Introduction
Head and neck carcinoma is a major type of cancer
that causes mortality in humans, ranking sixth in the incidence
rate of all types of cancer (1).
Laryngeal cancer is one of the most common types of head and neck
carcinoma, ranking second in the incidence rate of all respiratory
tract neoplasms behind head and neck squamous cell carcinoma, which
accounts for 95% of all types of head and neck carcinoma (2). Concomitant with increased
industrialization and the exacerbation of air pollution, the
incidence of laryngeal cancer is increasing by ~25%/year, primarily
in middle aged and elderly males (3).
In 2008, the incidence of laryngeal cancer was 5.1/100,000
estimated in male patients around the world, and the mortality rate
was ~2.2/100,000 (4). In spite of
novel surgical methods, novel chemotherapeutic drugs and advanced
radiation therapy in the treatment of laryngeal cancer in the last
30 years, the overall survival rate of patients with laryngeal
cancer has not improved; instead, ithas exhibited a downward trend,
with a survival rate of ~50%, and a survival rate of <40% for
patients with advanced laryngeal cancer (3).
Early diagnosis of laryngeal cancer and
improvementsin effective treatment are required to improve our
understanding of the underlying molecular mechanism of the
development of laryngeal cancer, thereforenovel or early treatment
may be developed (5). Epidemiological
investigation has confirmed that the etiology of laryngeal cancer
includes smoking, alcohol consumption, air pollution and
occupational factors (6). With the
development of molecular biology techniques, it has
beendemonstrated that the development of laryngeal cancer involves
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt), apoptosis
regulator Bcl-2, c-Myc proto-oncogene protein, epidermal growth
factor receptor and other oncogenes (7). Each microRNA (miRNA/miR) is considered
to be able to regulate hundreds of genes and has
disease-dependency, tissue-specificity and expression stability. In
numerous diseases, the expression profile of miRNAshave certain
characteristic alterations, particularly in the tumor; the
expression of miRNAs in tumor tissues and adjacent wild-type
tissues was significantly different (8). It has been demonstrated that analysis of
miRNA levels in different tumors can assist with early diagnosis
and evaluation of prognosis (9).
miRNAs are emerging as molecular markers for the diagnosis,
recurrence, metastasis and prognosis of numeroustypes of malignant
tumor (9).
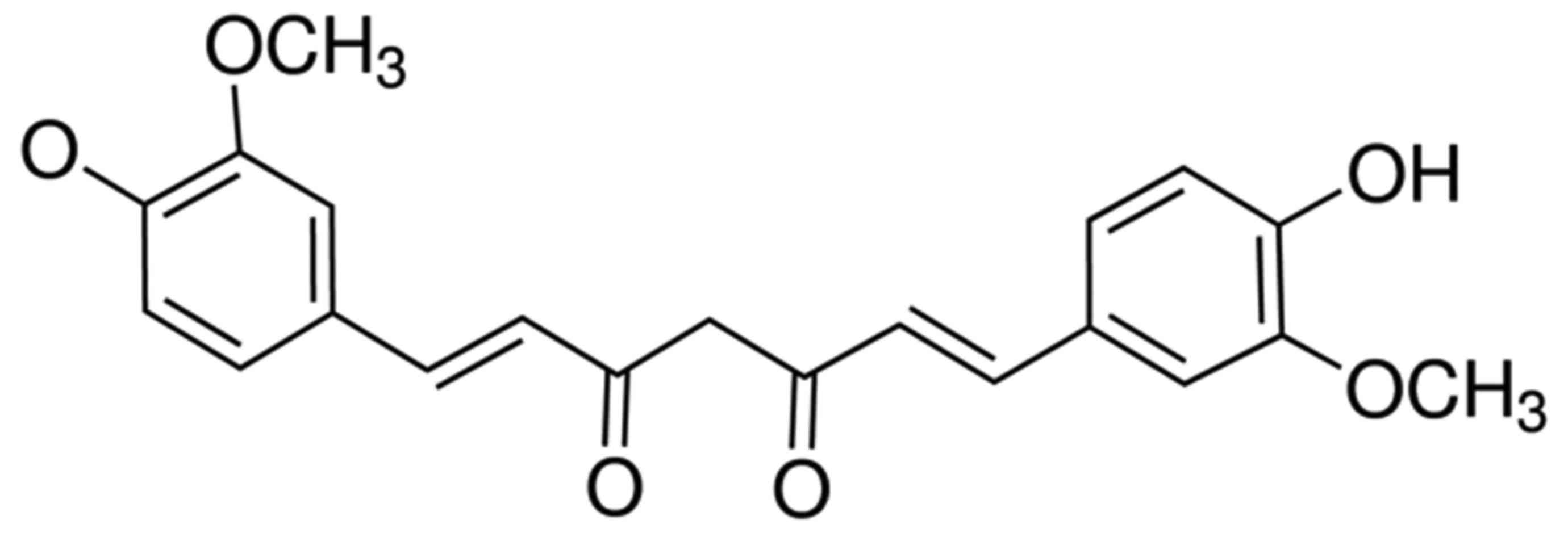
Curcumin, a polyphenolic compound (Fig. 1) of relatively low molecular mass, was
initially isolated from Curcuma longa in 1870 (10). In 1910, following the elucidation of
the chemical structure of double feruloyl methane, research into
the physiological and pharmacological effects of curcumin made
marked progress (11). Curcumin has
been demonstrated to exhibit anti-inflammatory, antioxidant,
lipid-lowering, antivirus, anti-infection, anti-tumor, anti-liver
fibrosis and anti-atherosclerosis effects, in addition to other
pharmacological activity; furthermore, curcumin demonstrates low
toxicity without serious adverse reactions (11,12). It
has been demonstrated that the underlying molecular mechanism of
the anti-tumor effects of curcumin primarily involves apoptosis of
tumor cells, inhibition of the signal transduction pathway of tumor
cell growth, oxidationresistance and inhibition of tumor
angiogenesis (13). In the present
study, it was identified that curcumin inhibits cell proliferation
and promotes apoptosis of laryngeal cancer through a novel
mechanism involving altered regulation of Bcl-2- and
PI3K/Akt-targeting miRNAs.
Materials and methods
Cell culture
Laryngeal squamous cell carcinoma cell line AMC-HN-8
was purchased from Shanghai Institute Chinese Academy of Sciences
(Shanghai, China) and cultured with Dulbecco's modified Eagle's
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.), supplemented with 100 µM penicillin and 100 µM
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2.
MTT assay
AMC-HN-8 cells [(1.0–2.0)x104 cells/well]
were cultured in 96-well plates with 0, 20 and 40 µM of curcumin at
37°C in a humidified atmosphere containing 5% CO2 for
0–3 days. A 50-µl volume of 5 mg/ml MTT dye (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was added to each well and incubated
for 4 h. Subsequently, 150 µl of dimethylsulfoxide (Invitrogen;
Thermo Fisher Scientific, Inc.) was added to each well prior to
agitation for 20 min. The optical density of samples was determined
at 490 nm using a Versamax microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA).
Flow cytometric detection of
apoptosis
AMC-HN-8 cells [(1.0–2.0)x106 cells/well]
were cultured in 6-well plates with 0, 10, 20 and 40 µM curcumin at
37°C in a humidified atmosphere containing 5% CO2 for 1
day. AMC-HN-8 cells were washed twice with ice-cold PBS prior to
being harvested. AMC-HN-8 cells (1×106 cells) were
resuspended with 1X binding buffer and stained with 5 µl Annexin
V-fluorescein isothiocyanate for 30 min in darkness at 4°C. A 10-µl
volume of propidium iodide was added to each well and apoptosis was
analyzed using flow cytometry (BD C6 flow cytometer; BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis of cell
lysates
AMC-HN-8 cells [(1.0–2.0)x106 cells/well]
were cultured in 6-well plates with 0, 10, 20 and 40 µM curcumin at
37°C in a humidified atmosphere containing 5% CO2 for 1
day. AMC-HN-8 cells were lysed in 1 ml radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) and harvested. Following centrifugation at 12,000 × g for 10
min at 4°C, the supernatant was collected and proteins were
quantified using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. Total
protein (~50 µg/lane) was separated by 10% SDS-PAGE and transferred
onto a 0.22 µm pore size nitrocellulose membrane (Sigma-Aldrich;
Merck Millipore). The nitrocellulose membrane was incubated with
anti-Bcl-2 (cat. no. sc-509; dilution, 1:1,000), anti-PI3K (cat.
no. sc-293172; dilution, 1:200), anti-phosphorylated (p)-Akt (cat.
no. sc-7985-R; dilution, 1:300), anti-Akt (cat. no. sc-8312;
dilution, 1:300; all from Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and anti-β-actin (cat. no. sc-70319; dilution, 1:500;
Sangon Biotech Co., Ltd., Shanghai, China) antibodies overnight at
4°C subsequent to blocking in Tris-buffered saline containing 0.1%
Tween-20 (TBST) with 5% dried skimmed milk at room temperature for
1 h. The nitrocellulose was incubated with anti-mouse or
anti-rabbit secondary antibodies (cat. nos. sc-2005 and sc-2357,
respectively; dilution, 1:1,000; Santa Cruz Biotechnology, Inc.) at
37°C for 1 h, detected using enhanced chemiluminescence reagent
(Thermo Fisher Scientific, Inc., USA) subsequent to washing with
TBST, and analyzed using Quantity One software 3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The whole procedure was
repeated three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
AMC-HN-8 cells [(1.0–2.0)x106 cells/well]
were cultured in 6-well plates with 0, 10, 20 and 40 µM curcumin at
37°C in a humidified atmosphere containing 5% CO2 for 1
day. Total RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse-transcribed into cDNA using a TaqMan MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. qPCR was performed
on 1.0 µl cDNA using a TaqMan MicroRNA assay (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol (including thermocycler settings), with the following
primers: miR-15a forward, 5′-GCTAGCAGCACATAATGGTTTGTG-3′ and
reverse, 5′-GTGCAGGGTCCGAGGTATTC-3′; U6 small nuclear
ribonucleoprotein forward, 5′-GTGCAGGGTCCGAGGTATTC-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The 2−ΔΔCq method was used
for quantification (14).
Anti-miRNA-15a and cell
transfection
Anti-miRNA-15a (sequence,
5′-TCATGGCAGCCTGGTCTACATGG-3′) and negative control (NC; sequence,
5′-CCCCCCCCCCCCCC-3′) oligonucleotides were synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China) and were transfected into
AMC-HN-8 cells at 100 nM using Lipofectamine™ 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol.
Statistical analysis
Results are presented as the mean ± standard
deviation for three replicates. The comparison between groups was
performed using one-way ANOVA, followed by Bonferroni's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Curcumin inhibits cell proliferation
of human laryngeal cancer cells
The potential anticancer effect of curcumin on cell
viability of human laryngeal cancer cells was investigated.
Cellular proliferation of AMC-HN-8 cells was decreased by treatment
with curcumin in a dose- and time-dependent manner (Fig. 2). A dosage of between 0 and 40 µM
curcumin and a time of <3 days were used for all assays.
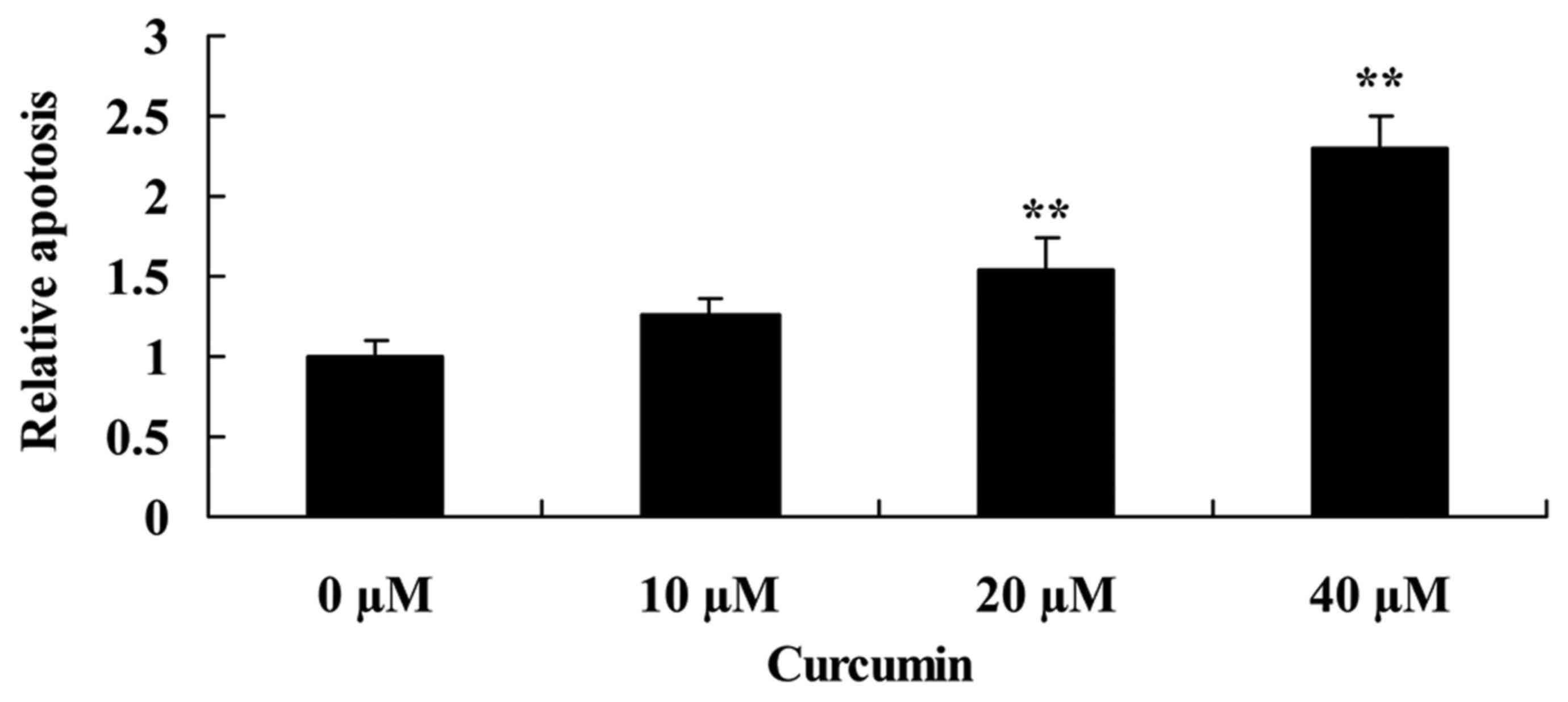
Curcumin increases apoptosis of human
laryngeal cancer cells
To determine that the possible anticancer effect of
curcumin was via apoptosis of human laryngeal cancer cells,
apoptosis of AMC-HN-8 cells was determined using flow cytometry.
When AMC-HN-8 cells were treated with 20 or 40 µM of curcumin for 2
days, apoptosis was significantly increased compared with the
untreated control cells (Fig. 3).
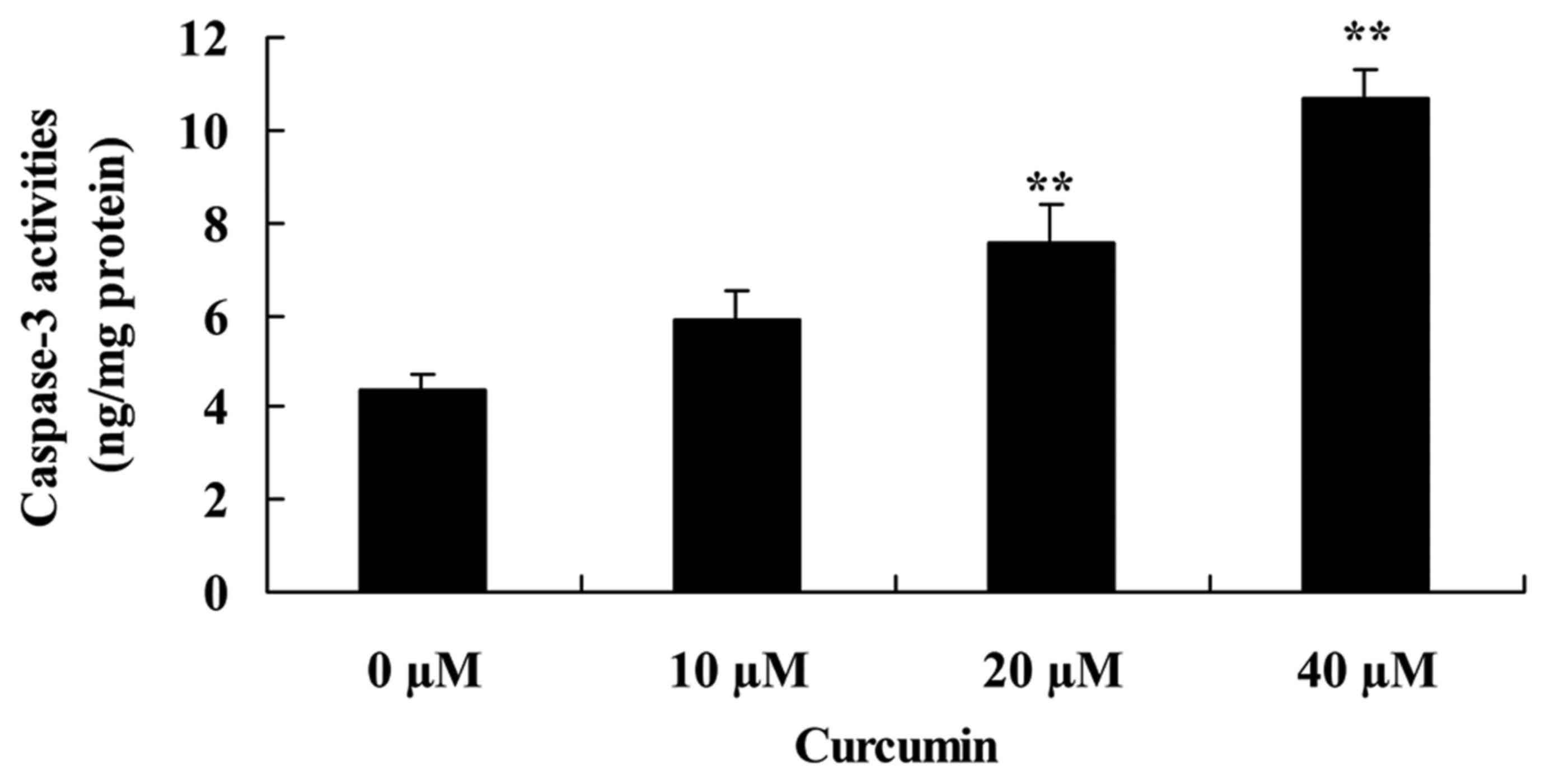
Curcumin increases capase-3 activity
of human laryngeal cancer cells
To investigate the effect of curcumin on capase-3
activity of human laryngeal cancer cells, capase-3 activity was
measured following treatment with curcumin. Curcumin at 20 and 40
µM significantly increased capase-3 activity of AMC-HN-8 cells
compared with the untreated control cells (Fig. 4).
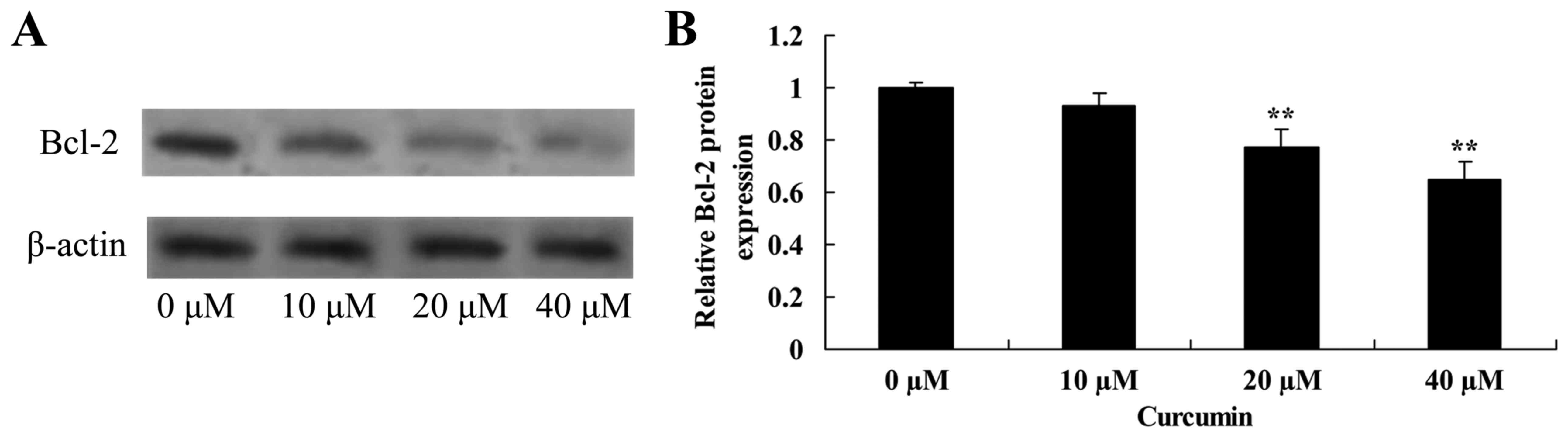
Curcumin decreases Bcl-2 protein
expression in human laryngeal cancer cells
The effect of curcumin on Bcl-2 protein expression
in human laryngeal cancer cells was determined using western blot
analysis. Curcumin at 20 and 40 µM significantly decreased the
expression of Bcl-2 protein inAMC-HN-8 cells compared with the
untreated control cells (Fig. 5).
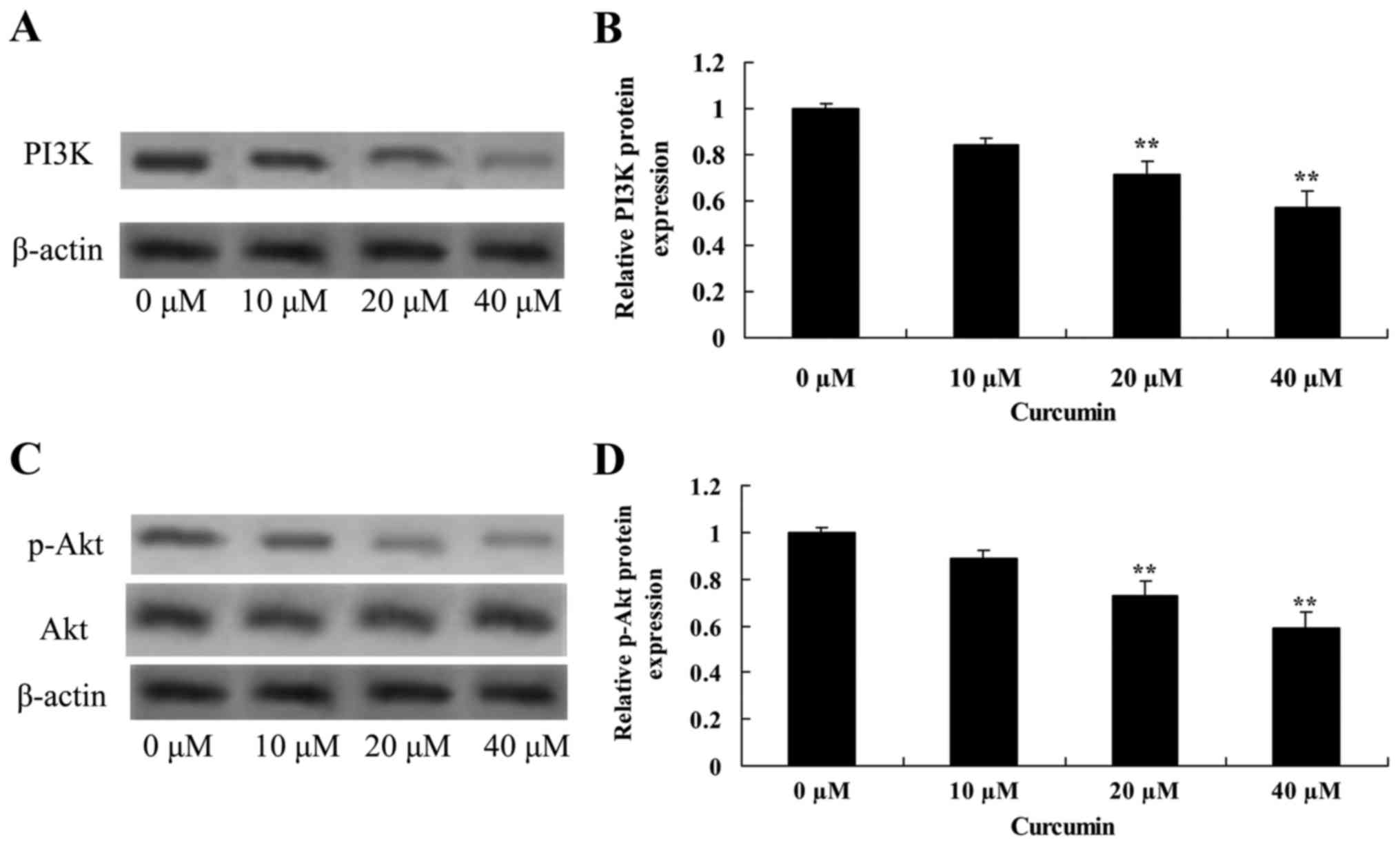
Curcumin decreases PI3K and Akt
protein expression in human laryngeal cancer cells
Treatment of AMC-HN-8 cells with 20 or 40 µM
curcumin significantly decreased PI3K and p-Akt protein expression,
and the p-Akt/Akt ratio was significantly decreased, compared with
the untreated control cells, which revealed that curcumin induced
apoptosis of laryngeal cancer via PI3K/Akt signaling pathway
(Fig. 6).
Curcumin increases miR-15a expression
in human laryngeal cancer cells
To determine the effect of curcumin on miR-15a
expression of human laryngeal cancer cell, RT-qPCR was employed to
analyze miR-15a expression in AMC-HN-8 cells. miR-15a expression
was significantly increased by 20 or 40 µM curcumin compared with
the untreated control cells (Fig.
7).
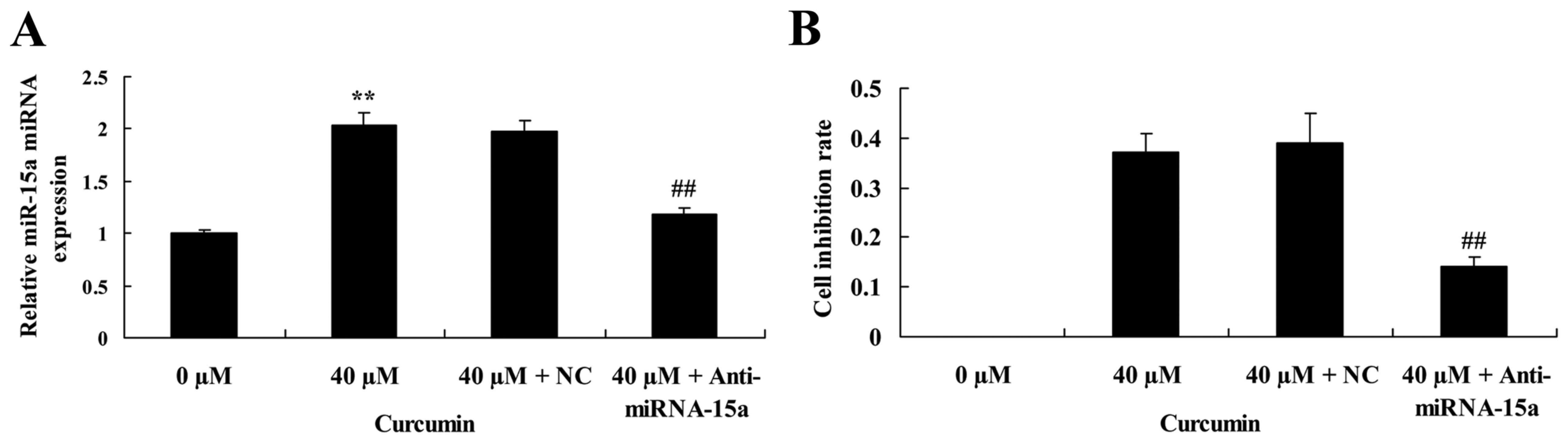
Suppression of miR-15a expression
partially reverses the anticancer effect of curcumin on cell
proliferation of human laryngeal cancer cells
The effect of miR-15a expression on the anticancer
effect of curcumin on cell proliferation of human laryngeal cancer
cell was investigated. Anti-miRNA-15a significantly decreased
miR-15a expression in AMC-HN-8 cells treated with 40 µM curcumin,
compared with 40 µM curcumin + NC treatment (Fig. 8A). In addition, suppression of
miRNA-15a expression led to a significant reversal of the
anticancer effect of 40 µM of curcumin on the cell inhibition rate
of AMC-HN-8 cells (Fig. 8B).
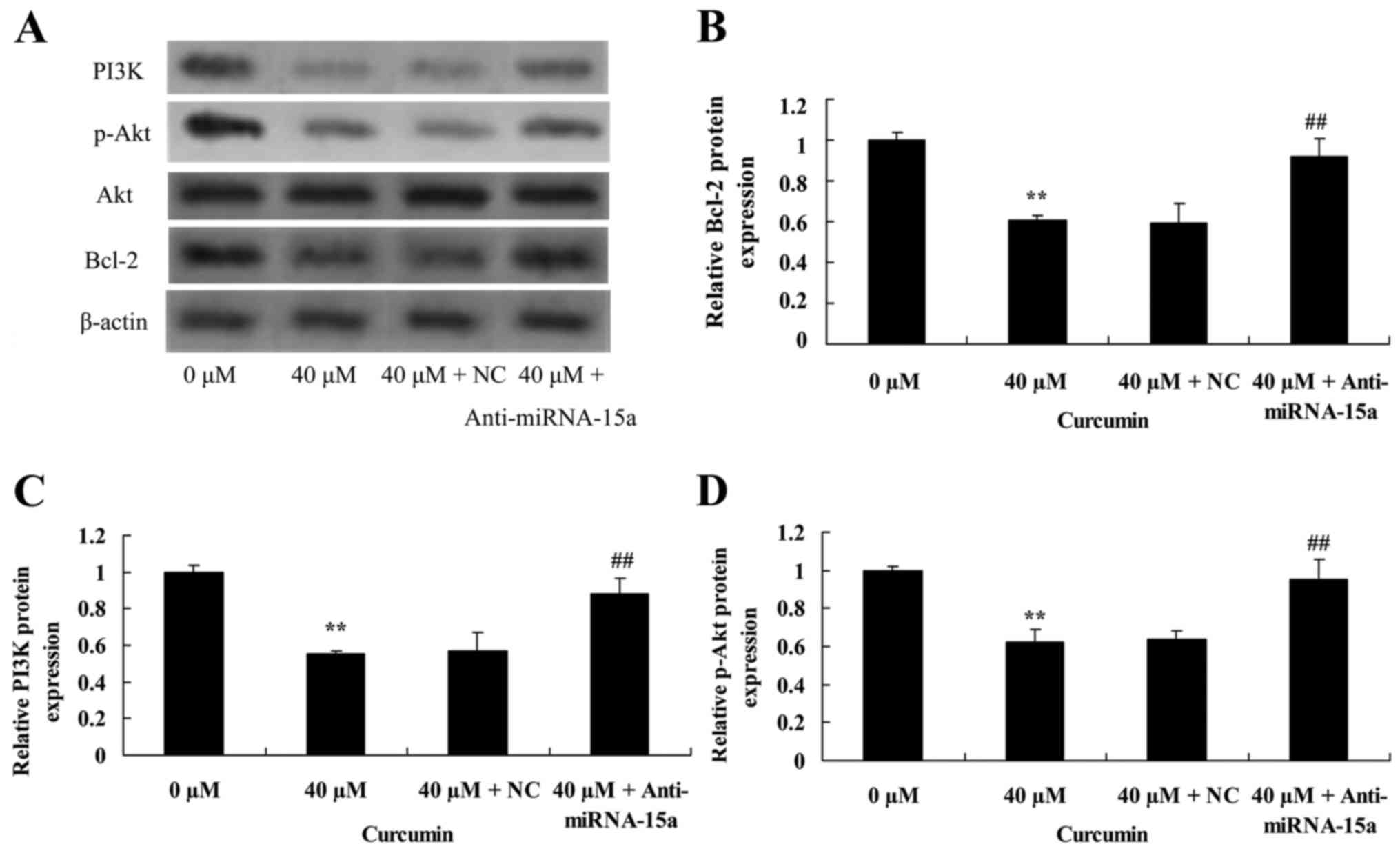
Suppression of miR-15a expression
reverses the anticancer effect of curcumin on Bcl-2, PI3K and p-Akt
protein expression in human laryngeal cancer cells
To validate the effect of suppression of miR-15a
expression on the anticancer effect of curcumin on Bcl-2, PI3K and
p-Akt protein expression of human laryngeal cancer cells, western
blot analysis was used to analyze Bcl-2, PI3K and p-Akt protein
expression in AMC-HN-8 cells. Compared with treatment with 40 µM of
curcumin + NC, Bcl-2 and PI3K protein expression, and the p-Akt/Akt
ratio were significantly increased by suppression of miR-15a
expression in AMC-HN-8 cells treated with 40 µM of curcumin
(Fig. 9).
Discussion
Laryngeal caner is one of the most common types of
malignant tumor in the head and neck (2). Concomitant with an increase in air
pollution in recent years, the incidence of laryngeal cancer is
increasing annually (4). Although it
is possible to treat the cancer using surgery, chemotherapy,
radiotherapy and other treatments, a number of patients still
succumbed due to local recurrence or metastasis after the radical
operation, radiotherapy and chemotherapy (15). Laryngeal cancer remains a serious
threat to the life and safety of patients; therefore, there is an
urgent requirement to identify more effective tumor prevention and
treatment (16). In the present
study, it was identified that curcumin inhibits cell proliferation
and promotes apoptosis of human laryngeal cancer cells. Tuorkey
(17) reported that curcumin is a
potent cancer preventive agent (17),
and Sordillo and Helson (18)
identified that curcumin exhibits asymmetrical effects on cancer
and wild-type stem cells. These findings implicated curcumin as a
clinically important drug for the treatment of human laryngeal
cancer cells.
Apoptosis of tumor cells is a complex process
involving the regulation of numerous genes, including members of
the Bcl-2 family, which are among the most important members as
they are able to inhibit the apoptosis of cells (19). Bcl-2 and Bcl-extra large are able to
inhibit apoptosis of cells, whereas Bcl-2-like protein (Bax) and
Bcl-2 homologous antagonist/killer promote apoptosis, and
alterations in expression of these genes affects the apoptosis of
wild-type cells and the apoptosis of tumor cells (20). For example, expression of Bcl-2 may
lead to survival of cells with damaged DNA and accumulation of
mutations, to promote tumor development (21). In the present study, curcumin was
demonstrated to be able to suppress Bcl-2 protein expression in
AMC-HN-8 cells. Furthermore, suppression of miR-15a expression led
to an increase in Bcl-2 protein expression in AMC-HN-8 cells
treated with 40 µM of curcumin. Zhu et al (13) demonstrated that curcumin triggers
apoptosis of SW872 human adipocytes through upregulation of caspase
activation and the Bax/Bcl-2 ratio.
The PI3K/Akt signaling pathway is an important
signaling pathway for growth factors in vivo, which are able
to activate the anti-apoptosis mechanism, and promote the
metabolism of glucose and protein synthesis, to promote the growth
and proliferation of cells (7). This
abnormal signal transduction pathway is able to lead to abnormal
increases in cell growth, proliferation, metabolism and
anti-apoptosis effect, which are involved in the development of the
majority of types of tumor (22).
Therefore, the PI3K/Akt signaling pathway is considered the primary
pathway for the survival of cancer cells, called ‘the pathway of
anti-apoptosis’ (23). The results of
western blot analysis in the present study revealed that curcumin
significantly inhibited PI3K protein expression and p-Akt protein
expression in AMC-HN-8 cells treated with 40 µM of curcumin.
Suppression of miR-15a expression was able to reverse the effects
of curcumin in AMC-HN-8 cells. Seo et al (24) reported that curcumin significantly
promoted NVP-BEZ235 (a PI3K/Akt and mammalian target of rapamycin
inhibitor)-induced apoptosis through Bcl-2 expression in human
renal carcinoma Caki cells. Zhao et al (25) indicated that curcumin induces
apoptosis via inhibition of the PI3K/Akt signaling pathway in
pancreatic cancer cells.
miRNAs serve a negative role primarily through the
inhibition of its target genes; a previous study has demonstrated
that miR-15a induces the apoptosis of laryngeal carcinoma cells by
activating Bcl-2 (26). It has been
confirmed that miRNA-15a is able to inhibit the proliferation of
laryngeal cancer cells by directly inhibiting PI3K/Akt expression
(27). In the present study, it was
identified that curcumin significantly increased miR-15a expression
in AMC-HN-8 cells. Suppression of miRNA-15a expression
significantly reversed the anticancer effect of curcumin on cell
viability of AMC-HN-8 cells. Yang et al (28) reported that curcumin upregulated
miR-15a and reduced the expression of Bcl-2 in MCF-7 breast cancer
cells. Gao et al (29)
suggested that curcumin decreases the expression of Wilms' tumor
protein through miR-15a and miR-16-1 in leukemic cells. Therefore,
it is hypothesized that miR-15a is able to reverse the anticancer
effect of curcumin on cell viability and apoptosis of human
laryngeal cancer AMC-HN-8 cells.
In conclusion, the present study demonstrates that
curcumin inhibits cell proliferation and promotes apoptosis of
laryngeal cancer through Bcl-2 and PI3K/Akt, and upregulating
miR-15a. The results of the present study also demonstrated a
functional link between miR-15a and the anticancer effect of
curcumin on human laryngeal cancer cells. Further studies are
required to determine the anticancer effects of curcumin on human
laryngeal cancer in vivo to evaluate the potentially
improved clinical significance for human laryngeal cancer in the
future.
References
|
1
|
Klatka J, Grywalska E, Klatka M, Wasiak M,
Andrzejczak A and Rolinski J: Expression of selected regulatory
molecules on the CD83+ monocyte-derived dendritic cells generated
from patients with laryngeal cancer and their clinical
significance. Eur Arch Otorhinolaryngol. 270:2683–2693. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilbert K, Dalley RW, Maronian N and Anzai
Y: Staging of laryngeal cancer using 64-channel multidetector row
CT: Comparison of standard neck CT with dedicated breath-maneuver
laryngeal CT. AJNR Am J Neuroradiol. 31:251–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Divi V, Worden FP, Prince ME, Eisbruch A,
Lee JS, Bradford CR, Chepeha DB, Teknos TN, Hogikyan ND, Moyer JS,
et al: Chemotherapy alone for organ preservation in advanced
laryngeal cancer. Head Neck. 32:1040–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mouw KW, Solanki AA, Stenson KM, Witt ME,
Blair EA, Cohen EE, Vokes EE, List M, Haraf DJ and Salama JK:
Performance and quality of life outcomes for T4 laryngeal cancer
patients treated with induction chemotherapy followed by
chemoradiotherapy. Oral Oncol. 48:1025–1030. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ampil FL and Nguyen NP: Defining ‘upper
mediastinal irradiation’ in secondary subglottic laryngeal cancer.
Oral Oncol. 50:e15–e16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janssens GO, van Bockel LW, Doornaert PA,
Bijl HP, van den Ende P, de Jong MA, van den Broek GB, Verbist BM,
Terhaard CH, Span PN and Kaanders JH: Computed tomography-based
tumour volume as a predictor of outcome in laryngeal cancer:
Results of the phase 3 ARCON trial. Eur J Cancer. 50:1112–1119.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vachhani P, Bose P, Rahmani M and Grant S:
Rational combination of dual PI3K/mTOR blockade and Bcl-2/−xL
inhibition in AML. Physiol Genomics. 46:448–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu X and Li Z: The role of MicroRNAs
expression in laryngeal cancer. Oncotarget. 6:23297–23305. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Chen Y, Yu J, Liu G and Huang Z:
Integrated transcriptome analysis reveals miRNA-mRNA crosstalk in
laryngeal squamous cell carcinoma. Genomics. 104:249–256. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu W, Geng H, Liu Z, Li H and Zhu Z:
Effect of curcumin on rats/mice with diabetic nephropathy: A
systematic review and meta-analysis of randomized controlled
trials. J Tradit Chin Med. 34:419–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koprowski S, Sokolowski K, Kunnimalaiyaan
S, Gamblin TC and Kunnimalaiyaan M: Curcumin-mediated regulation of
Notch1/hairy and enhancer of split-1/survivin: Molecular targeting
in cholangiocarcinoma. J Surg Res. 198:434–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He Y, Yue Y, Zheng X, Zhang K, Chen S and
Du Z: Curcumin, inflammation and chronic diseases: How are they
linked? Molecules. 20:9183–9213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu L, Han MB, Gao Y, Wang H, Dai L, Wen Y
and Na LX: Curcumin triggers apoptosis via upregulation of
Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes.
Mol Med Rep. 12:1151–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zubillaga-Guerrero MI, Alarcón-Romero Ldel
C, Illades-Aguiar B, Flores-Alfaro E, Bermúdez-Morales VH, Deas J
and Peralta-Zaragoza O: MicroRNA miR-16-1 regulates CCNE1 (cyclin
E1) gene expression in human cervical cancer cells. Int J Clin Exp
Med. 8:15999–16006. 2015.PubMed/NCBI
|
|
15
|
Koskinen WJ, Brøndbo K, Dahlstrand Mellin
H, Luostarinen T, Hakulinen T, Leivo I, Molijn A, Quint WG,
Røysland T, Munck-Wikland E, et al: Alcohol, smoking and human
papillomavirus in laryngeal carcinoma: A Nordic prospective
multicenter study. J Cancer Res Clin Oncol. 133:673–678. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamilton DW, de Salis I, Donovan JL and
Birchall M: The recruitment of patients to trials in head and neck
cancer: A qualitative study of the EaStER trial of treatments for
early laryngeal cancer. Eur Arch Otorhinolaryngol. 270:2333–2337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tuorkey MJ: Curcumin a potent cancer
preventive agent: Mechanisms of cancer cell killing. Interv Med
Appl Sci. 6:139–146. 2014.PubMed/NCBI
|
|
18
|
Sordillo PP and Helson L: Curcumin and
cancer stem cells: Curcumin has asymmetrical effects on cancer and
normal stem cells. Anticancer Res. 35:599–614. 2015.PubMed/NCBI
|
|
19
|
Hosseini A, Sharifi AM, Abdollahi M,
Najafi R, Baeeri M, Rayegan S, Cheshmehnour J, Hassani S, Bayrami Z
and Safa M: Cerium and yttrium oxide nanoparticles against
lead-induced oxidative stress and apoptosis in rat hippocampus.
Biol Trace Elem Res. 164:80–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Besbes S, Mirshahi M, Pocard M and Billard
C: New dimension in therapeutic targeting of BCL-2 family proteins.
Oncotarget. 6:12862–12871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moldoveanu T, Follis AV, Kriwacki RW and
Green DR: Many players in BCL-2 family affairs. Trends Biochem Sci.
39:101–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bitting RL and Armstrong AJ: Targeting the
PI3K/Akt/mTOR pathway in castration-resistant prostate cancer.
Endocr Relat Cancer. 20:R83–R99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simpson DR, Mell LK and Cohen EE:
Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of
the head and neck. Oral Oncol. 51:291–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seo BR, Min KJ, Cho IJ, Kim SC and Kwon
TK: Curcumin significantly enhances dual PI3K/Akt and mTOR
inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma
Caki cells through down-regulation of p53-dependent Bcl-2
expression and inhibition of Mcl-1 protein stability. PLoS One.
9:e955882014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Z, Li C, Xi H, Gao Y and Xu D:
Curcumin induces apoptosis in pancreatic cancer cells through the
induction of forkhead box O1 and inhibition of the PI3K/Akt
pathway. Mol Med Rep. 12:5415–5422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Zhang ZM, Liu Y, Wei MH, Xue LY, Zou
SM, Di XB, Han NJ, Zhang KT, Xu ZG and Gao YN: DNA
microarrays-based microRNA expression profiles derived from
formalin-fixed paraffin-embedded tissue blocks of squammous cell
carcinoma of larynx. Zhonghua Bing Li Xue Za Zhi. 39:391–395.
2010.(In Chinese). PubMed/NCBI
|
|
27
|
Skawran B, Steinemann D, Becker T, Buurman
R, Flik J, Wiese B, Flemming P, Kreipe H, Schlegelberger B and
Wilkens L: Loss of 13q is associated with genes involved in cell
cycle and proliferation in dedifferentiated hepatocellular
carcinoma. Mod Pathol. 21:1479–1489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J, Cao Y, Sun J and Zhang Y: Curcumin
reduces the expression of Bcl-2 by upregulating miR-15a and miR-16
in MCF-7 cells. Med Oncol. 27:1114–1118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao SM, Yang JJ, Chen CQ, Chen JJ, Ye LP,
Wang LY, Wu JB, Xing CY and Yu K: Pure curcumin decreases the
expression of WT1 by upregulation of miR-15a and miR-16-1 in
leukemic cells. J Exp Clin Cancer Res. 31:272012. View Article : Google Scholar : PubMed/NCBI
|