Introduction
Breast cancer (BC) is one of the most common types
of cancer in women in the developed and developing world, and
remains a leading cause of mortality globally, with ~1,300,000 new
cases and ~450,000 mortalities reported annually (1). BC represented 10% of all newly diagnosed
cancers, 30% of female malignancies and up to 15% of
cancer-associated mortality in 2013 worldwide (2,3). BC is a
heterogeneous disease in relation to molecular changes,
clinical-pathologic characteristics, responses to therapy and
clinical outcomes. The tumorigenesis of BC is a complicated process
characterized by genetic and epigenetic alterations that affect
major cellular pathways involved in BC development (4). Although notable advances in early
detection and therapeutic strategy for BC have been achieved,
substantial challenges still remain to improve the prevention and
treatment of BC (5). In China, the
number of incidences of mortality in patients with BC has doubled
during the last three decades (6).
Further exploration of the molecular mechanisms underlying BC
tumorigenesis and tumor progression is urgently required, as this
may contribute to the identification of novel prognostic biomarkers
and therapeutic targets for BC.
Lysosome associated membrane protein-1 (LAMP1), also
known as CD107a, is a heavily glycosylated lysosomal membrane
protein which is involved in protecting the lysosomal membrane from
intracellular proteolysis (7,8). Although LAMP1 is primarily expressed in
the endosome-lysosomal membrane of cells, it is also expressed in
the plasma membrane (9,10). Furthermore, elevated LAMP1 expression
at the cell surface has also been detected during platelet and
granulocytic cell activation, as well as in metastatic tumor cells
(10–12). Thus, cell-surface expressed LAMP1 may
serve as a ligand for selectins and may be modulated by tumor cells
(13). In a previous study, Furuta
et al (14) reported high
LAMP1 expression in colorectal neoplasm compared with normal
mucosa, indicating LAMP1's potential function in cell adhesion and
migration. LAMP1 has also been implicated to facilitate cancer
progression and tumor metastasis (13,15). As
for the prognostic role of LAMP1, Künzli et al (16) reported a positive association between
LAMP1 expression and survival status of patients with pancreatic
carcinoma. Nevertheless, the relationship between LAMP1 expression
and the clinicopathological significance of BC has not been
investigated.
In the present retrospective study, the expression
of LAMP1 was detected by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) in fresh BC samples and
immunohistochemistry (IHC) in BC tissue microarrays (TMA).
Furthermore, associations between LAMP1 expression and the
clinicopathological attributes of patients with BC, particularly
its prognostic significance, were further evaluated.
Materials and methods
Patient sample collection
A total of 20 fresh-frozen BC tissues and
corresponding non-cancerous tissues were collected from the
Department of Pathology, The Affiliated Hospital of Nantong
University (Nantong, China). Simultaneously, another 143
paraffin-embedded BC tissue samples and 143 matched non-cancerous
tissue samples were also obtained from the Department of Pathology,
The Affiliated Hospital of Nantong University, from January 2002 to
May 2010. Diagnosis of BC was validated by two pathologists in the
department, according to the latest World Health Organization
criteria (17). All patients
underwent mastectomy and/or axillary dissection (radical or
functional, based on clinical and surgical findings). Lymph node
metastasis was confirmed by postoperative histological examination.
The original clinical data were obtained from hospital medical
records, including patient age, histological grade, hormone
receptor (ER/PR status), erb-b2 receptor tyrosine kinase 2 (HER2)
expression, tumor size, lymph node metastasis and
tumor-node-metastasis (TNM) stage (18). None of the patients received
preoperative radiotherapy or chemotherapy prior to surgery.
Ethics statement
The Ethics Committee of Nantong University (Nantong,
China) and The Affiliated Hospital of Nantong University (Nantong,
China) in the present study approved the study protocol. Written
informed consent was acquired from all of the patients who were
enrolled in the present study.
RT-qPCR in fresh BC tissues
A total of 20 fresh-frozen BC and corresponding
non-cancerous tissues were included in the present study. Total RNA
from BC tissues and non-cancerous tissues was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and reverse transcribed to cDNA using a Revert
Aid™ First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's
instructions. The primers for LAMP1, designed by Primer
Express software (version 2.0; Thermo Fisher Scientific, Inc.) were
as follows: Forward, 5′-GTTTCTTCATTCTTTACTG-3′ and reverse,
5′-TCTCTACTGTTGTAATGT-3′. β-actin was included as an internal
control, using the following primers: Forward,
5′-TAATCTTCGCCTTAATACTT-3′ and reverse, 5′-TAATCTTCGCCTTAATACTT-3′.
One-step PCR was performed using the DyNAmo Flash SYBR Green qPCR
kit (cat. no. F-415XL; Thermo Fisher Scientific, Inc.) on an ABI
7500 thermal cycler (Applied Biosystems; Thermo Fisher Scientific,
Inc.) as previously described (19).
Briefly, the PCR conditions were as follows: Initial denaturation
at 95°C for 10 min; denaturation at 95°C for 15 sec, annealing and
extension at 60°C for 1 min, 40 cycles. All experiments were
performed in triplicate. Relative quantification was performed
using the 2−ΔΔCq method (20).
IHC using the BC TMA
A total of 143 BC tissue samples and corresponding
non-cancerous tissue samples were all fixed in 10% buffered
formalin overnight at room temperature and then embedded in
paraffin wax. Core tissue biopsies (2 mm in diameter) were taken
from individual paraffin embedded sections (5 µm) to make a TMA.
IHC analysis was performed as described previously (20–22).
Following deparaffinization, endogenous peroxidase activity was
blocked with 3% H2O2 for 10 min at room
temperature, then washed by PBS (3 times, 5 min each) and incubated
with 1% goat normal serum in PBS for 30 min at room temperature.
Subsequently, TMA sections were overnight at 4°C incubated with a
primary monoclonal mouse anti-LAMP1 antibody (cat. no. ab25630,
1:200; Abcam, Cambridge, MA, USA) in TBS and then incubated with
horseradish peroxidase-conjugated goat anti-mouse IgG at room
temperature (cat. no. A21010, 1:1,000; Abbkine, Inc, Redlands, CA,
USA), followed by washing with TBS. LAMP1 immunostaining was
evaluated by two trained pathologists from the Department of
Pathology, the Affiliated Hospital of Nantong University under
blinded experimental conditions. The percentage of LAMP1 positive
cells were scored as follows: 0, 0–19%; 1, 20–39%; 2, 40–59% and 3,
60–100%. LAMP1 staining intensity was also scored as follows: 0,
negative; 1, weakly positive; 2, moderately positive and 3,
strongly positive. A combined score was generated by taking into
consideration the percentage of positive cells and the staining
intensity. The cut-off point for a statistically significant LAMP1
expression score in terms of overall survival was set using the
X-tile software program (version 3.6.1; Rimm Lab, Yale University,
New Haven, CT, USA) (http://www.tissuearray.org/rimmlab) (23). The sum of the percentage and intensity
scores was used as the final LAMP1 staining score and was defined
as follows: <4 suggesting low or no expression and ≥4 indicating
high expression.
Statistical analysis
The Wilcoxon signed rank nonparametric test was
performed to compare the expression of LAMP1 mRNA in
fresh-frozen BC tissues with corresponding non-cancerous tissues. A
χ2 test was used to evaluate the associations between
clinicopathologic variables and LAMP1 protein expression.
Univariate and multivariate analyses were conducted using Cox
proportional hazard regression models to determine factors that
were independently associated with patients' overall survival.
Kaplan-Meier survival analysis and log-rank tests were used to
calculate survival curves. P<0.05 was considered to indicate a
statistically significant difference. All data were analyzed using
STATA 16.0 software (StataCorp LP, College Station, TX, USA).
Results
LAMP1 mRNA expression in BC and
corresponding non-cancerous tissues by RT-qPCR
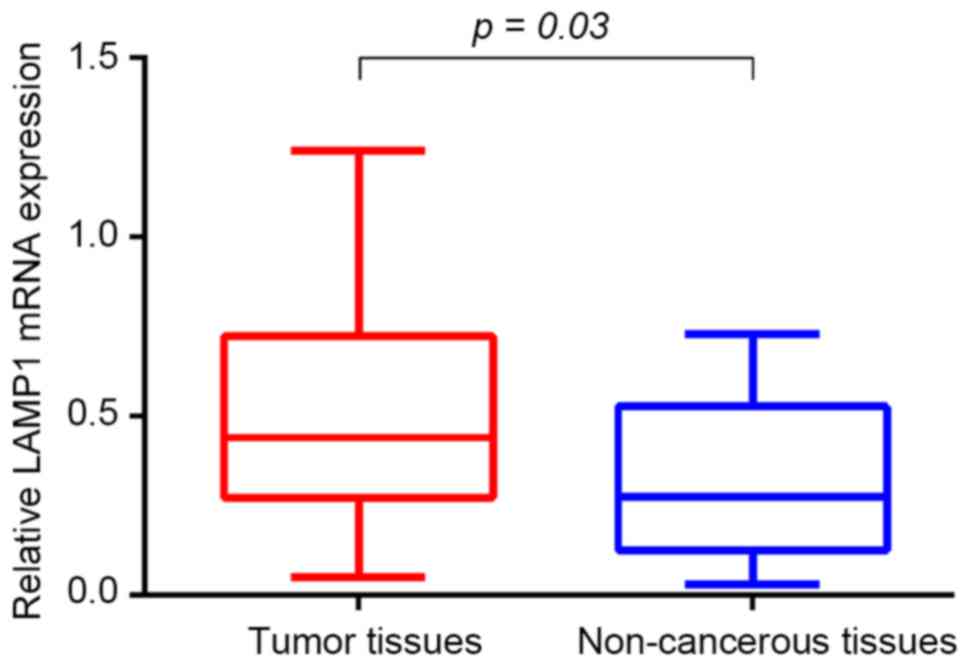
The expression of LAMP1 mRNA was analyzed by
RT-qPCR in BC and non-cancerous tissue specimens obtained from 20
patients. LAMP1 transcript levels were significantly higher
in BC tissues compared with corresponding non-cancerous tissues
(0.473±0.069 vs. 0.319±0.049, 1.5-fold; Fig. 1).
LAMP1 protein expression is increased
in BC tissues compared with corresponding non-cancerous
tissues
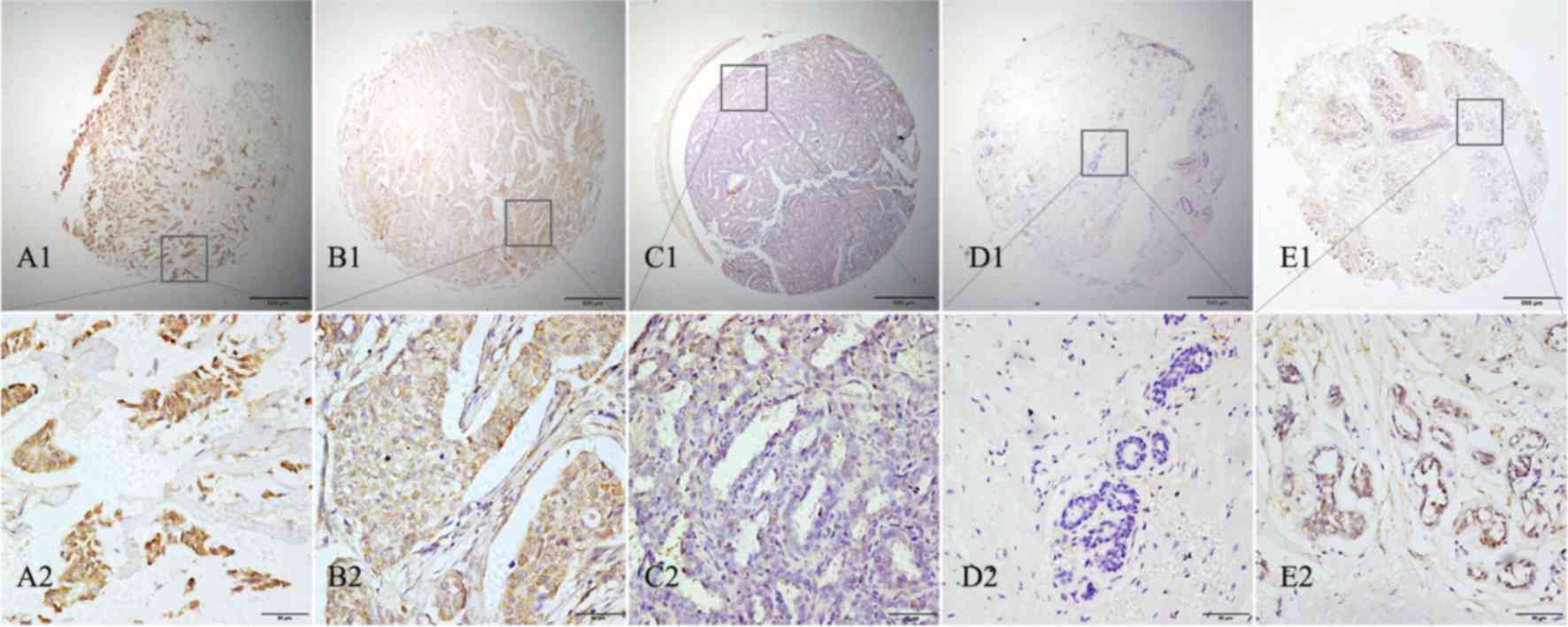
To investigate LAMP1 protein expression in BC, IHC
was conducted on a BC TMA. As presented in Fig. 2, LAMP1 was detected at different
intensities and percentages in BC, and was primarily located in the
cytoplasm of BC cells. High LAMP1 expression was detected in 64.3%
(82/143) of BC samples compared with 42.7% (61/143) of
non-cancerous tissue samples (Fig.
2). These results indicated that LAMP1 protein expression was
statistically increased in BC tissues compared with corresponding
non-cancerous tissues (χ2=13.5066; P=0.001).
Associations between LAMP1 protein
expression and clinical parameters of patients with BC
The relationship between LAMP1 protein expression
and important clinical parameters of patients with BC was
investigated. As presented in Table
I, high LAMP1 protein expression was significantly associated
with histological grade (P=0.047), estrogen receptor expression
(P=0.003), progesterone receptor expression (P=0.002), molecular
classification (P=0.022), lymph node metastasis (P=0.033) and TNM
stage (P=0.012). Specifically, the percentage of high LAMP1
expression in positive and negative lymph node metastasis was 65.4%
(51/78) and 47.7% (31/65) respectively, and this difference was
statistically significant (χ2=4.537; P=0.033). The data
demonstrated that patients with BC with positive lymph node
metastasis suffered high incidence of positive LAMP1 expression,
which indicates a correlation between high LAMP1 expression and
positive lymph node metastasis. In comparison, no significant
association was identified between LAMP1 expression and other
clinical characteristics, including age, HER2 expression, Ki-67
expression and tumor size.
 | Table I.Association of LAMP1 expression with
clinical parameters in breast cancer. |
Table I.
Association of LAMP1 expression with
clinical parameters in breast cancer.
|
|
| LAMP1 |
|---|
|
|
|
|
|---|
| Groups | No. | High expression, n
(%) | Low or no expression,
n (%) | χ2 | P-value |
|---|
| Total | 143 | 82 | 61 |
|
|
| Age (years) |
|
|
|
|
|
| ≤60 | 101 | 58 (57.4) | 43 (42.6) | 0.001 | 0.975 |
|
>60 | 42 | 24 (57.1) | 18 (42.9) |
|
|
| Histological
grade |
|
|
|
|
|
| I | 47 | 23 (48.9) | 24 (51.1) | 6.113 | 0.047a |
| II | 69 | 38 (55.1) | 31 (44.9) |
|
|
| III | 27 | 21 (77.8) | 6 (22.2) |
|
|
| ER expression |
|
|
|
|
|
|
Positive | 109 | 55 (50.5) | 54 (49.5) | 8.882 | 0.003a |
|
Negative | 34 | 27 (79.4) | 7 (20.6) |
|
|
| PR expression |
|
|
|
|
|
|
Positive | 97 | 47 (48.5) | 50 (51.5) | 9.741 | 0.002a |
|
Negative | 46 | 35 (76.1) | 11 (23.9) |
|
|
| HER-2
expression |
|
|
|
|
|
|
Positive | 42 | 29 (69.0) | 13 (31.0) | 3.331 | 0.068 |
|
Negative | 101 | 53 (52.5) | 48 (47.5) |
|
|
| Ki-67
expression |
|
|
|
|
|
|
High | 46 | 26 (56.5) | 20 (43.5) | 0.019 | 0.891 |
|
Low | 97 | 56 (57.7) | 41 (42.3) |
|
|
| Molecular
classification |
|
|
|
|
|
| Luminal
A | 69 | 34 (49.3) | 35 (50.7) | 9.638 | 0.022a |
| Luminal
B | 40 | 21 (52.5) | 19 (47.5) |
|
|
| Her-2
overexpression | 24 | 18 (75.0) | 6 (25.0) |
|
|
| Triple
negative | 10 | 9 (90.0) | 1 (10.0) |
|
|
| Tumor size (T
stage) |
|
|
|
|
|
| T1 | 76 | 37 (48.7) | 39 (51.3) | 5.599 | 0.061 |
| T2 | 54 | 35 (64.8) | 19 (35.2) |
|
|
|
T3+T4 | 13 | 10 (76.9) | 3 (23.1) |
|
|
| Lymph node
metastasis (N stage) |
|
|
|
|
|
| N0 | 65 | 31 (47.7) | 34 (52.3) | 4.537 | 0.033a |
|
N1+2+3 | 78 | 51 (65.4) | 27 (34.6) |
|
|
| TNM stage |
|
|
|
|
|
| I | 43 | 17 (39.5) | 26 (60.5) | 8.909 | 0.012a |
| II | 74 | 46 (62.2) | 28 (37.8) |
|
|
|
III | 26 | 19 (73.1) | 7 (26.9) |
|
|
Survival analysis
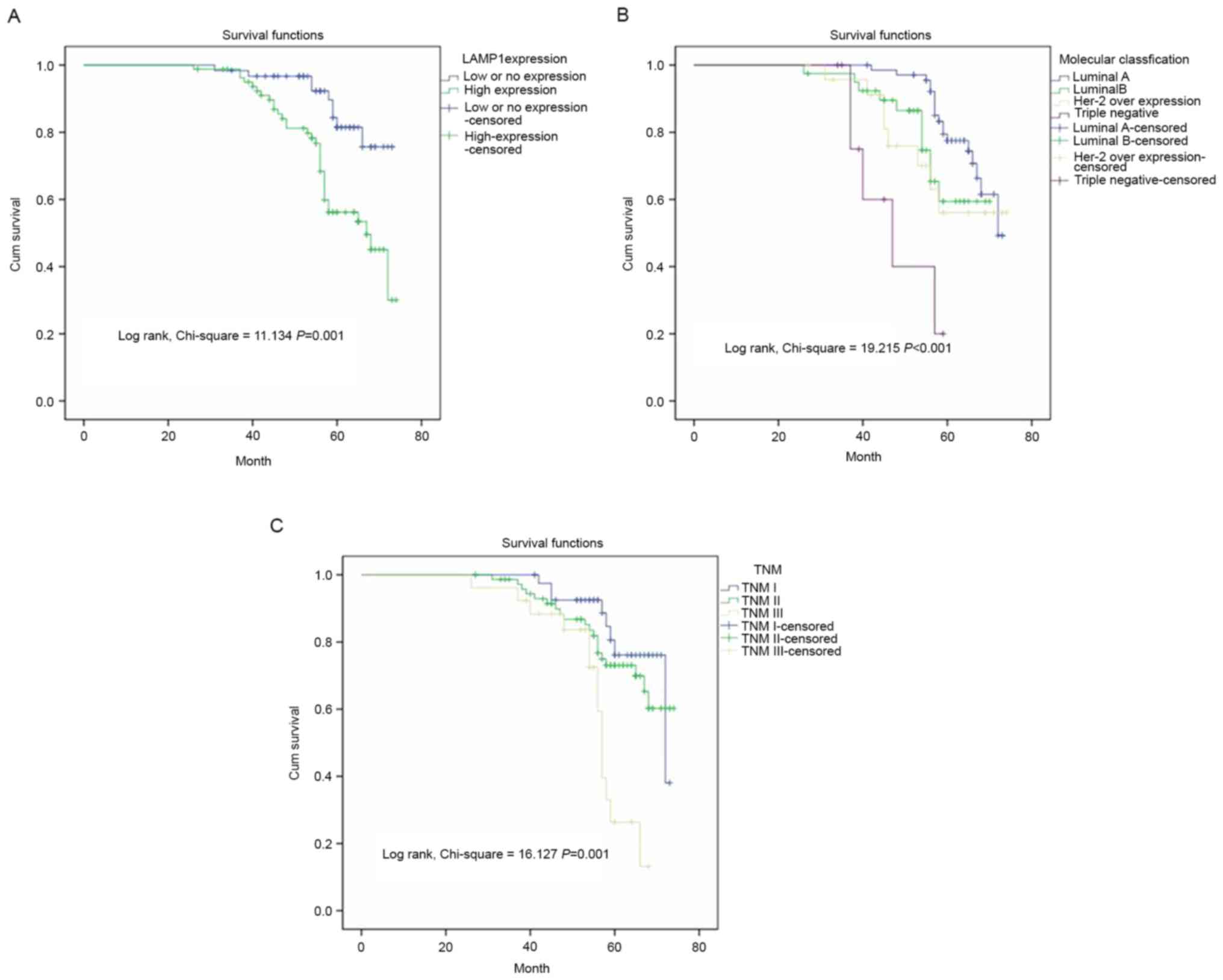
In univariate analysis, the overall survival of 143
patients with BC was associated with LAMP1 expression (P=0.002), ER
expression (P=0.017), PR expression (P=0.028), molecular
classification (P=0.001), histological grade (P=0.008), lymph node
metastasis (P=0.002) and TNM stage (P=0.001) (Table II). In multivariate analysis using
Cox regression model, only LAMP1 expression (P=0.037), molecular
classification (P=0.017) and TNM stage (P=0.003) may serve as
independent prognostic factors for overall survival. Kaplan-Meier
survival curves also demonstrated that patients with BC with high
LAMP1 expression, molecular classification and advanced TNM stage
were associated with unfavorable overall survival (Fig. 3).
 | Table II.Univariate and multivariate analysis
of prognostic factors in breast cancer for overall survival. |
Table II.
Univariate and multivariate analysis
of prognostic factors in breast cancer for overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | Yrs | HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| LAMP1
expression |
|
|
|
|
|
|
|
| Low or
no vs. high | 5 | 3.246 | 0.002a | 1.551–6.791 | 2.251 | 0.037a | 1.051–4.821 |
| Age (years) |
|
|
|
|
|
|
|
| ≤60 vs.
>60 | 5 | 1.856 | 0.053 | 0.993–3.470 |
|
|
|
| ER expression |
|
|
|
|
|
|
|
|
Positive vs. negative | 5 | 0.450 | 0.017a | 0.233–0.869 |
|
|
|
| PR expression |
|
|
|
|
|
|
|
|
Positive vs. negative | 5 | 0.489 | 0.028a | 0.259–0.925 |
|
|
|
| Her2
expression |
|
|
|
|
|
|
|
|
Positive vs. negative | 5 | 1.859 | 0.060 | 0.973–3.551 |
|
|
|
| Ki-67
expression |
|
|
|
|
|
|
|
| Low vs.
high | 5 | 1.076 | 0.837 | 0.538–2.149 |
|
|
|
| Molecular
classification |
|
|
|
|
|
|
|
| Luminal
A vs. luminal B vs. Her-2 overexpression vs. triple negative | 5 | 1.675 | 0.001a | 1.235–2.274 | 1.483 | 0.017a | 1.071–2.053 |
| Histological
grade |
|
|
|
|
|
|
|
| I vs.
II vs. III | 5 | 1.763 | 0.008a | 1.159–2.684 | 1.499 | 0.072 | 0.964–2.333 |
| T stage |
|
|
|
|
|
|
|
| T1 vs.
T2 vs. T3+T4 | 5 | 1.231 | 0.368 | 0.783–1.936 |
|
|
|
| Lymph node
metastasis (N stage) |
|
|
|
|
|
|
|
| N0 vs.
N1+2+3 | 5 | 1.822 | 0.002a | 1.256–2.643 |
|
|
|
| TNM stage |
|
|
|
|
|
|
|
| I vs.
II vs. III | 5 | 2.206 | 0.001a | 1.371–3.551 | 2.195 | 0.003a | 1.318–3.655 |
Discussion
The lysosomal pathway represents a novel regulator
of cell death in cancer through lysosomal membrane permeabilization
(24–26). In normal cells, the outcome of cell
death depends on the extent of lysosomal damage; limited lysosomal
damage leads to cell death by apoptosis while large number of
lysosomal breakdown results in cytosolic necrosis (27). The balance between apoptosis and
necrosis is crucial for cancer development because cancer cells
usually acquire mutations to protect themselves from cell death via
the classical apoptotic pathways (28). LAMP1 is among the most abundant
lysosomal membrane proteins, and it creates a glycocalyx on the
inner side of the lysosomal membrane to protect the membrane from
hydrolytic enzymes and degradation (18). High LAMP1 expression has been
implicated in cancer development and progression, including in
astrocytoma, colorectal cancer, pancreatic carcinoma and various
other cancer tissues (14,16,29,30). LAMP1
has also been suggested to facilitate cancer metastasis by acting
as a ligand for galectin-3, to increase translocation to the cell
membrane (31,32). The aforementioned studies all indicate
certain malignant characteristics of LAMP1 in human cancers.
However, little is known about the function of LAMP1 in BC.
In the present study, RT-qPCR analysis in a small
number of BC samples revealed a significantly higher level of
LAMP1 gene expression in BC tissues than in non-cancerous
tissues. Subsequently, IHC analysis was conducted in a constructed
TMA and the results demonstrated that LAMP1 protein expression in
BC was significantly higher than in non-cancerous tissues. These
data are consistent with previous studies demonstrating LAMP1
overexpression in various types of cancers (14,16,30). In
addition, high LAMP1 expression in BC was associated with certain
clinical attributes, including histological grade, ER/PR
expression, molecular classification, and TNM stage. Ozaki et
al (31) stated that LAMP1
expression on the cell surface was associated with the metastatic
potential of melanoma cells, and downregulation of LAMP1
significantly inhibited cancer metastasis. Agarwal et al
(33) also demonstrated that LAMP1
was well known to act as a carrier of β1,6 branched
N-oligosaccharides, which are aberrantly expressed in several human
cancers and have malignant potential. The results of the present
study are in line with these studies, and further confirm the
association between LAMP1 expression and malignant attributes of
BC.
To date, studies investigating the prognostic value
of LAMP1 are limited; therefore, the association between LAMP1
expression and overall survival was evaluated in patients with BC.
Univariate analysis revealed that in addition to cytoplasmic
expression of LAMP1, ER and PR expression, molecular
classification, histological grade, lymph node metastasis and TNM
stage were also associated with survival in patients with BC.
Multivariate analysis subsequently demonstrated that high LAMP1
expression, molecular classification and TNM stage were independent
predictors of poor prognosis in patients with BC. Kaplan-Meier
analysis further verified that patients with BC with high LAMP1
expression suffered a significantly reduced life span. However, the
results of the present study contrast with those of a previous
study, in which Künzli et al (16) reported that patients with pancreatic
carcinoma with higher LAMP1 expression had longer survival time.
This inconsistency may be due to the differences in the tumor types
(pancreatic carcinoma vs. BC), experimental methods (northern blot
vs. IHC) or evaluation system (mRNA expression vs. protein
expression).
In addition, there are several limitations faced by
the present study. For example, the use of archived, convenient BC
samples may introduce bias into this retrospective, observational
study, hence future studies that include larger sample sizes are
necessary to confirm the results of the present study. Secondly,
the construction of TMA using small sections of tissue blocks to
analyze target protein expression may not be representative of the
whole tissue block. Thirdly, the mechanisms underlying of how LAMP1
protein influences the tumor microenvironment in BC remains to be
fully elucidated. A series of in vitro and in vivo
experiments, including overexpression and knockdown studies, are in
progress. We anticipate that our research group will publish
related studies to illustrate the mechanisms underlying LAMP1
activity in BC development. Fourthly, blood samples were not
collected but this problem may be addressed when the construction
of the Biobank at our hospital is completed.
To the best of our knowledge, the present study was
the first to report on the differential expression of LAMP1 in BC,
at the gene and protein level. the results indicated that LAMP1 may
be a novel prognostic biomarker in patients with BC. Further in
vitro mechanistic studies concerning LAMP1 in BC are being
conducted by our research group.
Acknowledgements
The present study was supported by the Clinic Master
Grant (no. 2014-221) from the Medical Research Program of Nantong
University (Jiangsu, China); the Technology Innovation and
Demonstration Project of Nantong Science (grant no. HS 2014047);
and The Science and Technology Program of The Hospital Affiliated
to Nantong University (grant nos. Tfj14004 and Y2010-14).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
2
|
Li XY, Luo QF, Li J, Wei CK, Kong XJ,
Zhang JF and Fang L: Clinical significance of NOB1 expression in
breast infiltrating ductal carcinoma. Int J Clin Exp Pathol.
6:2137–2144. 2013.
|
|
3
|
Xu X, Tang X, Lu M, Tang Q, Zhang H, Zhu
H, Xu N, Zhang D, Xiong L, Mao Y and Zhu J: Overexpression of
MAGE-A9 predicts unfavorable outcome in breast cancer. Exp Mol
Pathol. 97:579–584. 2014. View Article : Google Scholar
|
|
4
|
Arnutti P, Kotepui M, Asanprakit W,
Punyarit P, Chavalitshewinkoon-Petmitr P, Harnroongroj T and
Petmitr S: Determination of whole transcription profiles and
specific pathways in invasive ductal breast carcinoma. Int J Clin
Exp Pathol. 6:1112–1120. 2013.
|
|
5
|
Parker JS, Mullins M, Cheang MC, Leung S,
Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al:
Supervised risk predictor of breast cancer based on intrinsic
subtypes. J Clin Oncol. 27:1160–1167. 2009. View Article : Google Scholar
|
|
6
|
Wang S, Li H, Wang J and Wang D:
Expression of microRNA-497 and its prognostic significance in human
breast cancer. Diagn Pathol. 8:1722013. View Article : Google Scholar
|
|
7
|
Kundra R and Kornfeld S: Asparagine-linked
oligosaccharides protect Lamp-1 and Lamp-2 from intracellular
proteolysis. J Biol Chem. 274:31039–31046. 1999. View Article : Google Scholar
|
|
8
|
Saftig P and Klumperman J: Lysosome
biogenesis and lysosomal membrane proteins: Trafficking meets
function. Nat Rev Mol Cell Biol. 10:623–635. 2009. View Article : Google Scholar
|
|
9
|
Parkinson-Lawrence EJ, Dean CJ, Chang M,
Hopwood JJ, Meikle PJ and Brooks DA: Immunochemical analysis of
CD107a (LAMP-1). Cell Immunol. 236:161–166. 2005. View Article : Google Scholar
|
|
10
|
Kannan K, Stewart RM, Bounds W, Carlsson
SR, Fukuda M, Betzing KW and Holcombe RF: Lysosome-associated
membrane proteins h-LAMP1 (CD107a) and h-LAMP2 (CD107b) are
activation-dependent cell surface glycoproteins in human peripheral
blood mononuclear cells which mediate cell adhesion to vascular
endothelium. Cell Immunol. 171:10–19. 1996. View Article : Google Scholar
|
|
11
|
Andrejewski N, Punnonen EL, Guhde G,
Tanaka Y, Lüllmann-Rauch R, Hartmann D, von Figura K and Saftig P:
Normal lysosomal morphology and function in LAMP-1-deficient mice.
J Biol Chem. 274:12692–12701. 1999. View Article : Google Scholar
|
|
12
|
Sarafian V, Jadot M, Foidart JM, Letesson
JJ, Van den Brûle F, Castronovo V, Wattiaux R and Coninck SW:
Expression of Lamp-1 and Lamp-2 and their interactions with
galectin-3 in human tumor cells. Int J Cancer. 75:105–111. 1998.
View Article : Google Scholar
|
|
13
|
Sawada R, Jardine KA and Fukuda M: The
genes of major lysosomal membrane glycoproteins, lamp-1 and lamp-2.
5′-flanking sequence of lamp-2 gene and comparison of exon
organization in two genes. J Biol Chem. 268:9014–9022. 1993.
|
|
14
|
Furuta K, Ikeda M, Nakayama Y, Nakamura K,
Tanaka M, Hamasaki N, Himeno M, Hamilton SR and August JT:
Expression of lysosome-associated membrane proteins in human
colorectal neoplasms and inflammatory diseases. Am J Pathol.
159:449–455. 2001. View Article : Google Scholar
|
|
15
|
Saitoh O, Wang WC, Lotan R and Fukuda M:
Differential glycosylation and cell surface expression of lysosomal
membrane glycoproteins in sublines of a human colon cancer
exhibiting distinct metastatic potentials. J Biol Chem.
267:5700–5711. 1992.
|
|
16
|
Kunzli BM, Berberat PO, Zhu ZW, Martignoni
M, Kleeff J, Tempia-Caliera AA, Fukuda M, Zimmermann A, Friess H
and Büchler MW: Influences of the lysosomal associated membrane
proteins (Lamp-1, Lamp-2) and Mac-2 binding protein (Mac-2-BP) on
the prognosis of pancreatic carcinoma. Cancer. 94:228–239. 2002.
View Article : Google Scholar
|
|
17
|
Lin H, Huang JF, Qiu JR, Zhang HL, Tang
XJ, Li H, Wang CJ, Wang ZC, Feng ZQ and Zhu J: Significantly
upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of
invasive ductal breast cancer. Exp Mol Pathol. 94:73–78. 2013.
View Article : Google Scholar
|
|
18
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar
|
|
19
|
Ni S, Xu L, Huang J, Feng J, Zhu H, Wang G
and Wang X: Increased ZO-1 expression predicts valuable prognosis
in non-small cell lung cancer. Int J Clin Exp Pathol. 6:2887–2895.
2013.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Han L, Jiang B, Wu H, Zhang S and Lu X:
Expression and prognostic value of MAGE-A9 in laryngeal squamous
cell carcinoma. Int J Clin Exp Pathol. 7:6734–6742. 2014.
|
|
22
|
Wang Q, Ni Q, Wang X, Zhu H, Wang Z and
Huang J: High expression of RAB27A and TP53 in pancreatic cancer
predicts poor survival. Med Oncol. 32:3722015. View Article : Google Scholar
|
|
23
|
Zhu H, Lu J, Wang X, Zhang H, Tang X, Zhu
J and Mao Y: Upregulated ZO-1 correlates with favorable survival of
gastrointestinal stromal tumor. Med Oncol. 30:6312013. View Article : Google Scholar
|
|
24
|
Huang J, Zhang J, Li H, Lu Z, Shan W,
Mercado-Uribe I and Liu J: VCAM1 expression correlated with
tumorigenesis and poor prognosis in high grade serous ovarian
cancer. Am J Transl Res. 5:336–346. 2013.
|
|
25
|
Groth-Pedersen L and Jäättelä M: Combating
apoptosis and multidrug resistant cancers by targeting lysosomes.
Cancer Lett. 332:265–274. 2013. View Article : Google Scholar
|
|
26
|
Jäättelä M: Multiple cell death pathways
as regulators of tumour initiation and progression. Oncogene.
23:2746–2756. 2004. View Article : Google Scholar
|
|
27
|
Kirkegaard T and Jäättelä M: Lysosomal
involvement in cell death and cancer. Biochim Biophys Acta.
1793:746–754. 2009. View Article : Google Scholar
|
|
28
|
Boya P and Kroemer G: Lysosomal membrane
permeabilization in cell death. Oncogene. 27:6434–6451. 2008.
View Article : Google Scholar
|
|
29
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar
|
|
30
|
Jensen SS, Aaberg-Jessen C, Christensen KG
and Kristensen B: Expression of the lysosomal-associated membrane
protein-1 (LAMP-1) in astrocytomas. Int J Clin Exp Pathol.
6:1294–1305. 2013.
|
|
31
|
Ozaki K, Nagata M, Suzuki M, Fujiwara T,
Ueda K, Miyoshi Y, Takahashi E and Nakamura Y: Isolation and
characterization of a novel human lung-specific gene homologous to
lysosomal membrane glycoproteins 1 and 2: Significantly increased
expression in cancers of various tissues. Cancer Res. 58:3499–3503.
1998.PubMed/NCBI
|
|
32
|
Krishnan V, Bane SM, Kawle PD, Naresh KN
and Kalraiya RD: Altered melanoma cell surface glycosylation
mediates organ specific adhesion and metastasis via lectin
receptors on the lung vascular endothelium. Clin Exp Metastasis.
22:11–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agarwal AK, Srinivasan N, Godbole R, More
SK, Budnar S, Gude RP and Kalraiya RD: Role of tumor cell surface
lysosome-associated membrane protein-1 (LAMP1) and its associated
carbohydrates in lung metastasis. J Cancer Res Clin Oncol.
141:1563–1574. 2015. View Article : Google Scholar : PubMed/NCBI
|