Introduction
Osteosarcoma, one of the primary malignant tumors of
bone, is the most common bone cancer and accounts for 20% of
primary malignant tumors (1).
Osteosarcoma occurs mainly in youth, with a higher incidence in
males (2). At present, the treatment
of osteosarcoma primarily includes surgery, radiotherapy and
chemotherapy and the 5-year survival rate of osteosarcoma ranges
from 20 to 75% (3). However, there
are many chemotherapy-insensitive osteosarcoma patients with a high
risk of tumor metastasis and recurrence. Therefore, it is highly
important to identify a more effective and stable method for the
diagnosis and treatment of osteosarcoma. The activation of
proto-oncogenes and the inactivation of tumor suppressor genes can
affect the cell cycle and apoptosis, which in turn lead to
tumorigenesis (4). Therefore,
studying osteosarcoma-related tumor suppressor genes at the
molecular level to improve the diagnosis and treatment of
osteosarcoma has high clinical value.
Tumor suppressor genes can inhibit tumor cell
proliferation, oncogenesis, invasion, metastasis, apoptosis and
even death and have become a research focus in the field of
osteosarcoma gene therapy. The WWOX and p53 genes are classical
tumor suppressor genes. WWOX, which is located on chromosome 16q23,
spans the entire chromosomal fragile site, FRA16D (5). WWOX contains nine exons. The first four
exons of WWOX encode the WW domain and exons 5–8 encode the
oxidoreductase domain (6). WWOX can
inhibit tumor cell activity and this inhibitory effect has been
reported in a variety of tumors, such as glioma (7), lung cancer (8) and thyroid cancer (9). The p53 gene, which is located on the
short arm of human chromosome 17, encodes a protein that can
promote DNA repair, transient and sustained cell growth arrest,
apoptosis and senescence (10). There
are two types of the p53 gene, wild-type and mutant. Wild-type p53,
which has anticancer effects, can cause apoptosis to eliminate
tumor cells (11). The common
mutation site of mutant p53 is position 143 (from A to G) and
mutant p53 can cause tumorigenesis (12).
The function of the WWOX gene in osteosarcoma has
not been well studied and comparison studies of the wild-type and
mutant p53 genes are also rare. In the present study, we examined
the effects of stable expression of the WWOX and p53 genes in
transfected cell lines. The effects of the WWOX and p53 (wild-type)
genes on the proliferation and cell cycle of osteosarcoma cells
were assessed by MTT assay and flow cytometry to investigate the
relationship between osteosarcoma and the expression of the WWOX
and p53 (wild-type) genes. Additionally, the expression of the
mutant p53 gene in osteosarcoma tissue was detected by
immunohistochemistry to investigate the relationship between the
mutant p53 gene and the degree of malignancy of osteosarcoma.
Materials and methods
Reagents
The human osteosarcoma cell line, MG-63, was
purchased from the Institute of Basic Medical Sciences of the
Chinese Academy of Medical Sciences (Beijing, China); plasmids were
constructed at the early stage of this project by the team members;
Lipofectamine® 2000 was from Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA); MTT and RIPA lysis buffer both
from Beijing PPLYGEN Technology Co., Ltd. (cat. no. C1053; Beijing,
China); TRIzol kit was from Invitrogen; Thermo Fisher Scientific,
Inc. (cat. no. 15596026); TransScript® One-Step gDNA
Removal and cDNA Synthesis SuperMix kit both from TransGen Biotech
Co., Ltd. (cat. no. AT311-02; Beijing, China); FastStart Universal
SYBR-Green Master (Rox) kit was from Roche Diagnostics (cat. no
04913914001; Indianapolis, IN, USA); BCA kit was from Thermo
Labsystems (cat. no 23225; Santa Rosa, CA, USA); Cell cycle assay
kit for flow cytometry was from Baomanbio Co., Ltd. (cat. no.
GMS10021.1; Shanghai, China).
Mouse monoclonal wild-type p53 antibody (dilution,
1:500; cat. no. 178924), mouse monoclonal mutant p53 antibody
(dilution, 1:500; cat. no. 178379) and HRP-labeled goat anti-mouse
secondary antibody (dilution, 1:2,000; cat. no. 197302) were from
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing,
China).
Construction of WWOX and
p53-overexpressing MG-63 cell lines through transfection
The recombinant eukaryotic overexpression plasmids
of WWOX and p53 were, respectively transfected into MG-63
osteosarcoma cells by liposome transfection to establish cell lines
that stably expressed WWOX and p53. These cell lines were named the
MW and MP cell line, respectively.
Cell culture
MG-63 cells were cultured in MEM medium supplemented
with 10% fetal bovine serum (FBS). G418 was added to DMEM medium
containing 10% FBS to a final concentration of 1,000 µg/ml for
culture of transfected MW and MP cells. The cells were cultured at
37°C with 5% CO2. When cells were 80–90% confluent, they
were subcultured according to the required dilution ratio.
Quantitative polymerase chain reaction
(qPCR)
MW, MP and MG-63 cells under healthy growth
conditions were collected at the logarithm growth period. Total
cellular RNA was extracted using TRIzol reagent and RNA was reverse
transcribed into cDNA according to the instructions of the
TransScript® One-Step gDNA Removal and cDNA Synthesis
SuperMix kit. The primer sequences of WWOX, p53 (wild-type) and the
endogenousv GAPDH gene were designed with reference to a large
number of studies (Table I). All
primers were synthesized by a third party. qPCR was carried out
using the FastStart Universal SYBR-Green PCR Master (Rox) kit and
the relative expression of each gene was calculated using the
2−ΔΔCq method.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| WWOX |
TCCTCAGAGTCCCATCGATTT |
CGGCAGCAGTTGTTGAAGTA |
| p53 |
TACTCCCCTGCCCTCAACAAG |
CGCTATCTGAGCAGCGCTCAT |
| GAPDH |
CATGCCATCACTGCCACCCA |
TTGACAAAGTGGTCGTTGAG |
Western blot analysis
MW, MP and MG-63 cells under healthy growth
conditions were collected at the logarithm growth period. Cells
were lysed with lysis buffer and total protein was extracted. Total
protein concentration was determined using a BCA kit. Protein
samples were subjected to (SDS-PAGE), after which protein was
transferred to PVDF membranes using a semi-dry method. The
membranes were blocked with 5% skimmed milk, followed by incubation
with primary mouse monoclonal wild-type p53 antibody (dilution,
1:500; cat. no. 178924) overnight at 4°C, after washing. Membranes
were then incubated with HRP-labeled goat anti-mouse secondary
antibody (dilution, 1:2,000; cat. no. 197302) for 1 h at room
temperature. After washing, a gel imaging system was used to detect
protein signals. β-actin was used as the endogenous control and
ImageJ software was used to quantify protein expression.
MTT assay
MW, MP and MG-63 cells under healthy growth
conditions were collected at the logarithm growth period and
digested with trypsin to prepare cell suspensions. Cells in
suspension were seeded in 96-well plates and MTT reagent was added
1–6 days later. After adding the MTT reagent, cells were cultured
at 37°C with 5% CO2 for an additional 4 h, followed by
the addition of DMSO to stop the reaction. The 96-well plates were
placed in a microplate reader and the absorbance value of each well
was measured at 490 nm to calculate the survival rate and construct
the growth curve.
Flow cytometry
MW, MP and MG-63 cells were digested with trypsin,
followed by centrifugation to remove the medium and obtain cell
pellets. The cell cycle assay kit for flow cytometry and the flow
cytometer were used for the cell cycle analysis.
Immunohistochemistry
The expression of mutant p53 in osteosarcoma was
detected by immunohistochemistry. A total of 65 paraffin-embedded
osteosarcoma tissue samples were selected in Jingmen No. 2 People's
hospital. The samples were diagnosed by pathological diagnosis and
were classified into different differentiation levels: High, 17
cases; moderate, 25 cases; and low differentiation, 23 cases.
Paraffin-embedded osteosarcoma tissue samples were sectioned at a
thickness of 5 µm. Immunohistochemical staining was performed using
the SP method (13) to detect the
expression of mutant p53 protein in osteosarcoma tissues with
different pathological grades. PBS was used as the negative control
of the primary antibody. A double-blind method was used to observe
the signals. Five high-power microscopic fields were randomly
selected for observation. Brown particles within the nucleus
represented positive signals. Specific criteria for the
determination of mutant p53 expression are shown in Table II.
 | Table II.Criteria for the determination of
mutant p53 expression. |
Table II.
Criteria for the determination of
mutant p53 expression.
| The proportion of
positive cells (%) | Classification |
|---|
| <5 | − |
| 5–25 | + |
| 26–50 | ++ |
| >50 | +++ |
Statistical analysis
Data are presented as percentage and one-way ANOVA
was performed using SPSS 13.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) for comparisons between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of WWOX and p53 (wild-type)
mRNA in the three groups of cells
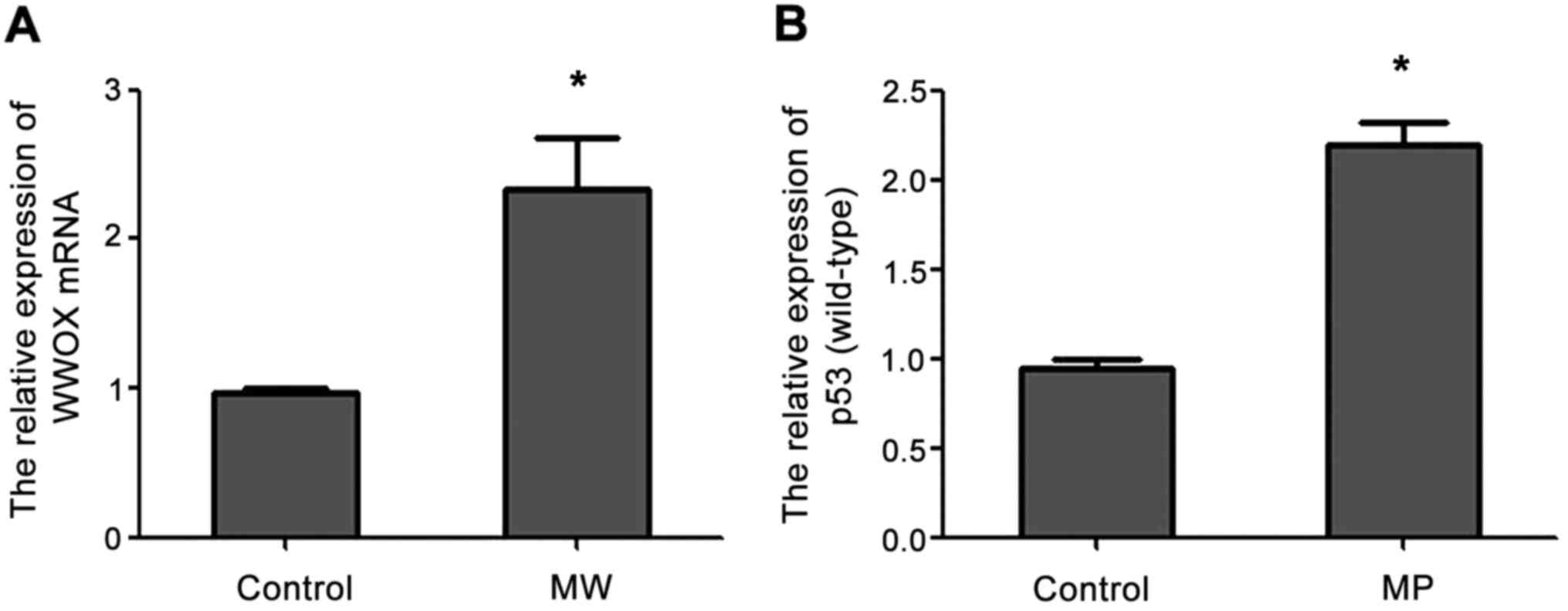
qPCR analysis showed that the expression of WWOX
mRNA was increased in MW cells compared with the blank control
group and the expression of p53 (wild-type) mRNA was increased in
MP cells compared with the blank control group (Fig. 1).
Expression of WWOX and p53 (wild-type)
protein in the three groups of cells
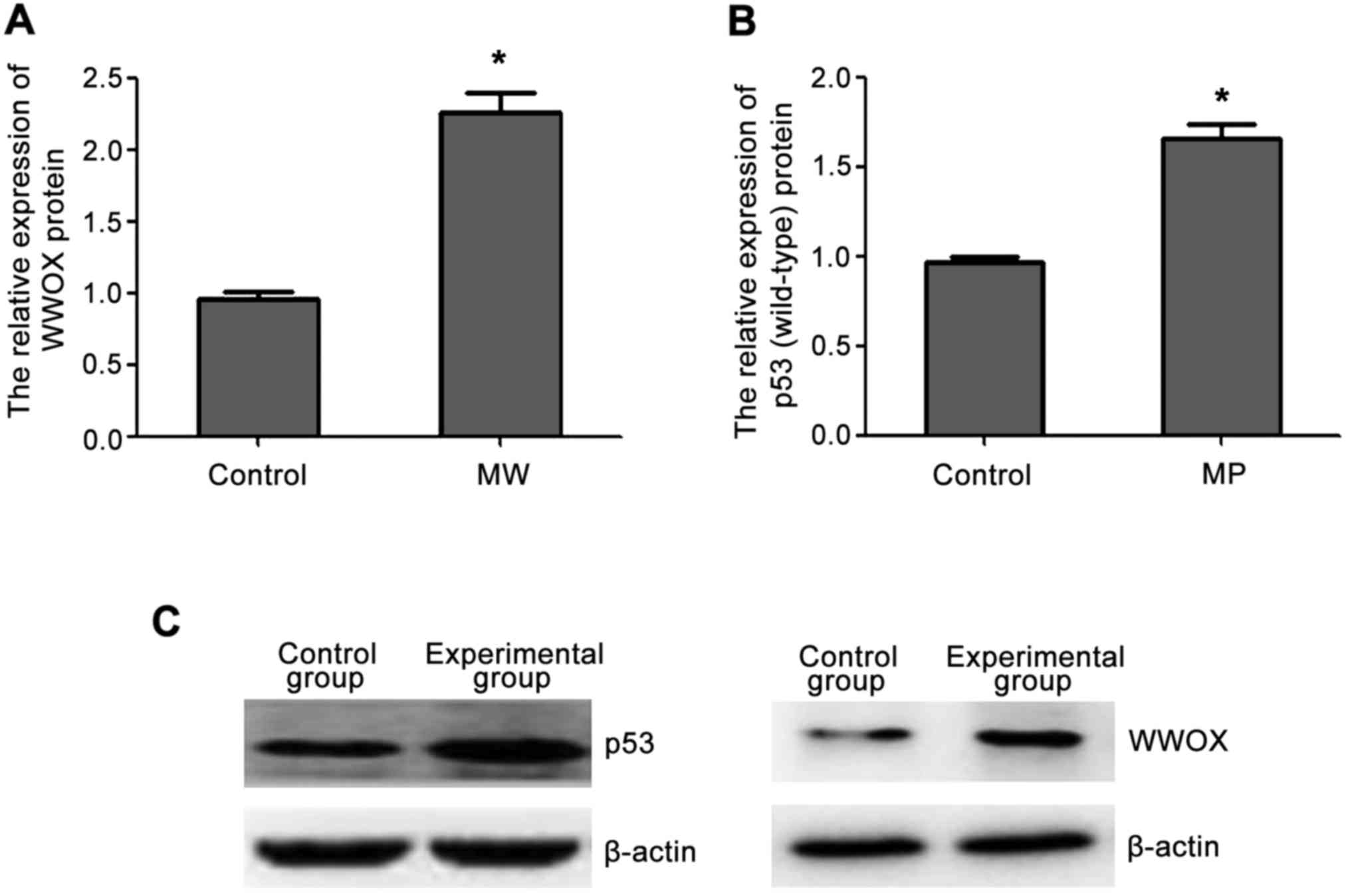
Western blot analysis showed that the expression of
WWOX and p53 (wild-type) protein in the transfected cells was
higher than those in the control group and the increased protein
expression was consistent with the increased mRNA expression
(Fig. 2).
The effect of WWOX and p53 (wild-type)
on the proliferation of MG-63 cells as determined by MTT assay
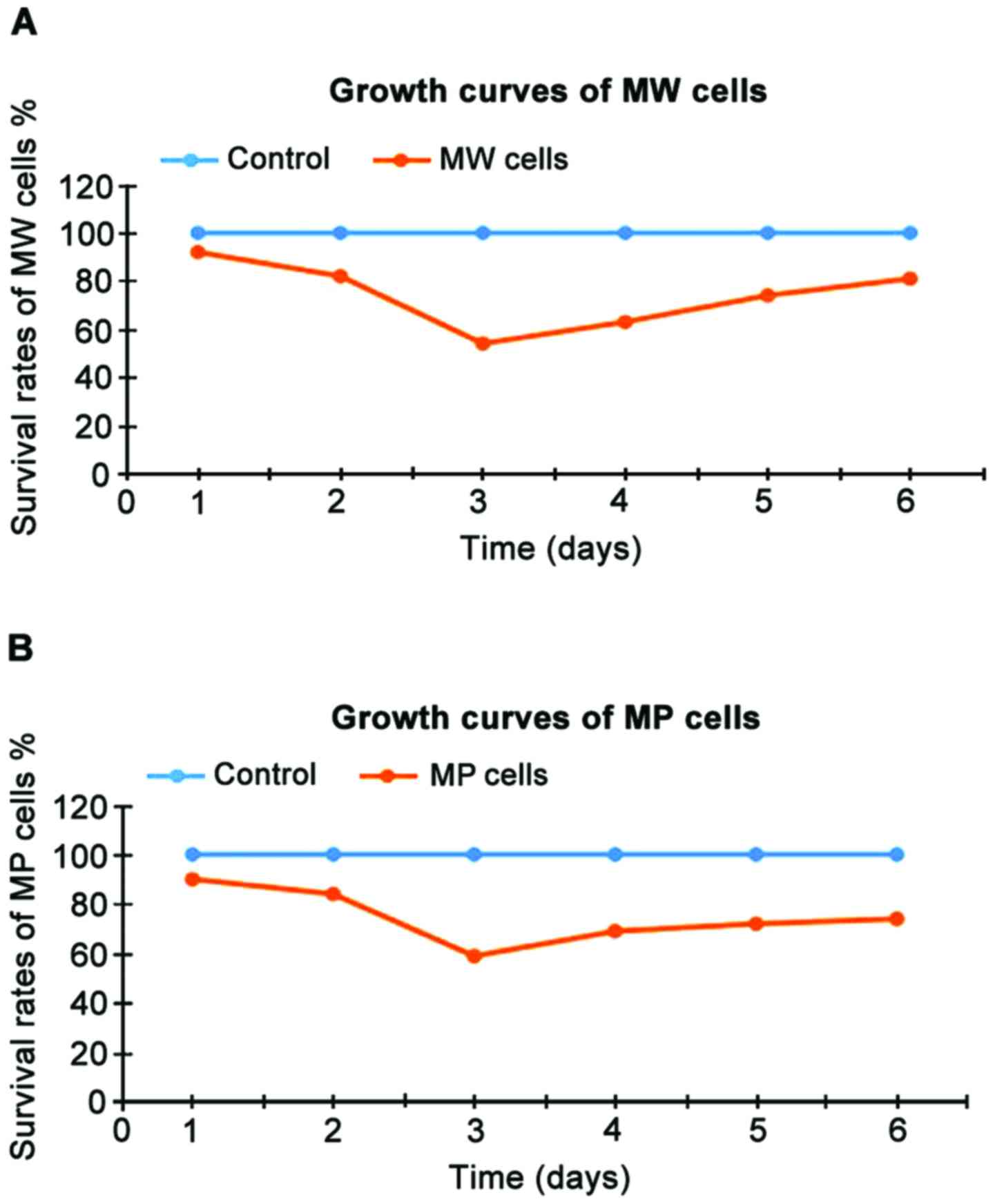
The cell survival rates of MW and MP cells at 1–6
days after the beginning of incubation are shown in Table III and the growth curve is shown in
Fig. 3. The survival rates of MW and
MP cells were significantly lower than those of the blank control
group at each time point (P<0.05). As shown in Table III and Fig. 3, the two genes showed significant
inhibitory effects on MG-63 osteosarcoma cells at 3 days after the
beginning of incubation.
 | Table III.Survival rates of MW and MP cells
(%). |
Table III.
Survival rates of MW and MP cells
(%).
| Days of
incubation | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Blank control
group | 100 | 100 | 100 | 100 | 100 | 100 |
| MW | 92a | 82a | 54a | 63a | 74a | 81a |
| MP | 90a | 84a | 59a | 69a | 72a | 74a |
Changes of the cell cycle after WWOX
and p53 (wild-type) gene transfection
Flow cytometric analysis showed that MW and MP cells
were blocked in the G0/G1 phase, suggesting that overexpression of
the WWOX and p53 (wild-type) genes causes cell cycle arrest of
MG-63 osteosarcoma cells at the G0/G1 phase (Table IV).
 | Table IV.The cell cycle of MW and MP cells
(mean ± standard deviation). |
Table IV.
The cell cycle of MW and MP cells
(mean ± standard deviation).
| Groups | G0/G1 (%) | S (%) | G2/M (%) |
|---|
| Control | 24.07±1.235 | 45.49±0.987 | 25.45±0.912 |
| MW | 78.49±2.392 | 12.01±1.209 |
8.30±0.462 |
| MP | 66.76±0.235 | 19.96±0.689 | 11.34±0.764 |
Immunohistochemical analysis of the
expression of mutant p53 in osteosarcoma tissue
The mutant p53 protein is located in the nucleus and
the brown particles observed after immunohistochemical staining
represented positive signals. Microscopic observation showed that
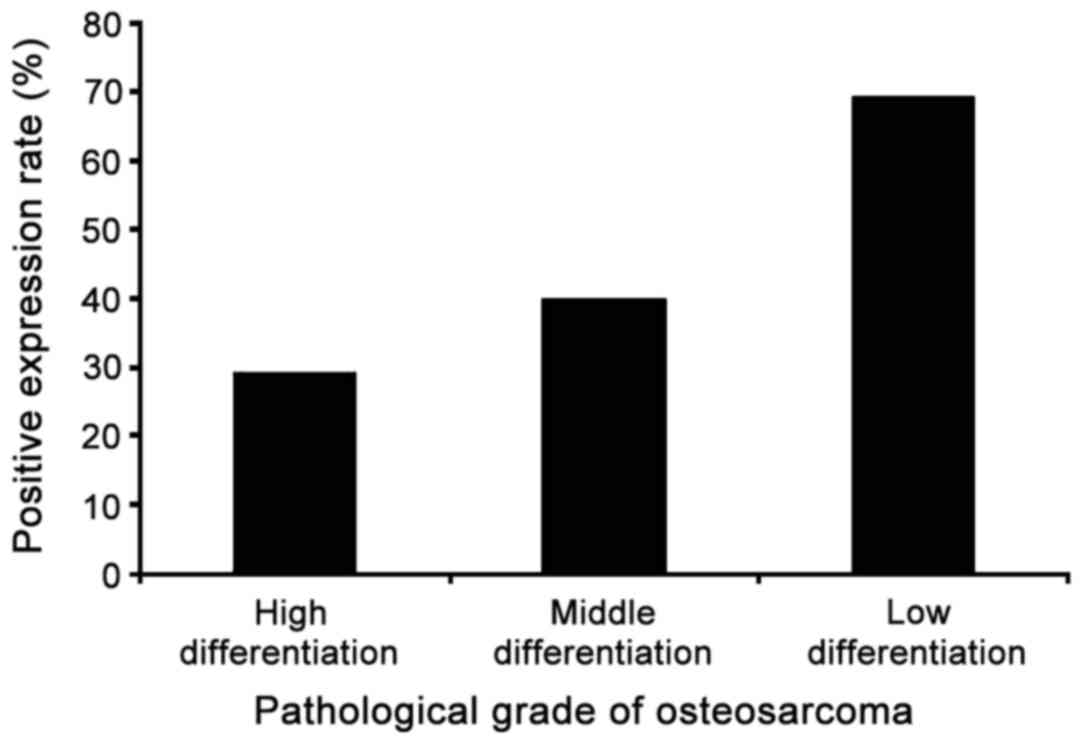
the positive expression rate of mutant p53 protein in 65 cases of
osteosarcoma was 47.7% and the positive expression rate was related
to pathological grade. According to the criteria for determination,
the expression status of the mutant p53 protein in each
pathological grade of osteosarcoma is summarized in Table V and the positive expression rates of
the mutant p53 protein in each pathological grade are shown in
Fig. 4. The highest positive
expression rate of mutant p53 was found in low differentiation
osteosarcoma tissue and the positive expression rate of mutant p53
protein decreased with increased degree of tumor malignancy.
 | Table V.The expression status of p53 (mutant)
protein in each pathological grade of osteosarcoma. |
Table V.
The expression status of p53 (mutant)
protein in each pathological grade of osteosarcoma.
| Pathological
grade | Case | Positive (cases) | Negative (cases) | Positive rate
(%) |
|---|
| High
differention | 17 | 5 | 12 | 29.4 |
| Moderate
differention | 25 | 10 | 15 | 40.0 |
| Low differention | 23 | 16 | 7 | 69.6 |
| Total positive
expression rate |
|
|
| 47.7 |
Discussion
The diagnosis and treatment of osteosarcoma at the
genetic level is an area of intense research. Studies have shown
that tumor suppressor genes can inhibit tumorigenesis and tumor
growth (14). Therefore, in the
present study, we investigated the functions of two classic tumor
suppressor genes, p53 and WWOX, at the molecular level to explore
the correlation between the two genes and osteosarcoma.
WWOX is a tumor suppressor gene with a length of
approximately 1.1 Mb. Studies have shown that the WWOX gene plays
an important role in preventing tumorigenesis and tumor growth.
WWOX can function as a transcription factor to regulate tumor
metastasis and growth by regulating the expression of various genes
(15) and reduced expression of the
WWOX gene can cause tumorigenesis and promote the growth of
osteosarcoma (16).
P53 is another well-known tumor suppressor gene. The
main role of p53 is to induce apoptosis and affect the cell cycle
(17). The p53 gene exists as two
types - wild and mutant and the two types have different functions
(18). Because of the short
half-life, the p53 (wild-type) protein cannot be easily detected by
immunohistochemistry (19,20). However, mutant p53 can be detected by
immunohistochemistry because of a long half-life. In this study,
p53 (wild-type) and mutant p53 were studied. The two transfected
MG-63 osteosarcoma cell lines, which stably expressed the WWOX and
p53 (wild-type) genes, were constructed and named the MW and MP
cell lines, respectively. Measurement of the levels of mRNA and
protein in the two groups showed that WWOX and p53 were stably
expressed in the cell lines and the two groups of transfected cells
were therefore suitable for use in subsequent experiments.
Based on the successful establishment of the MW and
MP cell lines, we simultaneously studied the effects of the WWOX
and p53 (wild-type) genes on MG-63 osteosarcoma cells via in
vitro experiments. The growth rates of MG-63 cells transfected
with the WWOX or p53 (wild-type) gene overexpression plasmids were
reduced and flow cytometric analysis showed that overexpression of
the WWOX and p53 (wild-type) genes can arrest MG-63 cells in the
G0/G1 phase of the cell cycle. These results indicate that the WWOX
and p53 (wild-type) genes can inhibit the proliferation of
osteosarcoma cells by affecting the cell cycle.
Immunohistochemical analysis showed that the
wild-type and mutant p53 genes have completely opposite functions.
Mutant p53 was highly expressed in osteosarcoma and its expression
was positively related to the pathological grade of
osteosarcoma.
In conclusion, detection of the expression of the
WWOX and p53 genes has great clinical value in the diagnosis,
prevention and treatment of osteosarcoma. The WWOX gene can
effectively prevent the growth of osteosarcoma, which is beneficial
for the diagnosis and treatment of the disease. The p53 gene exists
as two different types, where the wild-type p53 gene has the same
function as WWOX in osteosarcoma, and the mutant p53 gene has
important clinical value in the determination of the degree of
malignancy of osteosarcoma.
References
|
1
|
Chen X, Bahrami A, Pappo A, Easton J,
Dalton J, Hedlund E, Ellison D, Shurtleff S, Wu G, Wei L, et al:
Recurrent somatic structural variations contribute to tumorigenesis
in pediatric osteosarcoma. Cell Rep. 7:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: where do we stand? Eur J
Cancer. 47:2431–2145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clark JC, Dass CR and Choong PF: A review
of clinical and molecular prognostic factors in osteosarcoma. J
Cancer Res Clin Oncol. 134:281–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abu-Odeh M, Salah Z, Herbel C, Hofmann TG
and Aqeilan RI: Wwox, the common fragile site FRA16D gene product,
regulates ATM activation and the DNA damage response. Proc Natl
Acad Sci USA. 111:E4716–E4725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang NS, Pratt N, Heath J, Schultz L,
Sleve D, Carey GB and Zevotek N: Hyaluronidase induction of a WW
domain-containing oxidoreductasethat enhances tumor necrosis factor
cytotoxicity. J Biol Chem. 276:3361–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu K, Fan J, Ding X, Li C, Wang J, Xiang Y
and Wang QS: Association study of a functional copy number
variation in the Wwox gene with risk of gliomas among Chinese
people. Int J Cancer. 135:1687–1691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang D, Qiu F, Yang L, Li Y, Cheng M,
Wang H, Ma G, Wang Y, Hu M, Ji W, et al: The polymorphisms and
haplotypes of Wwox gene are associated with the risk of lung cancer
in southern and eastern Chinese populations. Mol Carcinog.
52:E19–E27. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cancemi L, Romei C, Bertocchi S, Tarrini
G, Spitaleri I, Cipollini M, Landi D, Garritano S, Pellegrini G,
Cristaudo A, et al: Evidences that the polymorphism Pro-282-Ala
within the tumor suppressor gene Wwox is a new risk factor for
differentiated thyroid carcinoma. Int J Cancer. 129:2816–2824.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Z, Ma C, Shan Z, Ju Y, Li S and Zhao Q:
Histone deacetylase inhibitors suppress the growth of human
osteosarcomas in vitro and in vivo. J Buon. 18:1032–1037.
2013.PubMed/NCBI
|
|
11
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–757. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li D and Marchenko ND: ErbB2 inhibition by
lapatinib promotes degradation of mutant p53 protein in cancer
cells. Oncotarget. 8:5823–5833. 2017.PubMed/NCBI
|
|
13
|
Chen W, Cooper TK, Zahnow CA, Overholtzer
M, Zhao Z, Ladanyi M, Karp JE, Gokgoz N, Wunder JS, Andrulis IL, et
al: Epigenetic and geneticloss of HIC1 function accentuates the
role of p53 in tumorgenes. Cancer Cell. 6:387–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang NS, Hsu LJ, Lin YS, Lai FJ and Sheu
HM: WW domain-containingoxidoreductase: A candidate tumor
suppressor. Trends Mol Med. 13:12–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Del MS and Aqeilan RI: Tumor suppressor
Wwox inhibits osteosarcoma metastasis by modulating RUNX2 function.
Sci Rep. 5:129592015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abdeen SK, Del MS, Hussain S, Abu-Remaileh
M, Salah Z, Hagan J, Rawahneh M, Pu XA, Russell S, Stein JL, et al:
Conditional inactivation of the mouse Wwox tumor suppressor gene
recapitulates the null phenotype. J Cell Physiol. 228:1377–1382.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mercer J, Mahmoudi M and Bennett M: DNA
damage, p53, apoptosis and vascular disease. Mutat Res. 621:75–86.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Subramanian M, Jones MF and Lal A: Long
non-codingRNAs embedded in the Rb and p53 pathways. Cancers
(Basel). 5:1655–1675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bargonetti J and Manfred IJJ: Multiple
roles of the tumor suppressor p53. Curr Opin Oncol. 14:86–91. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mardanpour K, Rahbar M and Mardanpour S:
Coexistence of HER2, Ki67 and p53 in Osteosarcoma: A strong
prognostic factor. N Am J Med Sci. 8:210–214. 2016. View Article : Google Scholar : PubMed/NCBI
|