Introduction
Pituitary tumors are primarily adenomas and occur in
the pituitary gland (1). They consist
of three categories, according to their biological functions:
Benign tumors, invasive tumors and carcinomas (2). Pituitary carcinomas are the malignant
tumor type, occurring in only 0.1–0.2% of pituitary tumor cases
(3). Pituitary carcinomas are only
diagnosed when metastasis inside or outside the nervous system is
observed (1). Since the pituitary
gland is close to the brain, invasive pituitary adenomas may invade
the cranial bone and lead to side effects (4). They may induce increased intracranial
pressure and various types of headache (5,6).
Pituitary tumors are divided into secreting and
non-secreting tumors, with the majority of tumors being the
secreting type (7). They produce
abundant amounts of hormones, and hormone secretion may cause
several forms of hyperpituitarism (8). In pregnant females, excessive hormone
secretion may induce maternal or fetal morbidity (9). Surveillance for tumor growth and hormone
levels in the blood of pregnant patients aids the fetus to grow
healthily and be successfully delivered (10).
For these pregnant females with pituitary tumors,
pituitary tumors may secrete hormones to affect the health of the
mother and fetus; furthermore, pregnancy may also promote tumor
growth (11). Pregnant individuals
secrete various types of hormone, including progesterone and human
placental lactogen (12), which may
promote tumor growth (13,14). Surveillance for tumor size is also
essential for tumor growth itself, as pregnancy may promote
pituitary tumor progression (11).
However, how pregnancy affects pituitary tumor growth remains
unclear. It has been shown that brain-derived neurotrophic factor
(BDNF) is detected at an increased level in pregnant females
compared with non-pregnant females (15). BDNF is associated with lung cancer
prognosis (16). However, to the best
of our knowledge, it has not yet been reported whether BDNF is
involved in the effect that pregnancy exhibits in inducing
pituitary tumor cell growth. The present study established pregnant
mouse models. The time of tumor occurrence and tumor weight were
determined, and the BDNF levels in the pregnant or control mice
were measured. In addition, the effect of BDNF on the proliferation
of pituitary tumor cells was investigated. How BDNF affects the
growth of tumor cells growth was investigated using cell cycle
distribution analysis. The results of the present study revealed
whether pregnancy promoted pituitary tumor growth in the mouse
model, and identified the underlying molecular mechanism of how
pregnancy affects pituitary tumor growth. The results of the
present study indicate that it may be beneficial to monitor the
effect of pregnancy on pituitary tumors and therapy of pituitary
tumors.
Materials and methods
Animal experiments
A total of 16 female nude mice at 8 weeks-old were
purchased from Shanghai Laboratory Animal Center (Shanghai, China).
All the mice are kept in Specific-pathogen-free (SPF) animal
facility and fed with sterilized food and water. The average weight
of the mice was 30 g. All the mice were randomly separated into 2
groups with 8 mice in each group and numbered with identifiers.
Each mouse was subcutaneously injected with 5×106 AtT-20
cells. After 7 days, one group of mice was mated with male mice,
whereas the other group were raised in the same conditions as the
pregnant mice (SPF animal facility and fed with sterilized food and
water). The female mice were checked whether with pessaries in the
morning to indicate its possibility of pregnancy, and mice with
pessaries were selected for subsequent experiments. The pregnancy
of the female mice was checked at 14 days post-mating. A total of
17 female mice were used for mating, and 8 pregnant mice were
finally used in the pregnant group, and 8 female mice that were
unmated were used as control. The occurrence of the tumors was
checked every 3 days post-tumor cell injection. The mice were
euthanized 28 days after the tumor injection. The tumors were
isolated and weighed. The orbital blood of mice was collected at
day 14 for ELISA assay. All the animal experiments were approved by
the Institutional Animal Board of Shandong Traffic Hospital
(Shandong, China) and performed following the US Public Health
Service Policy on Humane Care and the Use of Laboratory Animals
(17).
Determination of BDNF levels
The BDNF level in mice serum was detected using the
BDNF ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA; cat.
no. DBNT00), containing horseradish peroxidase-conjugated detection
antibody, and dilution and wash buffers, according to the
manufacturer's protocol. The blood samples and standard proteins
were prepared prior to the experiment (18). Assay diluent (100 µl) was added to
each well of the 96-well plates, followed by addition of 50 µl
blood samples from pregnant mice or non-pregnant mice and
incubation at room temperature for 2 h. A total of 100 µl detection
antibody diluted in the reagent diluent was added into each well
and incubated at room temperature for 1 h. The 100 µl aliquots of
the working dilution of Streptavidin-HRP were incubated at room
temperature for 20 min following antibody incubation and washed 3
times with wash buffer. Subsequently, substrate solution was added
for 30 min at room temperature in the dark, followed by termination
with stop solution from the ELISA kit (R&D Systems, Inc.).
Absorbance at 450 nm was evaluated and calculated based on the
standard curve.
Cell culture
Mouse pituitary tumor AtT-20 cells were purchased
from the Cell Bank of Shanghai Institutes for Biological Sciences
(Shanghai, China). The cells were maintained in RPMI-1640 medium
(Thermo Fisher Scientific, Inc., Waltham, MA) with 10% fetal bovine
serum (Thermo Fisher Scientific, Inc.), supplemented with 100 µg/ml
streptomycin and 100 U/ml penicillin. The cells were split at a
ratio of 1:3 once they reached 90–100% confluence and cultured at
37°C in a humid atmosphere with 5% CO2.
Detection of cell proliferation
The AtT-20 cells were trypsinized and split into
96-well dishes at 3×103 cells/well. The cells were
treated with 50 ng/ml BDNF. The cells were maintained at 37°C for
2, 4 and 6 days, respectively. Cell viability was measured at
indicated days (2, 4 and 6 days) by the Cell Counting kit-8 assay
(CCK-8; Beyotime Institute of Biotechnology, Haimen, China),
according to the manufacturer's protocol. CCK-8 reaction reagent
(10 µl) was directly added to each well. The cells were incubated
for 4 h at 37°C. The cell viability was measured at 450 nm. The
proliferation curve was constructed with the absorbance of cells at
day 0 as control.
Cell cycle detection
The AtT-20 cells were split into 6-well dishes at
3×105 cells/well. The cells were treated with 50 ng/ml
BDNF at 37°C for 2 days. After 2 days of treatment, the cell
cultures were trypsinized into single cell suspensions and fixed
with 70% ethanol at 4°C for 30 min. The cells were centrifuged at
300 × g at room temperature for 5 min and washed with PBS three
times. The cells were then incubated with 50 µg/ml RNaseA
(Sigma-Aldrich; Merck KGaA) in a water bath at 37°C for 30 min and
stained with 50 µg/ml propidium iodide (Sigma-Aldrich; Merck KGaA)
at room temperature in the dark for 30 min. The cells were filtered
and subjected to flow cytometry for cell cycle detection. The
samples were detected using LSR II (BD Biosciences, Franklin Lakes,
NJ, USA) and analyzed using FlowJo software version 8 (Tree Star,
Inc., Ashland, OR, USA).
Statistical analysis
All the data are presented as the mean ± standard
deviation (SD) or the mean ± SD. Independent experiments were
performed three times. Comparisons between groups were analyzed
using Student's t-test and R software version 3.2.2 (https://www.r-project.org/). P<0.05 was considered
to indicate a statistically significant difference between
groups.
Results
Pregnancy promotes AtT20 cell tumor
growth
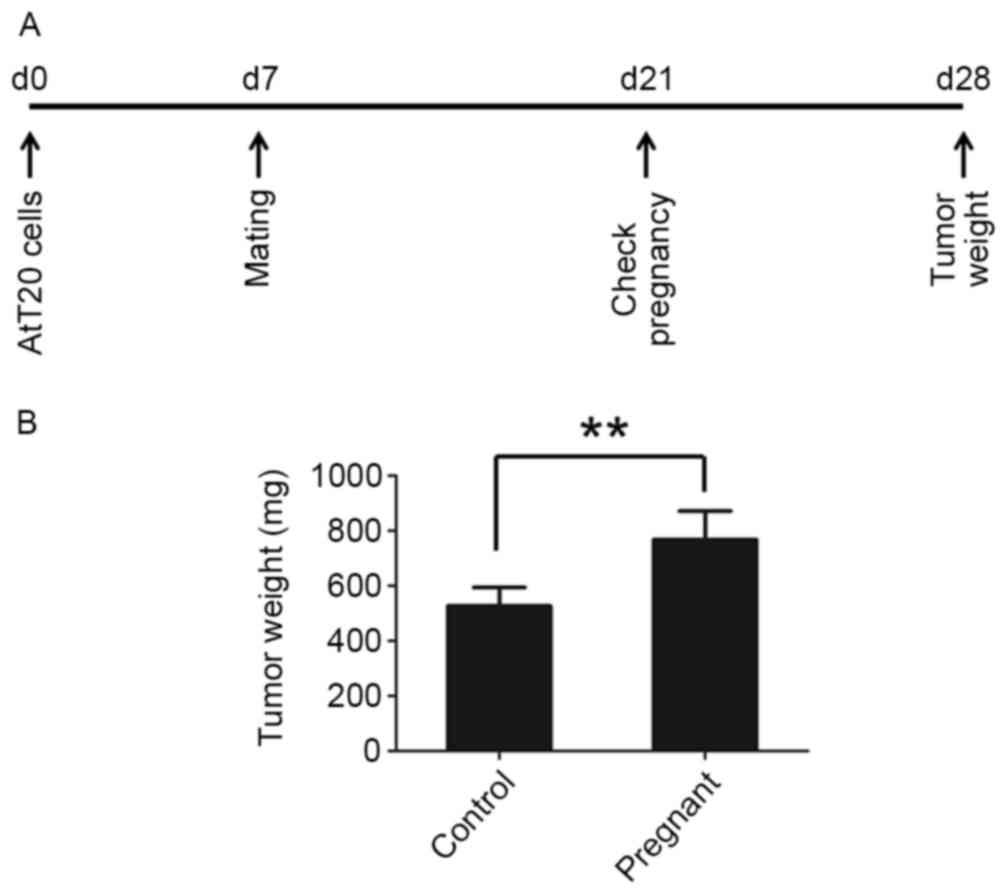
To test whether pregnancy promotes the progression
of pituitary tumors, pituitary tumor AtT-20 cells were
subcutaneously injected into 8 mice in each group and the mice were
mated 7 days later. The pregnancy of mice was verified 14 days
after mating and the tumors were collected 28 days post-tumor cell
injection (Fig. 1A). Pregnancy was
found to promote tumor growth in nude mice. Tumor occurrence in
pregnant mice was earlier compared with the control mice (Table I). The tumors grew larger in the
pregnant mice compared with mice that were unmated (Fig. 1B).
 | Table I.Time of tumor occurrence. |
Table I.
Time of tumor occurrence.
| Time, days | Control group, n | Pregnant group,
n |
|---|
| 7 | 0/8 | 0/8 |
| 10 | 0/8 | 0/8 |
| 14 | 2/8 | 6/8 |
| 17 | 6/8 | 8/8 |
| 21 | 8/8 | 8/8 |
| 24 | 8/8 | 8/8 |
| 28 | 8/8 | 8/8 |
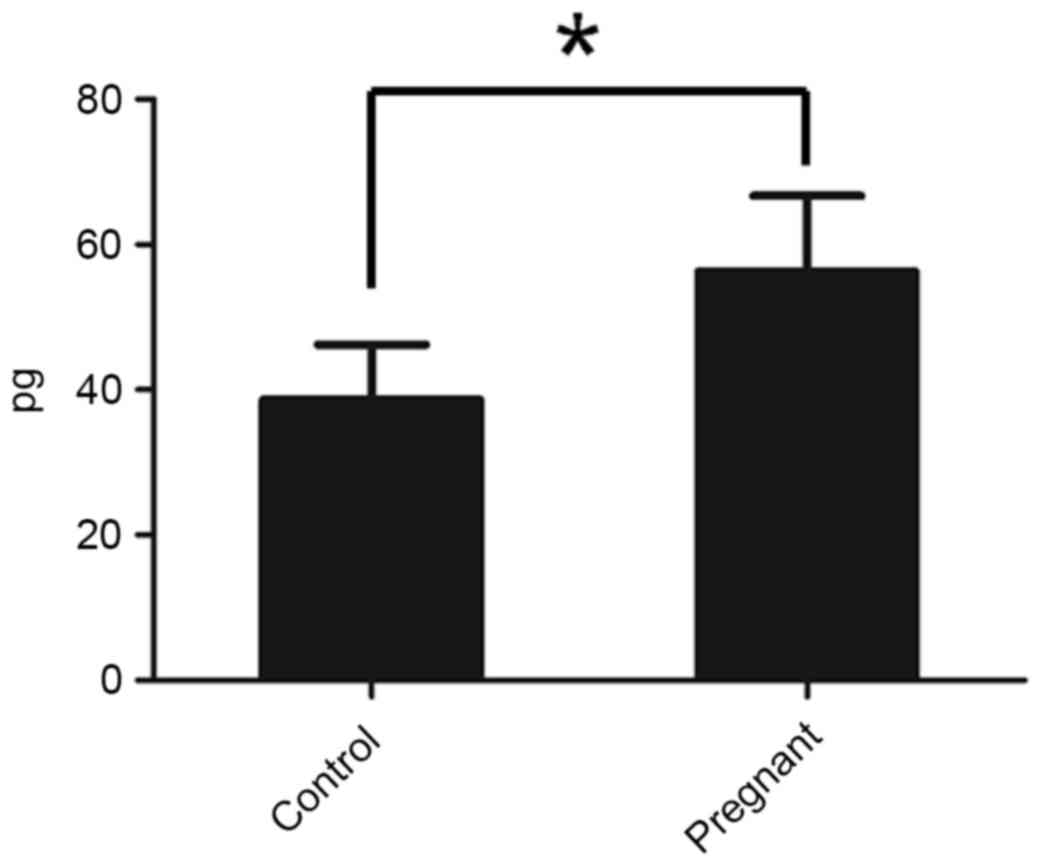
BDNF production is increased in
pregnant nude mice
To check whether the pregnant mice exhibited an
increased level of BDNF, the BDNF level in serum was determined
using an ELISA kit. It was identified that pregnant mice exhibited
increased levels of BDNF compared with the control mice (Fig. 2).
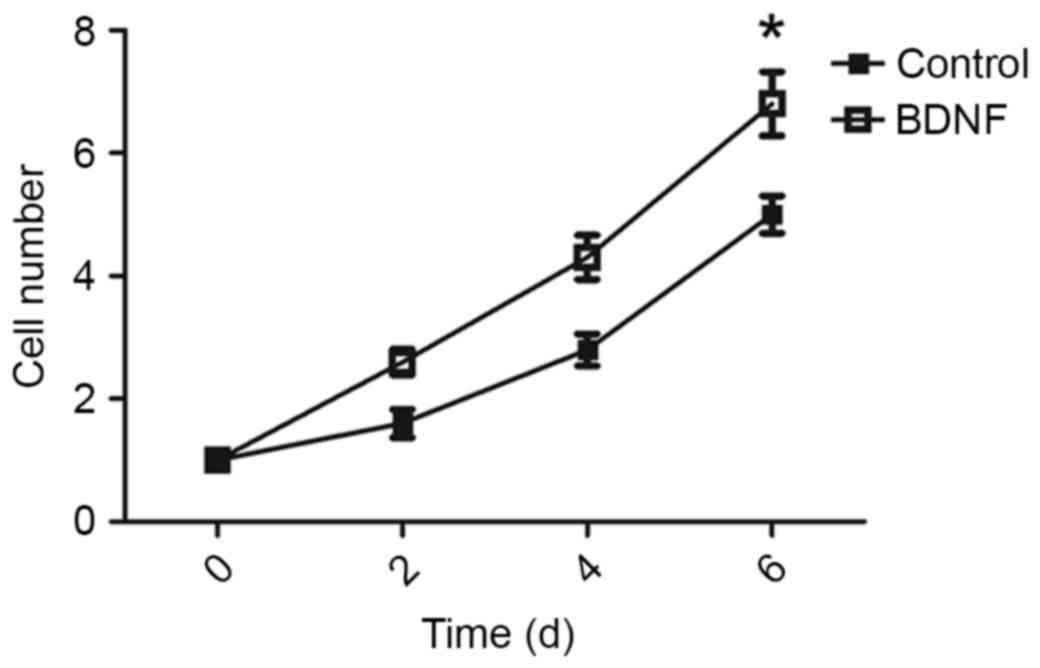
BDNF promotes AtT-20 cell growth
To test whether BDNF was able to promote tumor
growth, BDNF levels of AtT-20 cells were analyzed in vitro.
The AtT-20 cells were treated with or without BDNF, and cell
viability was determined at 2, 4 and 6 days following BDNF
treatment. BDNF increased the proliferation rate of AtT-20 cells
(Fig. 3).
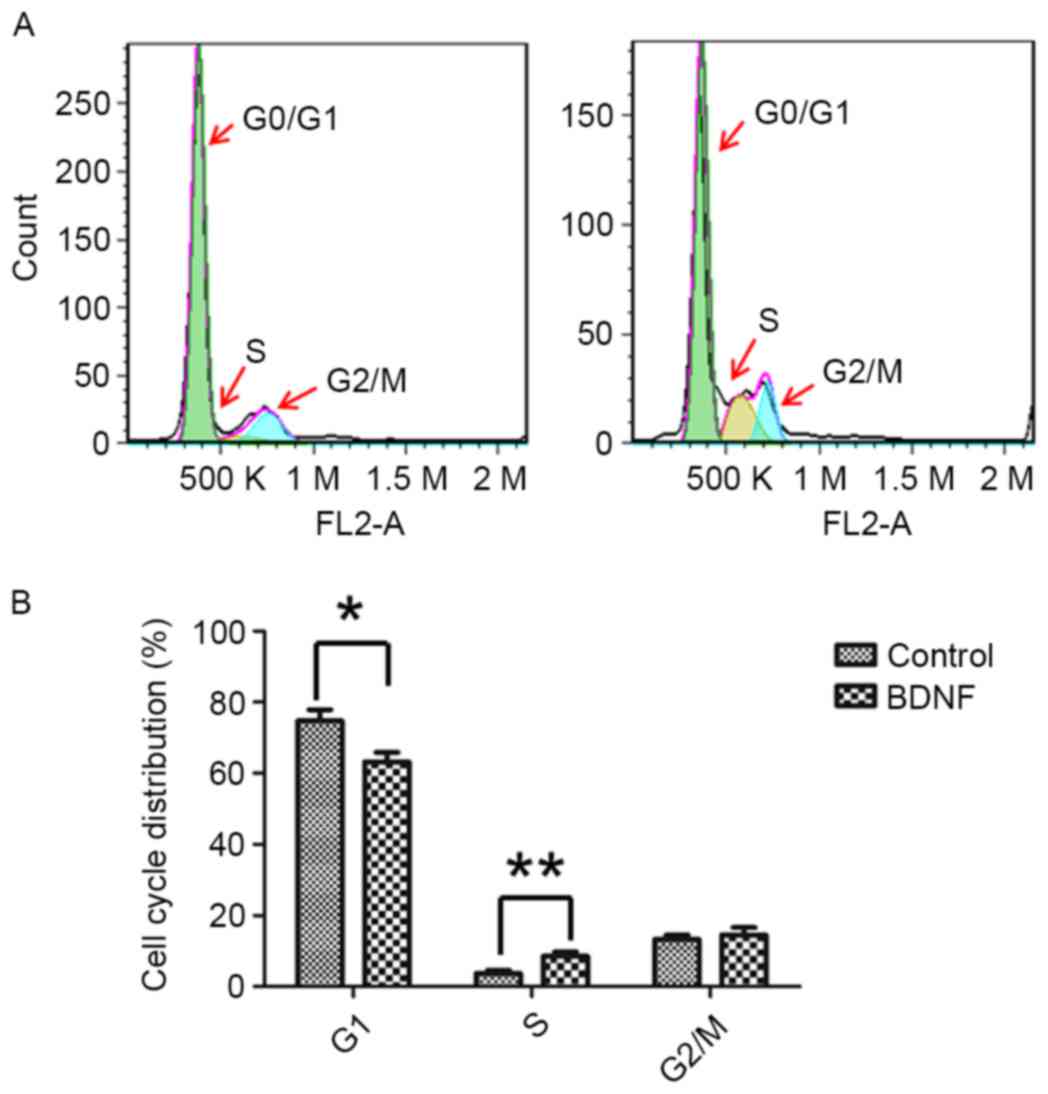
BDNF alters AtT20 cell cycle
distribution
To elucidate how BDNF regulated AtT-20 cell growth,
the cell cycle of AtT-20 cells was examined. It was identified that
BDNF treatment was able to alter the cell cycle distribution of
AtT-20 cells. Decreased G0/G1 phase cells were present in
BDNF-treated cells (Fig. 4). As cells
in the G0 phase are quiescent cells (19), BDNF treatment may promote quiescent
AtT-20 cells entering the cell cycle and proliferating. BDNF
treatment increases the rate of the cell cycle.
Discussion
Pituitary tumors are primarily benign tumors, and
rarely metastasize to other organs (4). Only 0.1–0.2% are classified as
carcinomas (1). Pituitary tumors
originate from the pituitary gland (1). The majority of pituitary tumors secrete
excessive hormones (11). The
increased level of hormones may have certain side effects on the
patients, particularly for pregnant females (11).
Not only pituitary tumors secrete hormones;
pregnancy may also promote the secretion of an abundance of
hormones (13). Pituitary tumors are
hormone-secreting tumors, and they are also affected by the
secreted hormones from pregnancy. Pituitary tumors are disordered
by pregnancy (20,21). However, how pregnancy affects
pituitary tumor growth is not well investigated.
BDNF has been reported to be associated with
pregnancy (22). Increased levels of
BDNF have been identified in the blood of pregnant females
(9). To identify whether BDNF is the
reason that pregnancy promotes pituitary tumor growth, a mouse
model was established in the present study. Subcutaneous xenograft
was established on the nude female mice with pituitary tumor AtT-20
cells. The female mice were mated with male mice, and the tumor
occurrence time, tumor weight and BDNF level in the blood were
detected. Increased levels of BDNF were identified in pregnant mice
compared with in the control mice (Fig.
2). Tumors occurred earlier in pregnant mice compared with the
control mice (Table I). In comparison
with the control mice, the tumors grew faster in the pregnant mice
and larger tumors had developed at the end of the experiment
(Fig. 1). This indicated that BDNF
may be associated with promoting pituitary tumor growth. To confirm
this hypothesis, the AtT-20 cells were treated with BDNF in
vitro and the proliferation rate of the cells was measured.
Increased proliferation ability was identified in the BDNF-treated
cells (Fig. 3). As proliferation is
markedly associated with cell cycle (23), to elucidate how BDNF affects the
proliferation rate of AtT-20 cells, the cell cycle distribution of
BDNF-treated cells and control cells was detected. BDNF altered the
cell cycle distribution of AtT-20 cells. A decreased ratio of G0/G1
cells and increased number of S phase cells were identified in the
experiments (Fig. 4). Cells at the G0
phase are in a state of quiescence (19). BDNF decreased this subpopulation and
increased the number of cells in the S phase. This indicated that
quiescent cells were driven by BDNF into entering the cell cycle.
Following BDNF treatment, more cells were proliferated. This may
lead to earlier occurrence of tumors and larger tumors in the
mice.
In the present study, BDNF was identified to alter
the cell cycle distribution of AtT-20 cells. The number of
quiescent cells were decreased by BDNF treatment. Quiescence is
associated with cancer stem cells, a subpopulation of cancer cells
that are critical for tumor progression (24–26). Tumor
stem cells were reported primarily at the status of quiescence
(27,28). Differentiation of tumor stem cells may
lead to the loss of quiescent status (29,30). The
existence of pituitary tumor stem cells is widely accepted
(31,32). BDNF expression is associated with
neuron stem cells (33). It is
possible that BDNF treatment enhances the rate of pituitary tumor
stem cell self-renewal, promoting the cells to enter the cell cycle
for tumor growth.
The results of the present study demonstrated that
pregnancy may promote pituitary tumor growth in the mice model.
BDNF serves a critical role in promoting tumor progression by
increasing the rate of the cell cycle, leading to growth of the
pituitary tumor cells in vitro and in vivo. The
results of the present study suggest that monitoring the effect of
pregnancy on pituitary tumors may be beneficial for the therapy of
pituitary tumors.
References
|
1
|
Ezzat S, Asa SL, Couldwell WT, Barr CE,
Dodge WE, Vance ML and McCutcheon IE: ‘The prevalence of pituitary
adenomas’: A systematic review. Cancer. 101:613–619. 2004.
View Article : Google Scholar
|
|
2
|
Karavitaki N: Prevalence and incidence of
pituitary adenomas. Ann Endocrinol (Paris). 73:79–80. 2012.
View Article : Google Scholar
|
|
3
|
Heaney AP: Clinical review: Pituitary
carcinoma: Difficult diagnosis and treatment. J Clin Endocrinol
Metab. 96:3649–3660. 2011. View Article : Google Scholar
|
|
4
|
Scheithauer BW, Kovacs KT, Laws ER Jr and
Randall RV: Pathology of invasive pituitary tumors with special
reference to functional classification. J Neurosurg. 65:733–744.
1986. View Article : Google Scholar
|
|
5
|
Levy MJ, Matharu MS, Meeran K, Powell M
and Goadsby PJ: The clinical characteristics of headache in
patients with pituitary tumours. Brain. 128:1921–1930. 2005.
View Article : Google Scholar
|
|
6
|
Milos P, Havelius U and Hindfelt B:
Clusterlike headache in a patient with a pituitary adenoma. With a
review of the literature. Headache. 36:184–188. 1996. View Article : Google Scholar
|
|
7
|
Kirk LF Jr, Hash RB, Katner HP and Jones
T: Cushing's disease: Clinical manifestations and diagnostic
evaluation. Am Fam Physician. 62(1119–1127): 1133–1134. 2000.
|
|
8
|
Kumar V, Abbas A, Fausto N and Aster J:
Robbins and Cotran pathologic basis of disease. 9th. Elsevier
Health Sciences. ISBN 9780323296397 (eBook).
|
|
9
|
Lee NM and Saha S: Nausea and vomiting of
pregnancy. Gastroenterol Clin North Am. 40:309–334, vii. 2011.
View Article : Google Scholar
|
|
10
|
Sharma JB, Roy KK, Mohanraj P, Kumar S,
Karmakar D and Barua J: Pregnancy outcome in pituitary tumors. Arch
Gynecol Obstet. 280:401–404. 2009. View Article : Google Scholar
|
|
11
|
Molitch ME: Pituitary tumors and
pregnancy. Growth Horm IGF Res. 13:Suppl A. S38–S44. 2003.
View Article : Google Scholar
|
|
12
|
Kumar P and Magon N: Hormones in
pregnancy. Niger Med J. 53:179–183. 2012. View Article : Google Scholar
|
|
13
|
Karaca Z, Tanriverdi F, Unluhizarci K and
Kelestimur F: Pregnancy and pituitary disorders. Eur J Endocrinol.
162:453–475. 2010. View Article : Google Scholar
|
|
14
|
Molitch ME: Pituitary disorders during
pregnancy. Endocrinol Metab Clin North Am. 35:99–116, vi. 2006.
View Article : Google Scholar
|
|
15
|
Mirshokraei P, Hassanpour H, Rahnama A and
Foster WG: Gene expression of BDNF and its receptors, TrkB and p75
in the uterus and oviduct of pregnant and non-pregnant ewes. Res
Vet Sci. 95:164–168. 2013. View Article : Google Scholar
|
|
16
|
Okamura K, Harada T, Wang S, Ijichi K,
Furuyama K, Koga T, Okamoto T, Takayama K, Yano T and Nakanishi Y:
Expression of TrkB and BDNF is associated with poor prognosis in
non-small cell lung cancer. Lung Cancer. 78:100–106. 2012.
View Article : Google Scholar
|
|
17
|
U.S. National Institutes of Health:
Laboratory animal welfare: Public health service policy on humane
care and use of laboratory animals by awardee institutions; notice.
Fed Regist. 50:19584–19585. 1985.
|
|
18
|
Ma H, Guo J, Xia J, Niu C, Shen X, Sun H
and Zheng Y: Preparation and application of rabbit anti-mouse Setd8
polyclonal antibody. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
33:246–251. 2017.(In Chinese).
|
|
19
|
Tomura M, Sakaue-Sawano A, Mori Y,
Takase-Utsugi M, Hata A, Ohtawa K, Kanagawa O and Miyawaki A:
Contrasting quiescent G0 phase with mitotic cell cycling in the
mouse immune system. PLoS One. 8:e738012013. View Article : Google Scholar
|
|
20
|
Prager D and Braunstein GD: Pituitary
disorders during pregnancy. Endocrinol Metab Clin North Am.
24:1–14. 1995.
|
|
21
|
Nader S: Pituitary disorders and
pregnancy. Semin Perinatol. 14:24–33. 1990.
|
|
22
|
Fung J, Gelaye B, Zhong QY, Rondon MB,
Sanchez SE, Barrios YV, Hevner K, Qiu C and Williams MA:
Association of decreased serum brain-derived neurotrophic factor
(BDNF) concentrations in early pregnancy with antepartum
depression. BMC Psychiatry. 15:432015. View Article : Google Scholar
|
|
23
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar
|
|
24
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar
|
|
25
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: An evolving concept. Nat Rev Cancer. 12:133–143.
2012.
|
|
26
|
Dean M: Cancer stem cells: Implications
for cancer causation and therapy resistance. Discov Med. 5:278–282.
2005.
|
|
27
|
Yang Y, Xu H, Huang W, Ding M, Xiao J,
Yang D, Li H, Liu XY and Chu L: Targeting lung cancer stem-like
cells with TRAIL gene armed oncolytic adenovirus. J Cell Mol Med.
19:915–923. 2015. View Article : Google Scholar
|
|
28
|
Martín V, Sanchez-Sanchez AM, Herrera F,
Gomez-Manzano C, Fueyo J, Alvarez-Vega MA, Antolín I and Rodriguez
C: Melatonin-induced methylation of the ABCG2/BCRP promoter as a
novel mechanism to overcome multidrug resistance in brain tumour
stem cells. Br J Cancer. 108:2005–2012. 2013. View Article : Google Scholar
|
|
29
|
Li N, Yang Y, Ding M, Huang W, Li H, Ye J,
Xiao J, Zha X and Xu H: GFP stable transfection facilitated the
characterization of lung cancer stem cells. Mol Biotechnol.
56:1079–1088. 2014. View Article : Google Scholar
|
|
30
|
Chen K, Huang YH and Chen JL:
Understanding and targeting cancer stem cells: Therapeutic
implications and challenges. Acta Pharmacol Sin. 34:732–740. 2013.
View Article : Google Scholar
|
|
31
|
Andoniadou CL, Matsushima D, Gharavy
Mousavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet
C, Mollard P, Jacques TS, Le Tissier P, et al: Sox2(+)
stem/progenitor cells in the adult mouse pituitary support organ
homeostasis and have tumor-inducing potential. Cell Stem Cell.
13:433–445. 2013. View Article : Google Scholar
|
|
32
|
Tunici P and Yu JS: Pituitary adenoma stem
cells. Methods Mol Biol. 568:195–201. 2009. View Article : Google Scholar
|
|
33
|
Chen SQ, Cai Q, Shen YY, Cai XY and Lei
HY: Combined use of NGF/BDNF/bFGF promotes proliferation and
differentiation of neural stem cells in vitro. Int J Dev Neurosci.
38:74–78. 2014. View Article : Google Scholar
|