerable effort has been devoted to identifying
factors of prognostic and therapeutic significance in invasive
ductal carcinoma. The immunohistochemical expression of estrogen
receptor (ER), progesterone receptor (PR) and human epithelial
growth factor receptor 2 (HER2) has been widely used for predicting
the prognosis of breast cancer and for providing therapeutic
strategies (3). Since Perou et
al (4) reported the molecular
features of breast cancer cells in 2000, the improvements in
molecular techniques have provided a framework to establish
molecular subtypes, namely luminal A, luminal B HER2−,
luminal B HER2+, triple-negative, HER2 type, 5 negative
phenotype and basal phenotype breast cancer (5,6). Breast
cancer-expressed hormonal receptors, including ER and PR, or
amplification of HER2, have been used in various targeted treatment
approaches (7,8).
A targeted therapy has not yet been established for
TNBC. Therefore, chemotherapy with a platinum-based agent remains
in use as a common treatment of choice for TNBC (9). Excision repair cross-complementation
group 1 (ERCC1)-xeroderma pigmentosum complementation group F (XPF)
complex repairs DNA damaged by anticancer agents; studies have
reported that ERCC1 expression is an important factor in
determining the poor response of chemotherapy (10,11).
Certain studies have also reported that the expression of ERCC1 in
TNBC may be a predictive factor of a poor response to
platinum-based chemotherapy (12,13). By
contrast, others studies have reported that there is no association
between ERCC1 expression and TNBC (14,15).
Another study reported that TNBC showed the lowest ERCC1 expression
among other breast cancer subtype based on the expression of
hormonal receptor (16). Furthermore,
these studies showed that the high expression of ERCC1 was
correlated with the clinicopathological factors associated with a
good prognosis (14,15).
Thus, the expression of ERCC1 in breast cancer has
provided ambivalent results. Therefore, the present study evaluated
the association between various clinicopathological parameters and
ERCC1 expression in invasive ductal breast carcinoma. Furthermore,
the study also analyzed the prognosis, depending on the level of
ERCC1 expression, in this carcinoma.
Materials and methods
Patient selection
A total of 224 patients with invasive ductal breast
cancer, who were diagnosed and treated at the Kangbuk Samsung
Hospital (Sungkyunkwan University School of Medicine, Seoul, South
Korea) between January 2006 and April 2010 were enrolled. Patients
who received preoperative treatment and had other diseases were
excluded. Patients who performed biopsy for pathologic diagnosis
were also excluded. All studies were conducted with the prior
approval of the Institutional Review Board of Kangbuk Samsung
Hospital. The requirement for patient consent for publication of
this study was waived. The following clinicopathological parameters
were included: Patient age, presence of an extensive intraductal
component (EIC), skin or chest wall invasion, Paget's disease,
lymphovascular invasion (LVI), tumor borders, ER positivity, PR
positivity, HER-2 positivity, triple negativity,
Tumor-Node-Metastasis (TNM) stage (17), presence of lymph node metastasis,
distant metastasis and mortality due to breast cancer. Histological
grades were assigned using tubule formation, nuclear pleomorphism,
and mitotic counts based on the modified Bloom-Richardson grading
system (18). The tissue samples were
formalin fixed at room temperature for more than 8 h and they were
paraffin embedded representatively. Tissue section (3-µm-thick)
were stained with hematoxylin (at room temperature for 90 sec) and
eosin (at room temperature for 40 sec) using Dako Coverstainer
fully automated system (Dako, Agilent Technologies, Inc., Santa
Clara, CA, USA) and slides from all patients were reviewed by two
pathologists in a blind manner with an Olympus BX51 microscope, and
the histological data such as T and N stage, and lymphatic
invasion, were confirmed again. The discrepant cases were reviewed
by the two pathologists together to achieve a consensus result.
Tissue microarray (TMA)
construction
The surgically resected specimens were fixed in 10%
buffered formalin at room temperature for 24 h, processed and
embedded in paraffin using a standard protocol. All H&E-stained
slides were reviewed and the most representative tumor area was
carefully selected and marked on individual paraffin blocks. The
most representative tissue core was obtained from each tumor
specimen. The TMA specimens were assembled using a tissue-array
instrument (TMA Master; 3D HISTECH Kft., Budapest, Hungary)
consisting of thin-walled stainless steel punches and stylets for
emptying and transferring the needle contents. The assembly was
held in an X-Y position guide with a 1-mm increment between the
individual samples, a 4-mm punch depth stop device and
semiautomatic micrometers. The instrument was used to create holes
in the recipient block with defined array cores. The fit needle was
used to transfer the tissue cores into the recipient block. Taking
into consideration the limitations of the representative areas of
the tumor, duplicate 2-mm-diameter tissue cores were used from each
donor block. The percentage of tissue cores with tumor was
≥70%.
Immunohistochemistry and
immunohistochemical evaluation
Immunohistochemistry analysis was performed using
Leica BOND-MAX™ fully automated immunohistochemistry system,
according to the manufacture's protocol (Leica Microsystems GmbH,
Wetzlar, Germany). Briefly, 4-µm-thick sections were deparaffinized
and pre-treated with the Epitope Retrieval Solution 2 (EDTA-buffer
pH 8.8) at 98°C for 20 min. After the tissue washed three times
with Bond TM Wash Solution 10X concentrate (cat. no. AR9590),
peroxidase blocking was performed for 10 min using the Bond Polymer
Refine Detection kit DS9800 (Leica Microsystems GmbH). Tissues were
again washed three times with Bond TM Wash Solution 10X concentrate
(cat. no. AR9590) and then incubated with the primary antibodies at
room temperature for 60 min. Subsequently, tissues were incubated
with polymer for 10 min and developed using 3,3-diaminobenzidine at
room temperature for 10 min. ER (cat. no. RM-9101-F; 1:200
dilution; SP1 clone; Labvision Corporation, Fremont, CA, USA), PR
(cat. no. M3569; 1:200 dilution; PgR636 clone; Dako; Agilent
Technologies, Inc.) and HER2 (cat. no. RM-9103-R7-A; 1:200
dilution; SP3 clone; Labvision Corporation) antibodies were used.
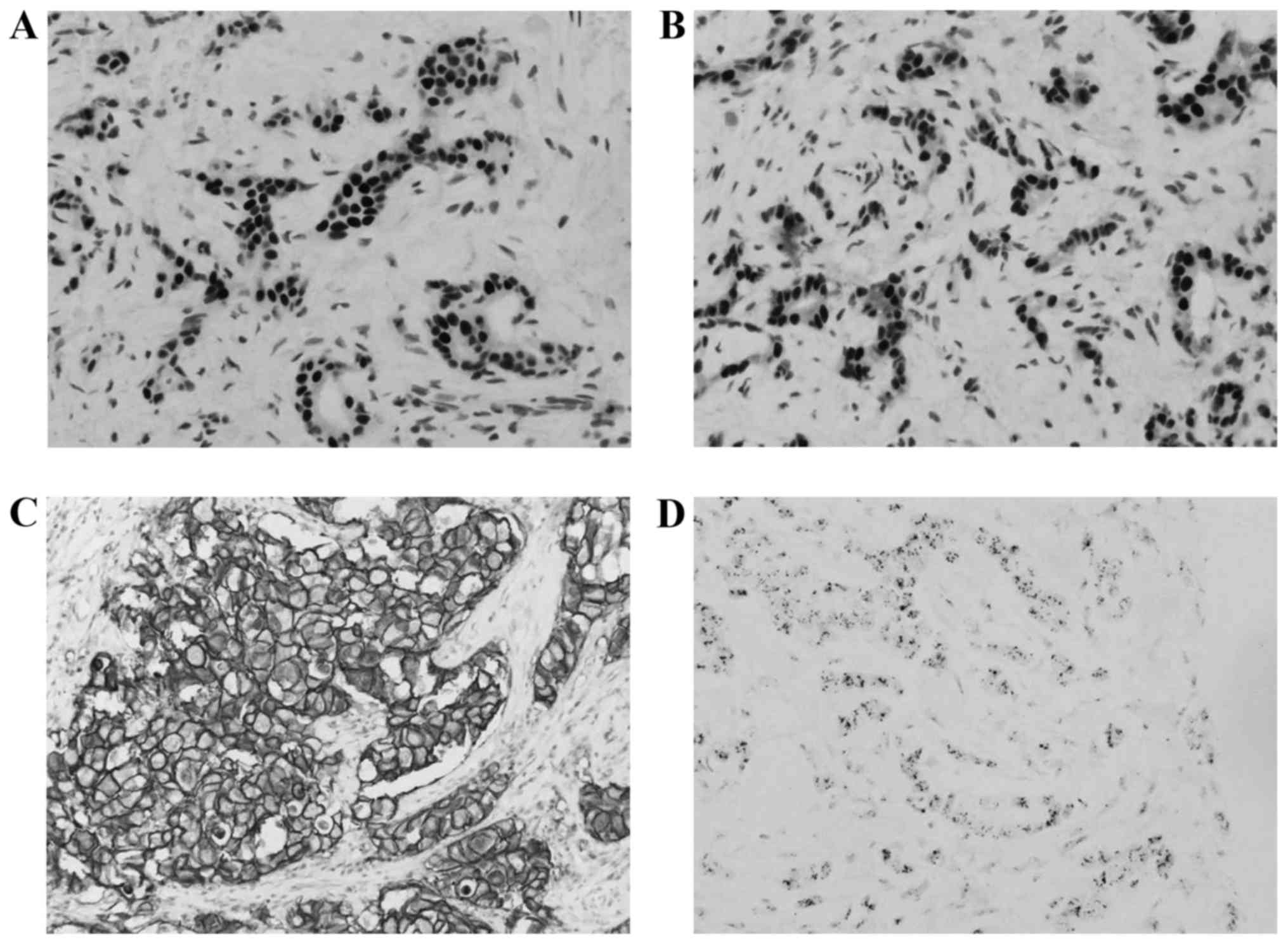
ER and PR expression was evaluated by Allred scoring (19) and HER2 expression was evaluated by
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations (20). In case with equivocal scores (HER2
score 2) (20), silver in situ
hybridization was performed for the determination of HER2 gene
status (Fig. 1).
ERCC1 immunohistochemistry staining
and immunohistochemical evaluation
Human tissues obtained were fixed in 10% formalin
solution at room temperature for 24 h, dehydrated through a graded
ethanol series, washed in xylene and processed for embedding in
paraffin wax, according to routine protocols. Sections were
incubated in a solution of 0.3% H2O2 at room
temperature for 15 min to inhibit endogenous peroxidase activity.
Antigen retrieval procedure was performed using 10 mM Tris + 1 mM
EDTA + 0.03% Tween-20 Solution for 30 min in a presser cooker
chamber. Non-specific blocking was quenched by incubation with 4%
bovine serum albumin for 30 min. Sections were then incubated for 1
h at room temperature with primary antibodies against ERCC1 (cat.
no. ab2356; Abcam, Cambridge, MA, USA) diluted to 1:100. The
detection system EnVision+ for secondary horseradish
peroxidase-conjugated mouse antibodies (cat. no. K4001; 1:2,000;
Dako; Agilent Technologies, Inc.) was applied according to the
manufacturer's instructions. The secondary antibodies were
incubated at room temperature for 8 min. Slides were stained with
liquid diaminobenzidine tetrahydrochloride, a high-sensitivity
substrate-chromogen system (cat. no. K5007; Dako; Agilent
Technologies, Inc.). Counterstaining was performed with Meyer's
hematoxylin at room temperature for 1 min.
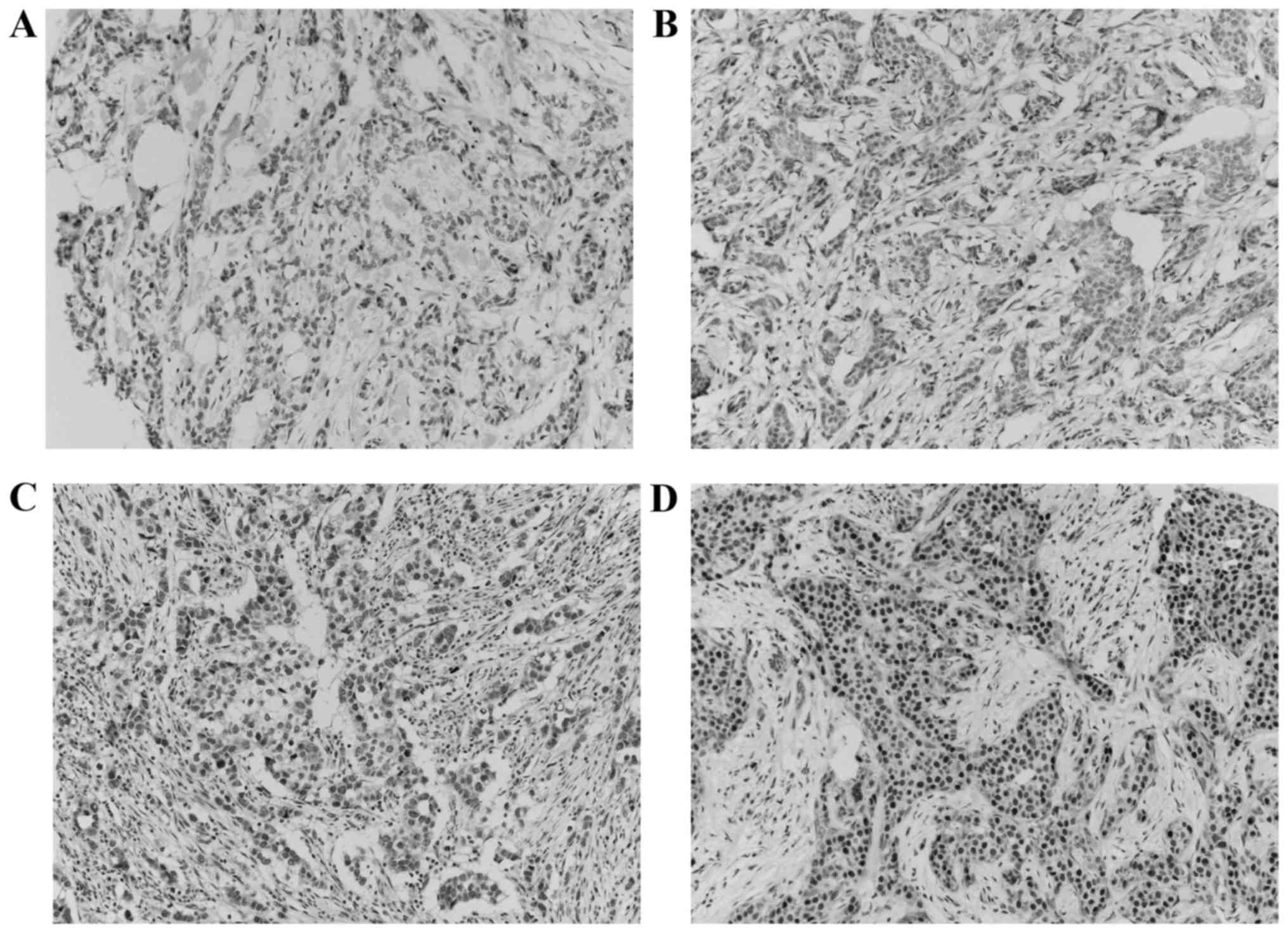
The images on the slides were visualized with an
Olympus BX51 light microscope (Olympus, Tokyo, Japan). Staining
intensity was scored on a scale of 0 to 3 (0, negative; 1, weak; 2,
moderate; and 3, strong) (Fig. 2).
The percentage of positive cells was also classified into one of
four categories: Score of 1, 0–25%; score of 2, 26–50%; score of 3,
51–75%; and score of 4, 76–100%. When a discrepancy occurred
between duplicate cores, the higher score of the two tissue cores
was used as the final score. The level of staining was analyzed as
an immunoreactive score (IRS), which was calculated by multiplying
together the score of the staining intensity and the percentage of
positive cells (21). The expression
was classified into low expression (IRS≤7) and high expression
(IRS>7) groups, according to a previous study (21).
Statistical analysis
Statistical analyses were performed with PASW
Statistics for Windows, version 18.0 (SPSS, Inc., Chicago, IL,
USA). The χ2 test, Fisher's exact test and Student's
t-test were used to evaluate the associations between ERCC1
expression and clinicopathological parameters. Multivariate
logistic regression analysis was used to identify the
clinicopathological predictors of ERCC1 high expression.
Disease-free survival (DFS) was defined from the day of surgery to
the day of recurrence. Overall survival (OS) was defined from the
day of diagnosis to the day of the patient mortality from breast
cancer or last known follow-up. Survival probability curves were
calculated by life table method, and Gehan's generalized Wilcoxon
method was applied for analyzing the univariate survival
clinicopathological parameters. P≤0.05 was considered to indicate a
statistically significant difference. Multivariate survival
parameters were detected among parameters that were statistically
significant in univariate analysis by applying the Cox proportional
hazards model (95% confidence interval) with a backward stepwise
elimination method.
Results
ERCC1 immunohistochemical staining was performed for
all 224 invasive ductal carcinoma cases. ERCC1 showed a nuclear
expression pattern in all cases. The cut-off value of IRS was 7,
and IRS>7 referred to high expression. Among the 224 cases, high
expression of ERCC1 was observed in 33 cases (14.7%).
Clinicopathological and immunohistochemical parameters, including
the expression of ERCC1, are shown in Table I.
 | Table I.Clinicopathological parameters and
immunohistochemical results. |
Table I.
Clinicopathological parameters and
immunohistochemical results.
| Parameter | No. | % |
|---|
| Age, years |
|
|
| ≤52 | 129 | 57.6 |
|
>52 | 95 | 42.4 |
| T stage |
|
|
| 1 | 111 | 49.6 |
| 2 | 102 | 45.5 |
| 3 | 11 | 4.9 |
| 4 |
0 | 0 |
| Tumor size, cm |
|
|
| ≤2.0 | 111 | 49.6 |
|
>2.0 | 113 | 50.4 |
| N stage |
|
|
| 0 | 120 | 53.6 |
| 1 | 66 | 29.5 |
| 2 | 21 | 9.4 |
| 3 | 17 | 7.6 |
| TMN stage |
|
|
| 1 | 77 | 33.4 |
| 2 | 107 | 47.8 |
| 3 | 40 | 17.9 |
| Lymph node
metastasis |
|
|
|
Absence | 120 | 53.6 |
|
Presence | 104 | 46.4 |
| Histological
grade |
|
|
| 1 | 54 | 24.1 |
| 2 | 99 | 44.2 |
| 3 | 71 | 31.7 |
| EIC |
|
|
|
Absence | 189 | 84.4 |
|
Presence | 35 | 15.6 |
| Skin and chest wall
invasion |
|
|
|
Absence | 197 | 87.9 |
|
Presence |
5 |
2.2 |
| Not
examined | 22 |
9.9 |
| Paget's
disease |
|
|
|
Absence | 205 | 91.5 |
|
Presence |
4 |
1.8 |
| Not
examined | 15 |
6.7 |
| Lympho-vascular
invasion |
|
|
|
Absence | 133 | 59.4 |
|
Presence | 91 | 40.6 |
| ER |
|
|
|
Negative | 65 | 29.0 |
|
Positive | 159 | 71.0 |
| PR |
|
|
|
Negative | 77 | 34.4 |
|
Positive | 147 | 65.6 |
| HER2 |
|
|
|
Negative | 167 | 74.6 |
|
Positive | 57 | 25.4 |
|
Triple-negative |
|
|
|
Yes | 32 | 14.3 |
| No | 192 | 85.7 |
| Distant
metastasis |
|
|
|
Absence | 202 | 90.2 |
|
Presence | 22 |
9.8 |
| ERCC1 |
|
|
|
Low | 191 | 85.3 |
|
High | 33 | 14.7 |
With regard to the clinicopathological parameters,
high expression of ERCC1 was statistically associated with smaller
tumor size (<2.0 cm; P=0.001), no lymph node metastasis
(P=0.044), lower pathological stage (stage I; P=0.001) and no LVI
(P=0.004). However, age (P=0.253), N stage (P=0.131), histological
grade (P=0.373), EIC (P=0.935), skin and chest wall invasion
(P=0.442), Paget's disease (P=0.999), the presence of metastasis
(P=0.750) and the recurrence rate (P=0.999) were not statistically
associated with ERCC1 expression (Table
II). To detect parameters that were independently associated
with high expression of ERCC1, the four parameters found to be
significant on univariate analysis were analyzed by multivariate
logistic regression analysis. It was found that smaller tumor size
(<2.0 cm; P=0.002 relative risk, 3.815; 95% confidence interval,
1.638–8.888), no lymph node metastasis (P=0.048; relative risk,
2.229; 95% confidence interval, 1.007–4.9340), lower pathological
stage (stage I; P=0.001; relative risk, 3.617; 95% confidence
interval, 1.685–7.764) and no LVI (P=0.007; relative risk, 3.608;
95% confidence interval, 1.424–9.141) were independent
clinicopathological parameters in accordance with the high
expression of ERCC1 (Table II).
 | Table II.Association between ERCC1 expression
and clinicopathological parameters. |
Table II.
Association between ERCC1 expression
and clinicopathological parameters.
|
| Univariate
analysis |
|
|
|---|
|
|
|
|
|
|---|
|
| ERCC low
expression | ERCC high
expression |
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | (IRS≤7), n (%) | (IRS>7), n
(%) | P-value | RR (95% CI) | P-value |
|---|
| Age, years |
|
| 0.253 |
|
|
|
≤52 | 107 (56.0) | 22 (66.7) |
| Not applicable |
|
|
>52 | 84 (44.0) | 11 (33.3) |
|
|
|
| Tumor size, cm |
|
| 0.001a |
| 0.002a |
|
≤2.0 | 86 (45.0) | 25 (75.8) |
| 3.815
(1.638–8.888) |
|
|
>2.0 | 105 (55.0) | 8 (24.2) |
|
|
|
| N stage |
|
| 0.131 |
|
|
| 0 | 97 (50.8) | 23 (69.7) |
| Not applicable |
|
| 1 | 58 (30.4) | 8 (24.2) |
|
|
|
| 2 | 19 (9.9) | 2 (6.1) |
|
|
|
| 3 | 17 (8.9) | 0 (0.0) |
|
|
|
| Lymph node
metastasis |
|
| 0.044a |
| 0.048a |
|
Absence | 97 (50.8) | 23 (69.7) |
| 2.229
(1.007–4.934) |
|
|
Presence | 94 (49.2) | 10 (30.3) |
|
|
|
| TNM stage |
|
|
|
|
|
| 1 | 57 (29.8) | 20 (60.6) | 0.001a | 3.617
(1.685–7.764) | 0.001a |
| 2 and
3 | 134 (70.2) | 13 (39.4) |
|
|
|
| Histological
grade |
|
| 0.373 |
|
|
| 1 | 45 (23.6) | 9 (27.3) |
| Not applicable |
|
| 2 | 82 (42.9) | 17 (51.5) |
|
|
|
| 3 | 64 (33.5) | 7 (21.2) |
|
|
|
| EIC |
|
| 0.935 |
|
|
|
Absence | 161 (84.3) | 28 (84.8) |
| Not applicable |
|
|
Presence | 30 (15.7) | 5 (15.2) |
|
|
|
| Skin and chest wall
invasiona |
|
| 0.442 |
|
|
|
Absence | 176 (97.8) | 21 (95.5) |
| Not applicable |
|
|
Presence | 4 (2.2) | 1 (4.5) |
|
|
|
| Paget's
diseaseb |
|
| 0.999 |
|
|
|
Absence | 176 (97.8) | 29 (100) |
| Not applicable |
|
|
Presence | 4 (2.2) | 0 (0) |
|
|
|
| Lymphovascular
invasion |
|
| 0.004a |
| 0.007c |
|
Absence | 106 (55.5) | 27 (81.8) |
| 3.608
(1.424–9.141) |
|
|
Presence | 85 (44.5) | 6 (18.2) |
|
|
|
| Distant
metastasis |
|
| 0.750 |
|
|
|
Absence | 171 (89.5) | 31 (93.9) |
| Not applicable |
|
|
Presence | 20 (10.5) | 2 (6.1) |
|
|
|
| Recurrence |
|
| 0.999 |
|
|
|
Negative | 163 (85.3) | 29 (87.9) |
| Not applicable |
|
|
Positive | 28 (14.7) | 4 (12.1) |
|
|
|
With regard to immunohistochemical parameters, high
expression of ERCC1 was associated with positive ER (P=0.006) and
PR (P=0.001) expression. Non-triple-negative breast carcinoma
(TNBC) occurred more frequently in the high expression group (97%)
than the low expression group, however, the difference was not
statistically significant (P=0.056). Additionally, HER2 expression
was also not associated with ERCC1 expression. Multivariate
logistic regression analysis was also applied for the detection of
independent parameters that were associated with the high
expression of ERCC1. It was found that positive ER (P=0.012;
relative risk, 4.806; 95% confidence interval, 1.412–16.359) and
positive PR (P=0.003; relative risk, 6.325; 95% confidence
interval, 1.864–21.466) expression are independent
immunohistochemical parameters that correspond to the high
expression of ERCC1 (Table
III).
 | Table III.Association between ERCC1 expression
and immunohistochemical results. |
Table III.
Association between ERCC1 expression
and immunohistochemical results.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Immunohistochemical
results | ERCC low expression
(IRS≤7) | ERCC high
expression (IRS>7) | P-value | RR (95% CI) | P-value |
|---|
| ER |
|
| 0.006a |
| 0.012a |
|
Negative | 62 (32.5) | 3 (9.1) |
| 4.806
(1.412–16.359) |
|
|
Positive | 129 (67.5) | 30 (90.9) |
|
|
|
| PR |
|
| 0.001a |
| 0.003a |
|
Negative | 74 (38.7) | 3 (9.1) |
| 6.325
(1.864–21.466) |
|
|
Positive | 117 (61.3) | 30 (90.9) |
|
|
|
| HER2 |
|
| 0.299 |
|
|
|
Negative | 140 (73.3) | 27 (81.8) |
| Not applicable |
|
|
Positive | 51 (26.7) | 6 (18.2) |
|
|
|
|
Triple-negative |
|
| 0.056 |
|
|
| No | 160 (83.8) | 32 (97.0) |
| Not applicable |
|
|
Yes | 31 (16.2) | 1 (3.0) |
|
|
|
The 5-year OS rate for all patients in this study
was 95.1% (213/224 patients). In the high expression group, the
5-year OS rate was 100% (33/33 patients). In the low expression
group, the 5-year OS rate was 94.2% (180/191 patients). ERCC1
expression was not statistically associated with the OS rate
(P=0.375). The 5-year DFS rate for all patients in this study was
85.7% (192/224 patients). In the high expression group, the 5-year
DFS rate was 87.9% (29/33 patients). In the low expression group,
the 5-year DFS rate was 85.3% (163/191 patients). ERCC1 expression
was also not statistically associated with the DFS rate
(P=0.999).
To evaluate OS, univariate analysis was performed.
Advanced T stage (T stage 2–3; P=0.006), presence of lymph node
metastasis (P=0.038), advanced pathological stage (stage 2–3;
P=0.023), presence of skin and chest wall invasion (P=0.015),
presence of LVI (P=0.011) and presence of distant metastasis
(P=0.001) were statistically associated with poor OS. Status of
ERCC1 expression and immunohistochemical parameters were not
associated with OS. Multivariate analysis for OS was performed
using these statistically significant parameters. It was found that
advanced T stage (P=0.034; hazard ratio, 9.283; 95% confidence
interval, 1.188–72.538), presence of lymph node metastasis (P=0.04;
hazard ratio, 4.989; 95% confidence interval, 1.078–23.097),
presence of skin and chest wall invasion (P=0.001; hazard ratio,
12.647; 95% confidence interval, 2.718–58.839), presence of LVI
(P=0.017; hazard ratio, 6.448; 95% confidence interval,
1.393–29.854) and presence of distance metastasis (P=0.000; hazard
ratio, 22.361; 95% confidence interval, 6.486–77.095) independently
predicted poor OS (Table IV).
 | Table IV.Factors associating with poor overall
and shorter disease-free survival time. |
Table IV.
Factors associating with poor overall
and shorter disease-free survival time.
|
| Overall
survival | Disease free
survival |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Factors | P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value |
|---|
| Age, years (>52
vs. ≤52) | 0.334 | Not applicable |
| 0.753 | Not applicable |
|
| T stage (2–3 vs.
1) | 0.006a | 9.283
(1.188–72.538) | 0.034a | 0.007a | 2.968
(1.333–6.607) | 0.008a |
| Lymph node
metastasis (presence vs. absence) | 0.038a | 4.989
(1.078–23.097) | 0.040a | 0.169 | Not applicable |
|
| TNM stage (2–3 vs.
1) | 0.023a | 38.061
(0.196–7394.970) | 0.176 | 0.081 | Not applicable |
|
| Histologic grade
(2–3 vs. 1) | 0.214 | Not applicable |
| 0.035a | 2.404
(0.843–6.854) | 0.101 |
| EIC (presence vs.
absence) | 0.985 | Not applicable |
| 0.927 | Not applicable |
|
| Skin and chest wall
invasion (presence vs. absence) | 0.015a | 12.647
(2.718–58.839) | 0.001a | 0.001a | 8.991
(2.713–29.796) |
0.001a |
| Paget disease
(presence vs. absence) | 0.261 | Not applicable |
| 0.062 | Not applicable |
|
| Lymphovascular
invasion (presence vs. absence) | 0.011a | 6.448
(1.393–29.854) | 0.017a | 0.022a | 2.22
(1.096–4.497) | 0.027a |
| Distant metastasis
(presence vs. absence) | 0.001a | 22.361
(6.486–77.095) | 0.001a | 0.001a | 16.016
(7.790–32.929) |
0.001a |
| ERCC1 (IRS>7 vs.
IRS≤7) | 0.215 | Not applicable |
| 0.989 | Not applicable |
|
| ER (negative vs.
positive) | 0.680 | Not applicable |
| 0.100 | Not applicable |
|
| PR (negative vs.
positive) | 0.255 | Not applicable |
| 0.020a | 2.091
(1.045–4.184) | 0.037a |
| HER2 (negative vs.
positive) | 0.116 | Not applicable |
| 0.237 | Not applicable |
|
| Triple-negative
(yes vs. no) | 0.171 | Not applicable |
| 0.002a | 3.020
(1.395–6.537) | 0.005a |
To evaluate DFS, univariate analysis was performed.
Advanced T stage (P=0.007), high histological grade (P=0.035),
presence of skin and chest wall invasion (P=0.001), presence of LVI
(P=0.022), presence of distance metastasis (P=0.001), loss of PR
expression (P=0.020) and triple-negative subtype (P=0.002) were
statistically associated with a shorter DFS time. Multivariate
analysis for DFS was performed using these statistically
significant parameters. It was found that advanced T stage
(P=0.008; hazard ratio, 2.968; 95% confidence interval,
1.333–6.607), presence of skin and chest wall invasion (P=0.001;
hazard ratio, 8.991; 95% confidence interval, 2.713–27.796),
presence of LVI (P=0.027; hazard ratio, 2.22; 95% confidence
interval, 1.096–4.497), presence of distance metastasis (P=0.001;
hazard ratio, 16.016; 95% confidence interval, 7.790–32.929), loss
of PR expression (P=0.037; hazard ratio, 2.091; 95% confidence
interval, 1.045–4.184) and triple-negative subtype (P=0.005; hazard
ratio, 3.020; 95% confidence interval, 1.395–6.537) independently
predicted a shorter DFS time (Table
IV).
Discussion
There are four major pathways to repair damaged DNA:
NER, base excision repair, mismatch repair and double strand break
repair (22). Among these pathways,
NER plays an important role in recognizing and repairing the DNA
adducts, particularly those induced by chemotherapeutic agents such
as cisplatin (10). The NER pathway
requires a number of factors. Among these factors, ERCC1 serves an
essential role for the incision step and completion of the NER
pathway (11). ERCC1 bind to XPF and
forms the ERCC1-XPF complex (10,11). The
ERCC1-XPF complex is important as a structure-specific endonuclease
in the NER pathway (11). Therefore,
certain studies have reported that the ERCC1-XPF complex can be an
important factor for repairing the DNA damage induced by
chemotherapeutic agents, including cisplatin; thus, the expression
of ERCC1 has been considered as a predictive factor for resistance
to platinum-based chemotherapy (10,11).
Certain studies have reported the association
between ERCC1 expression and TNBC. Sidoni et al (12) reported that one-third of the
triple-negative patients exhibited relevant ERCC1 expression.
Additionally, Ozkan et al (13) reported that two-thirds of the
triple-negative patients exhibited ERCC1 expression.
However, recently, good prognostic effects of ERCC1
expression have been reported by certain researchers. Goyal et
al (14) reported that the
overexpression of ERCC1 was associated with lower T stage, nodal
negativity, an age >50 years and ER positivity, but was not
associated with OS and DFS. Gerhard et al (15) reported that ERCC1 expression was
significantly associated with smaller tumor size and ER positivity,
but was not associated with OS and DFS. These two studies also
reported that the triple-negative immunohistochemical phenotype was
not statistically associated with ERCC1 expression. Furthermore,
one report demonstrated that the level of ERCC1 expression was the
lowest in triple-negative phenotypes compared with other
phenotypes, and that negativity for ERCC1 expression occurred more
frequently in TNBC and luminal B group breast cancer (16).
By contrast, other studies did not find any
association between ERCC1 expression and clinical and
immunohistochemical parameters. Fu et al (23) found that ERCC1 gene expression
detected by reverse transcription-polymerase chain reaction was not
significantly associated with age, tumor size, axillary lymph node
metastasis, pathological type, histological grade, ER, PR or HER-2.
Metro et al (24) also
reported that there was no significant association between in
situ protein expression of ERCC1 and various
clinico-pathological parameters, including age, tumor stage at
diagnosis, histology, hormone receptor status, HER-2 status,
presence of visceral disease and pretreatment of metastatic
disease.
Besides cases of breast cancer, in cases of
non-small cell lung cancer and gastric cancer, a association has
been reported between ERCC1 expression and good prognosis. Lee
et al (25) showed that in
patients with resected non-small cell lung cancer, ERCC1 expression
was an independent prognostic factor of longer survival, and that
EGFR mutation was more frequent in ERCC1-negative patients. Another
study also showed that in patients with resected non-small cell
lung cancer, the 5-year survival rate of ERCC1-positive patients
was longer than that of ERCC1-negative patients (76 vs. 49%,
P=0.004) (26). Wang et al
(27) reported that ERCC1 expression
may be a good prognostic factor in patients with resected gastric
cancer.
In addition, Han et al (28) reported that single nucleotide
polymorphism (SNP)-SNP interaction of NER pathway genes increased
the risk of breast cancer. Another study reported that ERCC
polymorphism was associated with the increase in the breast cancer
risk (29). Notably, Mo et al
(30) reported that the mRNA level of
ERCC1 expression was significantly associated with water arsenic
concentration and nail arsenic concentration. Moreover, the study
suggested that the DNA repair response was induced by arsenic
exposure. Therefore, high ERCC1 expression may be a compensatory
response against the DNA injury that is induced by various
carcinogens.
In the present study, the immunohistochemical
expression of ERCC1 was analyzed in patients with invasive ductal
carcinoma. ERCC1 high expression (IRS>7) was statistically
associated with the smaller tumor size (≤2 cm), no lymph node
metastasis, low pathological stage (TNM stage 1) and no LVI. In
addition, high ERCC1 expression was statistically associated with
positive estrogen receptor (ER) and progesterone receptor (PR)
expression. The triple-negative phenotype was frequently expressed
in the ERCC1 low expression group, but this result was not
statistically significant. ERCC1 expression was not statistically
associated with OS and DFS. Higher T stage (stage 2–3), the
presence of skin and chest wall invasion, and LVI were independent
of predictive factors of poor OS and shorter DFS. The presence of
lymph node metastasis was associated with poor OS only. No
immunohistochemical parameters influenced the OS, but the negative
expression of PR and triple-negative status were statistically
associated with a shorter DFS time. Although ERCC1 expression was
not a direct predictor of OS and DFS, low T stage (size ≤2 cm), no
lymph node metastasis and no LVI were significantly associated with
the high expression of ERCC1. Therefore, the high expression of
ERCC1 may be a more favorable factor of good OS and longer DFS
times than low level ERCC1 expression.
In conclusion, in the present study, high ERCC1
expression was associated with several clinical and
immunohistochemical parameters, namely lower T stage, smaller tumor
size, no lymph node metastasis, no LVI, and positivity for ER and
PR in invasive ductal carcinoma of the breast. However, no
association was shown between the expression of ERCC1 and OS and
DFS rate. Based on the results of previously reviewed studies, the
role of ERCC1 is not yet fully understood. In order to evaluate the
complete role of ERCC1 and the association between ERCC1 expression
and clinical outcomes, a greater number of large-scale studies may
be required.
References
|
1
|
Li CI, Anderson BO, Daling JR and Moe RE:
Trends in incidence rates of invasive lobular and ductal breast
carcinoma. JAMA. 289:1421–1424. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li CI, Uribe DJ and Daling JR: Clinical
characteristics of different histologic types of breast cancer. Br
J Cancer. 93:1046–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Subramaniam S, Bhoo-Pathy N, Taib NA, Tan
GH, See MH, Jamaris S, Ho GF, Looi LM and Yip CH: Breast cancer
outcomes as defined by the estrogen receptor, progesterone receptor
and human growth factor receptor-2 in a multi-ethnic Asian country.
World J Surg. 39:2450–2458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perou CM, Sorlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schnitt SJ: Classification and prognosis
of invasive breast cancer: From morphology to molecular taxonomy.
Mod Pathol. 23:(Suppl 2). S60–S64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engstrom MJ, Opdahl S, Hagen AI,
Romundstad PR, Akslen LA, Haugen OA, Vatten LJ and Bofin AM:
Molecular subtypes, histopathological grade and survival in a
historic cohort of breast cancer patients. Breast Cancer Res Treat.
140:463–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parise CA and Caggiano V: Breast Cancer
Survival Defined by the ER/PR/HER2 Subtypes and a Surrogate
Classification according to Tumor Grade and Immunohistochemical
Biomarkers. J Cancer Epidemiol. 2014:4692512014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gajria D and Chandarlapaty S:
HER2-amplified breast cancer: Mechanisms of trastuzumab resistance
and novel targeted therapies. Expert Rev Anticancer Ther.
11:263–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hudis CA and Gianni L: Triple-negative
breast cancer: An unmet medical need. Oncologist. 16:(Suppl 1).
S1–S11. 2011. View Article : Google Scholar
|
|
10
|
Kirschner K and Melton DW: Multiple roles
of the ERCC1-XPF endonuclease in DNA repair and resistance to
anticancer drugs. Anticancer Res. 30:3223–3232. 2010.PubMed/NCBI
|
|
11
|
McNeil EM and Melton DW: DNA repair
endonuclease ERCC1-XPF as a novel therapeutic target to overcome
chemoresistance in cancer therapy. Nucleic Acids Res.
40:9990–10004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sidoni A, Cartaginese F, Colozza M, Gori S
and Crinó L: ERCC1 expression in triple negative breast carcinoma:
The paradox revisited. Breast Cancer Res Treat. 111:569–570. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozkan C, Gumuskaya B, Yaman S, Aksoy S,
Guler G and Altundag K: ERCC1 expression in triple negative breast
cancer. J BUON. 17:271–276. 2012.PubMed/NCBI
|
|
14
|
Goyal S, Parikh RR, Green C, Schiff D,
Moran MS, Yang Q and Haffty BG: Clinicopathologic significance of
excision repair cross-complementation 1 expression in patients
treated with breast-conserving surgery and radiation therapy. Int J
Radiat Oncol Biol Phys. 76:679–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerhard R, Carvalho A, Carneiro V, Bento
RS, Uemura G, Gomes M, Albergaria A and Schmitt F:
Clinicopathological significance of ERCC1 expression in breast
cancer. Pathol Res Pract. 209:331–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim D, Jung W and Koo JS: The expression
of ERCC1, RRM1, and BRCA1 in breast cancer according to the
immunohistochemical phenotypes. J Korean Med Sci. 26:352–359. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edge SB: American Joint Committee On
Cancer, AJCC Cancer Staging Manual. 8th. Springer; New York, NY:
2010
|
|
18
|
O'Malley FP and Pinder SE: Breast
pathology, Churchill Livingstone. Elsevier; Edinburgh: 2006,
View Article : Google Scholar
|
|
19
|
Nadji M, Gomez-Fernandez C, Ganjei-Azar P
and Morales AR: Immunohistochemistry of estrogen and progesterone
receptors reconsidered: Experience with 5,993 breast cancers. Am J
Clin Pathol. 123:21–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
22
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu JM, Zhou J, Xie JS and Li H: Effect of
neoadjuvant chemotherapy on ERCC1 gene expression in breast cancer.
Nan Fang Yi Ke Da Xue Xue Bao. 28:603–605. 2008.(In Chinese).
PubMed/NCBI
|
|
24
|
Metro G, Zheng Z, Fabi A, Schell M,
Antoniani B, Mottolese M, Monteiro AN, Vici P, Lara Rivera S,
Boulware D, et al: In situ protein expression of RRM1, ERCC1, and
BRCA1 in metastatic breast cancer patients treated with
gemcitabine-based chemotherapy. Cancer Invest. 28:172–180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee KH, Min HS, Han SW, Oh DY, Lee SH, Kim
DW, Im SA, Chung DH, Kim YT, Kim TY, et al: ERCC1 expression by
immunohistochemistry and EGFR mutations in resected non-small cell
lung cancer. Lung Cancer. 60:401–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seyhan EC, Altin S, Cetinkaya E, Sökücü S,
Abali H, Buyukpinarbasili N and Fener N: Prognostic significance of
ERCC1 expression in resected non small cell lung carcinoma. Ann
Thorac Cardiovasc Surg. 17:110–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Zhou XQ, Li JY, Cheng JF, Zeng XN,
Li X and Liu P: Prognostic significance of ERCC1 expression in
postoperative patients with gastric cancer. Chin J Cancer Res.
26:323–330. 2014.PubMed/NCBI
|
|
28
|
Han W, Kim KY, Yang SJ, Noh DY, Kang D and
Kwack K: SNP-SNP interactions between DNA repair genes were
associated with breast cancer risk in a Korean population. Cancer.
118:594–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mojgan H, Massoud H and Ahmad E: ERCC1
intron 1 was associated with breast cancer risk. Arch Med Sci.
8:655–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mo J, Xia Y, Ning Z, Wade TJ and Mumford
JL: Elevated ERCC1 gene expression in blood cells associated with
exposure to arsenic from drinking water in Inner Mongolia.
Anticancer Res. 29:3253–3259. 2009.PubMed/NCBI
|