Introduction
Chronic alcoholic liver disease is typically caused
by long-term excessive alcohol consumption, and includes alcoholic
fatty liver, alcoholic hepatitis, alcoholic liver fibrosis and
alcoholic cirrhosis. According to statistics, chronic alcoholic
liver disease has become the leading cause of liver fibrosis and
liver cancer in Europe and America (1). Liver fibrosis is the excessive
accumulation and hyperplasia of extracellular matrix proteins
resulting from chronic damage to the liver and characterized by
abnormalities in the hepatic structure or the way it functions
(2). It can be caused by autoimmune
liver disease, drug-induced liver injury and chronic alcoholic
liver disease. Previously, researchers have identified that
transforming growth factor β (TGFβ) served an important role in the
occurrence and development of liver fibrosis (3–5). Platelets
in the liver cells can release large amounts of TGFβ following
liver damage, which continuously promotes the synthesis and
accumulation of extracellular matrix proteins, resulting in liver
fibrosis. TGFβ can also enhance the activity of hepatic stellate
cells (HSCs), which can in turn increase TGFβ synthesis and
secretion, thus contributing to the development of liver fibrosis
(6).
A complex community of microorganisms, termed
intestinal flora, live in the digestive tract of the human body;
>500 strains of intestinal flora exist in the human gut, the
most common types being obligate anaerobes such as
Bifidobacteria, E. coli, Enterococcus faecalis
and Bacteroides (3).
Intestinal flora performs a crucial role in human health. However,
under certain abnormal conditions, bacterial translocation or a
change of intestinal flora ratio can occur, resulting in various
pathophysiological manifestations. The liver is connected to the
intestinal flora system anatomically through the portal vein and
mesenteric lymphatic system and continuously receives intestinal
blood into the portal system.
The liver also performs a defensive role in the
detoxification of gut-derived toxins including lipopolysaccharides
and microbial products depending on its innate immune system
(7). Furthermore, the liver cannot
only regulate metabolism and immune responses, but can also
influence the intestinal function through bile secretion and the
enterohepatic cycle. The pathophysiological association between the
gut and the liver is described as the gut-liver axis. Over the past
10 years, understanding of the gut-liver axis has progressively
increased, meaning that the impact of intestinal flora on the
pathogenesis of chronic liver disease has received increased
attention (8). It has been reported
that patients with liver fibrosis often had abdominal distension,
diarrhea and other gastrointestinal symptoms, but their symptoms
alleviated following a period of treatment using probiotics,
implying that their symptoms are associated with an intestinal
flora imbalance (9). Therefore, it
can be speculated that intestinal flora is associated with liver
fibrosis.
The present study investigated the role of
intestinal flora in the occurrence and development of liver
fibrosis and explored its mechanism, providing a new theoretical
basis for the treatment of liver fibrosis.
Materials and methods
Ethics statement
The animal experiments in the present study were
approved by the committee on the Ethics of Qingdao University
(Qingdao, China) in accordance with national and institutional
policies. The animals received humane care and treatment in
accordance with the Guide for the Care and Use of Laboratory
Animals of Qingdao University.
Animals and treatments
Male C57B1/6 mice (6 weeks old; 30–35 g in weight)
were bought from the laboratory animal center of The Academy of
Military Medical Sciences (License number, SCXK Jing 2006–0009). A
total of 36 mice were randomly allocated into 3 groups: Group I
(alcohol injury group), group II (alcohol injury with flora
imbalance group) and group III (alcohol injury with corrected flora
imbalance group), using randomization software. The mice in group I
were fed with lieber-deCarli liquid (Dyets, Inc., Bethlehem, PA,
USA) diets containing 4% alcohol and 0.3% tetrachloromethane for 8
weeks to establish a mouse model with liver fibrosis. Subsequently,
the mice were fed with lieber-deCarli liquid diets with equal
energy of maltodextrin instead of alcohol for another 8 weeks. In
addition to alcohol, the mice in group II were given lincomycin
hydrochloride (6 g/kg/d) in the first 8 weeks and fed with the same
diets to the ones in group I in the following 8 weeks. In group
III, the mice received the same treatment to the ones in group II
in the first 8 weeks and then were supplemented with 0.25 ml
probiotics (Lactobacillus casei subsp. rhamnosus
strain, 480 mg/ml) continuously for an additional 8 weeks. All mice
were housed in cages by group at a temperature of 25°C and humidity
of 60–70% with a8/16 h light/dark cycle (lights were turned on at
9:00 am and off at 5:00 pm).
To evaluate the degree of liver fibrosis and liver
biochemical indicators in mice, half the mice in each group were
sacrificed through cervical dislocation to harvest liver tissues
and collect blood samples from the retro-orbital plexus; HSCs were
additionally isolated from the remaining mice, as described
below.
Pathologic assessments
The harvested liver tissues were fixed overnight in
10% formalin, embedded in paraffin, sectioned to a thickness of 4
µm and stained with hematoxylin and eosin (H&E). Following
staining, histopathological characteristics of the liver tissues
were examined under a light microscope. Masson staining (Shanghai
Bo Valley Biological Technology Co., Ltd, Shanghai, China) was
performed to evaluate the degree of liver fibrosis, according to
the manufacturer's protocol. For histomorphometry, 3 midsagittal
Masson's trichrome-stained sections were selected from each group.
Images were captured using an optical microscope (ECLIPSE 50i;
Nikon Corporation, Tokyo, Japan). The areas of blue (immature bone
or collagen fibers) and red (remodeled or lamellar bone) staining
were semiquantitatively determined using Image Pro Plus software
(version 6.0; Media Cybernetics, Inc., Rockville, MD, USA). The
area mean of total bone (red and blue) and remodeled bone staining
was normalized to the control group and compared between the three
groups. The hepatic pathologic assessments were qualitatively
graded according to an alcohol induced disease (AID) histology
score standard (10): i) Ballooning
degeneration (0, none; 1, mild to moderate; 2, severe); ii)
steatosis (0, ≤10%; 1, >10–30%; 2, >30-≤60%; 3, >60%);
iii) inflammation and necrosis (0=none, 1=mild, 2=moderate,
3=severe); iv) lobular fibrosis (0=none, 1=mild, 2=moderate,
3=severe).
Biochemical analysis
The levels of alanine aminotransferase (ALT),
aspartate aminotransferase (AST) and alkaline phosphatase (ALP) in
the mice sera were detected with ALT, AST or ALP kits purchased
from Jiancheng Technology Co., Ltd., (Nanjing, China).
HSC isolation
At 16 weeks, mice were anesthetized with 1% sodium
pentobarbital. The mice livers were perfused to prepare for the
digestion with D-Hanks (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) via intubation of the portal vein until
the livers turned white. Subsequently, mice livers were isolated
from the site, placed on a dish and continuously perfused with
0.05% pronase and 0.025% collagenase (60 drops/min). Following the
removal of the liver capsule and large blood vessels, the livers
were made into a homogenate, filtered through 3 layers of gauze and
centrifuged at 400 × g for 10 min at 25°C. The supernatants were
collected and then mixed with D-Hanks and DNase I until they were
clear. Supernatants were carefully transferred onto the top of the
cell separation solution and centrifuged at 1,400 × g for 20 min at
25°C to obtain HSCs. Following centrifugation HSCs were collected
and washed 3 times with D-Hanks for western blot analysis. The
purity of HSCs was identified by immunocytochemical staining of
Desmin and α smooth muscle actin (α-SMA), as described previously
(11).
Western blot analysis
Western blot analysis was performed in accordance
with the standard protocols. Total protein extracts of HSCs were
collected and lysed in ice-cold radioimmunoprecipitation assay
buffer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10
µl/ml proteinase inhibitor cocktail (Thermo Fisher Scientific,
Inc.) for 15 min. The lysates were centrifuged at 12,000 × g for 20
min at 4°C. The protein concentration of each sample was determined
by Coomassie brilliant blue staining. The cell lysates of each
sample were mixed with 2X Laemmli buffer (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and boiled for 5 min. The proteins (50
µg/lane) were separated using 8% SDS-PAGE and transferred onto
nitrocellulose membranes. The nitrocellulose blots were blocked
with 5% skimmed milk for 1 h, incubated at 25°C with primary
antibodies against mothers against decapentaplegic homologs smad3
(cat. no., sc-101154) and smad4 (cat. no., sc-7966; both Santa Cruz
Biotechnology Inc., Dallas, TX, USA), diluted with TBS/Tween-20 to
1:500, overnight at 4°C. Subsequent to washing 3 times, the
membranes were incubated for 40 min at 25°C with horseradish
peroxidase conjugated rabbit anti-goat IgG (dilution, 1:1,000; cat.
no., ab6721; Abcam, Cambridge, UK). The membranes were imaged using
an enhanced-chemiluminescent kit (GE Healthcare, Chicago, IL, USA).
Western blots were quantified using Image Pro Plus 5.1 (Media
Cybernetics, Inc.), according to the kit protocol. All results were
repeated 3 times.
Flow cytometry
Flow cytometry was utilized to measure the extent of
apoptosis. Cells were stained with an annexin-V fluorescein
isothiocyanate/propidium iodide kit (BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer's protocol. The
FlowSight instrument (BD Biosciences) was then used to perform flow
cytometry and the data were analyzed using FlowJo software (version
7.6.1; Tree Star, Inc. Ashland, OR, USA).
Statistical analysis
All measured data were presented as the mean ±
standard deviation and analyzed with SPSS 18.0 software (SPSS Inc.,
Chicago, IL, USA). A one-way analysis of variance followed by a
Fisher's least significant difference test and Kruskal-Wallis H
test were used to assess differences among 3 groups according to
the types of statistics. P<0.05 was considered to indicate a
statistically significant difference.
Results
ALT, ALP and AST levels in mice
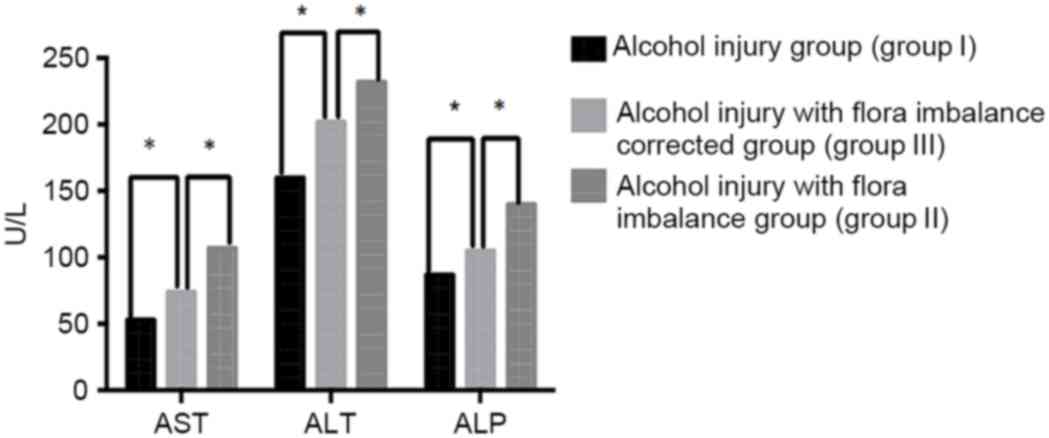
The levels of ALT, ALP and AST in the mice of each
group are shown in Fig. 1, which can
demonstrate the degree of liver fibrosis to some extent. The mean
values of AST, ALT and ALP in mice from the 3 groups were as
follows: Group I, 54.87, 156.21 and 87.34 U/l, respectively; group
II, 106.87, 234.21 and 139.34 U/l, respectively; and group III,
76.87, 200.21 and 104.34 U/l, respectively. Mice in group II
exhibited significantly higher serum ALT, ALP and AST levels
(P<0.05) compared with those in group I and group III. Serum
ALT, ALP and AST levels of the mice in group III were significantly
lower compared with that of the mice in group II (P<0.05). These
results indicate that intestinal flora imbalance can enhance the
damage of alcohol to the liver.
Intestinal flora imbalance promoted
alcohol induced liver fibrosis
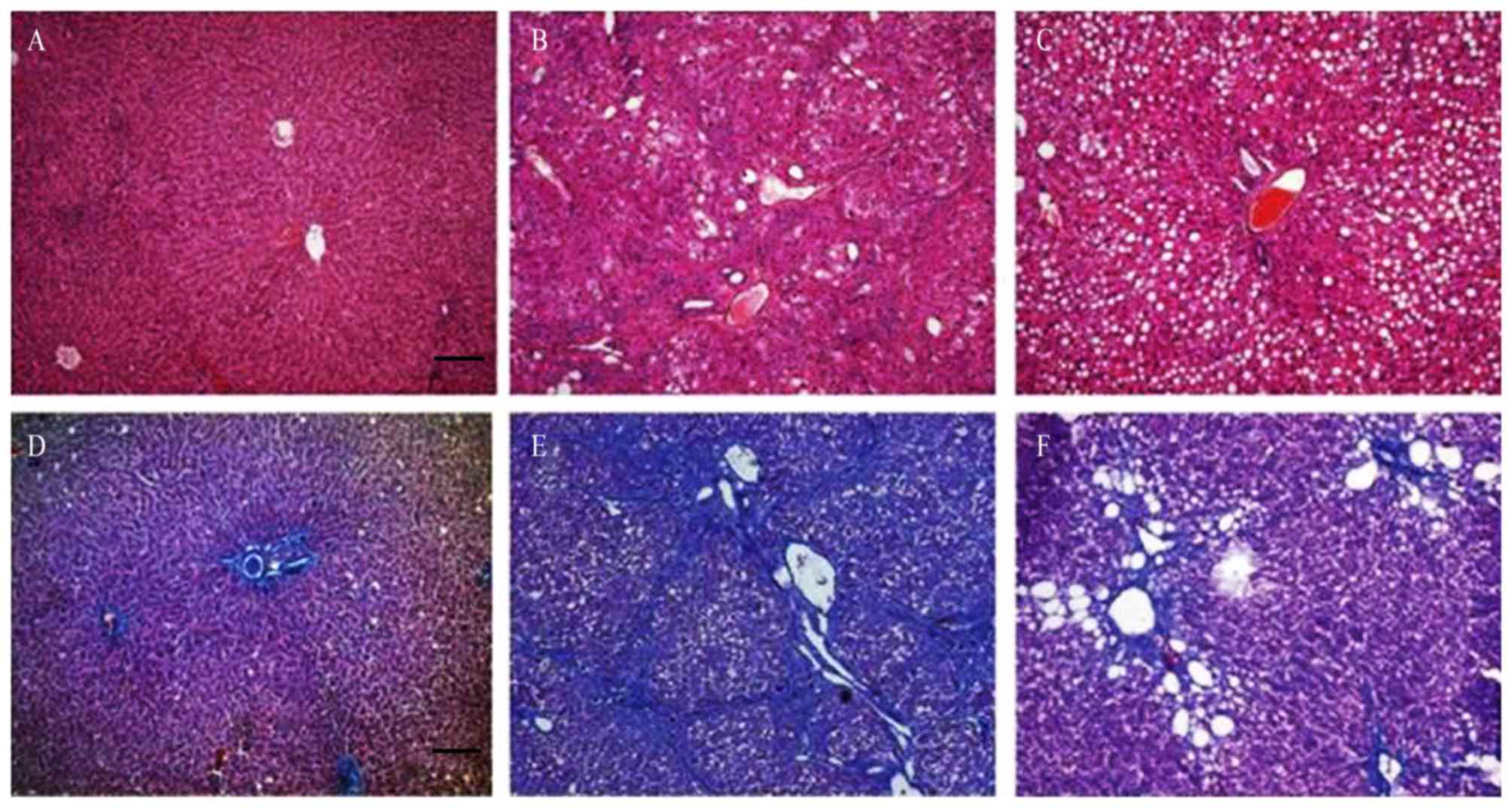
Hepatic architecture was analyzed by H&E
staining and the degree of liver fibrosis was evaluated by Masson
staining of liver tissue sections (Fig.
2). Although the mice in group I had normal hepatic lobule
structure, inflammatory cell infiltration and hyperplasia of
fibrous tissue existed around the portal area and central vein
(Fig. 2A and B), suggesting that the
lieber-deCarli liquid diets induced liver damage in mice. Compared
with the mice in group II, the structure of hepatic lobule of the
mice in group III exhibited a greater degree of balloon
degeneration, and decreased necrosis of the portal vein and
surrounding liver cells. The collagen fibers in and around the
lobules were also significantly reduced, and no fibrous septums
were observed (Fig. 2C and D).
Serious structural disorder of the hepatic lobule was observed in
group II mice, along with significant hepatic inflammation
including hepatocyte ballooning, degeneration and necrosis. The
hyperplasia of liver collagen fibers was evident around the portal
area and central vein, resulting in the formation of fibrous
septums (Fig. 2E and F).
Intestinal flora imbalance induced
hepatic pathological changes in mice in the 3 groups
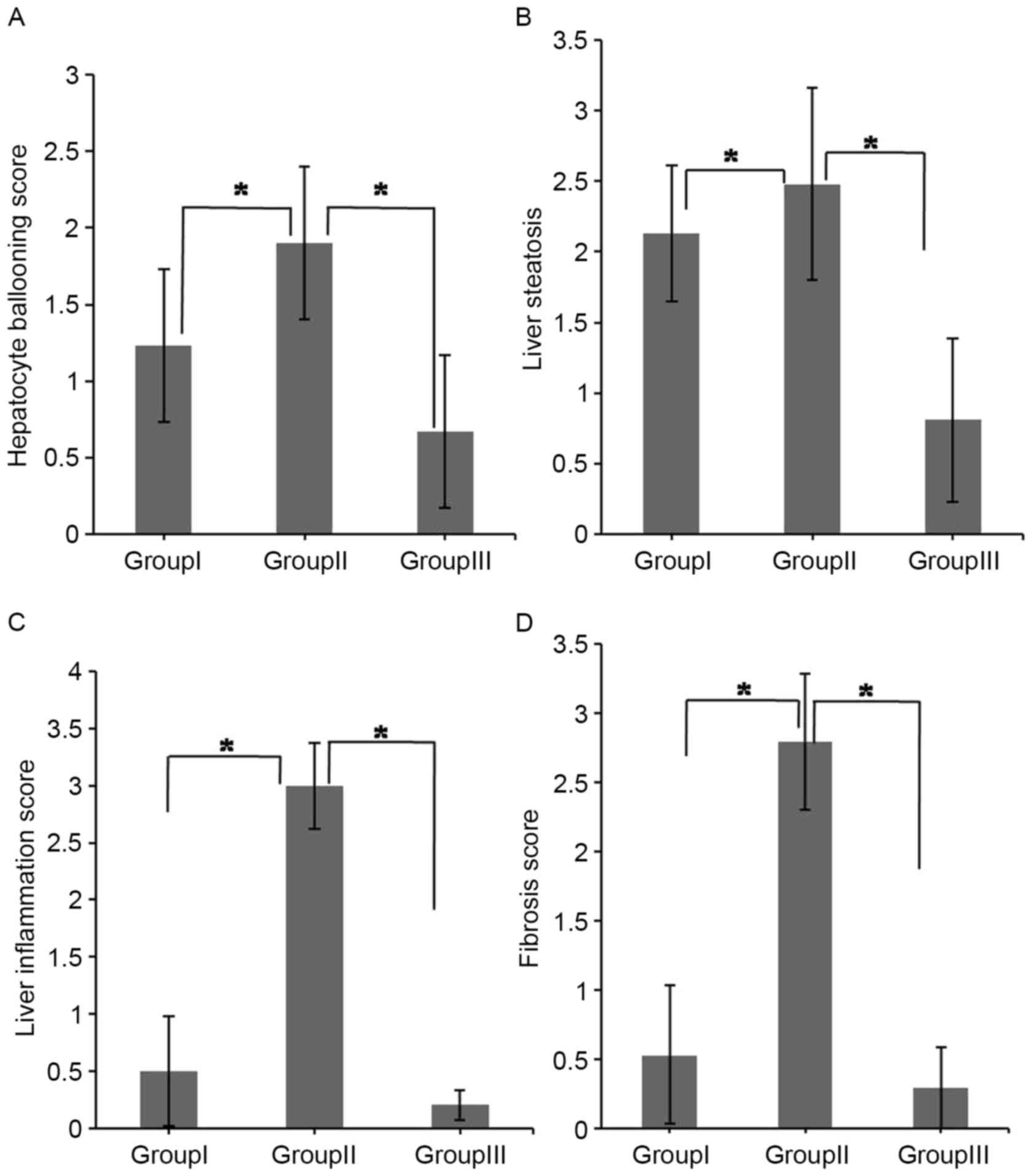
The pathological assessments of livers were
performed according to AID histology score standard. The scores of
hepatocyte ballooning, liver steatosis, liver inflammation and
fibrosis are summarized in Table I.
Fig. 3 shows that the degree of liver
damage was significantly higher in the mice of group II compared
with the mice in group I. However, these effects were limited
following the correction of intestinal flora imbalance in the mice
of group III (P<0.05).
 | Table I.Alcohol induced disease histology
score. |
Table I.
Alcohol induced disease histology
score.
| Group | Hepatocyte
ballooning | Liver steatosis | Liver
inflammation | Fibrosis |
|---|
| III | 0.67±0.44 | 0.81±0.68 | 0.21±0.38 | 0.29±0.49 |
| II | 1.90±0.33 | 2.48±0.58 | 3.00±0.13 | 2.79±0.29 |
| I | 1.23±0.48 | 2.13±0.48 | 0.50±0.61 | 0.53±0.55 |
Intestinal flora imbalance inhibited
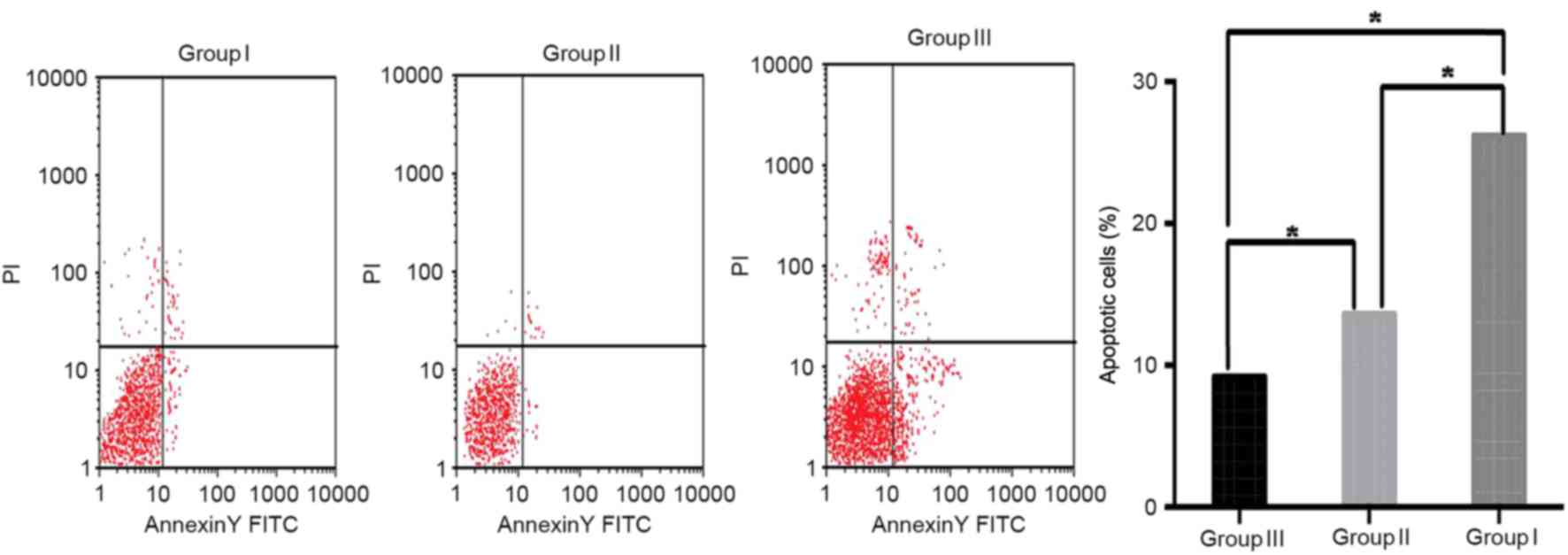
apoptosis of HSCs in mice
HSCs perform an important role in producing
collagen, which is an important process in development of liver
fibrosis. To analyze the apoptosis of HSCs in the mice among 3
groups, Annexin V-FITC/PI double staining was performed. Apoptosis
in HSCs was significantly inhibited in the livers of the mice in
group II as compared with that in group I (P<0.05). The present
study also observed that correcting the intestinal flora imbalance
in the mice of group III significantly increased apoptosis in HSCs
compared with that in group I (P<0.05; Fig. 4). These data indicate that an
intestinal flora imbalance may be associated with the proliferation
and activation of HSCs.
Association of intestinal flora
imbalance and TGFβ/smad signaling pathway
To investigate whether the TGFβ/smad signaling
pathway is involved in the role of intestinal flora imbalance in
alcohol-induced liver fibrosis, western blot analysis was performed
to detect the expression levels of smad3 and smad4 in HSCs among 3
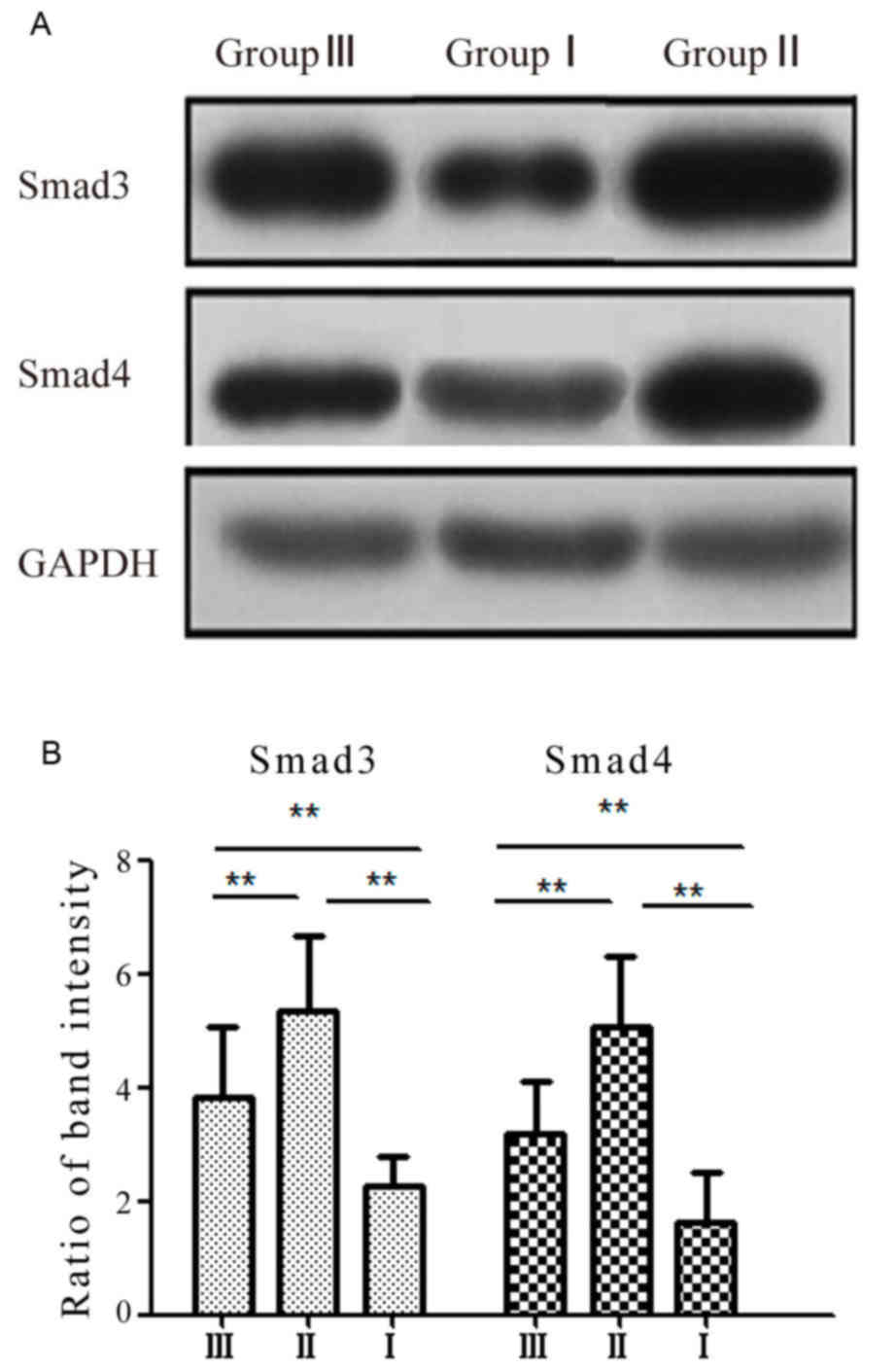
groups. The present data shows that the expression levels of smad3
and smad4 were significantly upregulated in HSCs of the mice in
group II compared with that in group I (P<0.01; Fig. 5A). By contrast, the expression levels
of smad3 and smad4 were significantly downregulated in HSCs of the
mice in group III compared to that in the mice of group II
(P<0.01; Fig. 5B).
Discussion
Several studies have indicated that there are
associations between intestinal flora imbalance and liver injury
(12–14). The present study investigated the
association between intestinal flora imbalance and liver fibrosis
in 4 ways: Analysis of serum AST, ALT and ALP levels; observing the
hepatic pathological changes; monitoring the proliferation rate of
HSCs; and monitoring the expression of smad3 and smad4.
It has previously been reported that the levels of
serum ALT, AST and ALP were positively correlated with liver injury
and fibrosis and used as indices of accessory diagnosis (15–17). The
present study found that the levels of serum AST, ALT and ALP in
mice of the alcoholic injury with intestinal flora imbalance group
(group II) were significantly increased compared with that in the
alcoholic injury group (group I) (P<0.05); while these diagnosis
indices in mice of the alcoholic injury with corrected intestinal
flora group (group III) were significantly decreased compared with
that in group II (P<0.05), which suggested that the reduction of
intestinal flora contributed to increased vulnerability to alcohol
induced liver fibrosis. Furthermore, in order to investigate the
role of intestinal flora imbalance in liver fibrosis, the present
study performed H&E staining and Masson staining of liver
tissue sections among 3 groups and found a relatively high degree
of liver fibrosis in the mice of group II compared with that in the
mice of group I and group III, primarily manifested increased
hepatocyte ballooning degeneration and necrosis, inflammatory cell
infiltration, and a significantly extended area of collagen fiber.
Liver fibrosis may be caused by impaired intestinal barrier
function following an imbalance in intestinal flora. When the
intestinal barrier is damaged, harmful substances such as bacteria
and bacterial metabolites in the intestine can invade the liver
through the enterohepatic cycle. This leads to over-activation of
the immune system and adverse immune reactions, which subsequently
cause vast inflammatory cell infiltration, hepatocyte degeneration
and necrosis (15).
A HCS is a type of mesenchymal cell in the liver
that has characteristics of muscle, adipose and fibroblast cells.
HSCs regulate blood flow and liver fibrosis, and help to maintain a
metabolic balance of Vitamin A (17,18). In
physiological conditions, HSCs grow slowly and are almost dormant,
producing little collagen. However, when pathological changes such
as alcoholic induced liver damage occur in the liver, liver cells
secrete numerous cytokines, including tumor necrosis factor-α
(TNF-α) and TGFβ, which promote HSCs to lose lipids and undergo
morphological changes into fibroblasts. Liver cells release excess
extracellular matrixes (ECMs), causing the proliferation and
activation of HSCs (19). Extensive
ECM accumulation in the sinusoids and perisinusoidal space is one
of pathological features of liver fibrosis, and so the
proliferation and activation of HSCs are cytological elements
causing liver fibrosis (20). The
present data show that apoptosis of HSCs in group II mice was
significantly inhibited compared with that of group I mice, meaning
that HSCs are more susceptible to proliferate and differentiate
into fibroblasts. Conversely, apoptosis of HSCs in group III mice
increased significantly compared with that of group II mice,
suggesting that liver fibrosis may be recovered following the
correction of the intestinal flora imbalance.
With the depth of research on pathological fibrosis,
an increasing number of studies consider that smad3 and smad4 are
critical in the development of pathological fibrosis. Huang et
al (21) identified that the
expression of smad3 in patients with renal fibrosis was increased
compared with that in controls. A study by Schwartze et al
(22) found that the expression of
smad3 and smad4 was significantly upregulated in patients with
pulmonary fibrosis. Additionally, Zhu et al (23) reported that the degree of liver
fibrosis was alleviated following inhibiting the expression of
smad3 in rats. Therefore, smad3 may perform an important role in
promoting pathological fibrosis in different organs. There is
increasing evidence that smad3 and smad4 serve as mediators in the
TGF-β signaling pathway and perform important roles in the
occurrence of liver fibrosis (24).
TGF-β is a major cell-signaling pathway involved in activating HSCs
and increasing the synthesis of collagen and other extracellular
matrix proteins by regulating downstream pathways such as smad
proteins. The present study detected the expression of smad3 and
smad4 in HSCs of mice among 3 groups and identified that
significantly increased expression levels of smad3 and smad4 were
observed in group II mice compared with the mice in group I.
Additionally, the expression levels of smad3 and smad4
significantly reduced in HSCs of group III mice compared with those
observed in group II mice. The present data indicated that the
TGFβ/smad signaling pathway is involved in the effect of intestinal
flora imbalance on liver fibrosis.
A previous study (24)
identified that intestinal flora imbalance can increase the level
of bacterial endotoxin, and subsequently upregulate the secretion
of inflammatory factors such as TNF-α, interleukin (IL)-1β and
IL-6. These inflammatory factors enhanced oxidative stress in the
liver, which led to liver fibrosis and lipid peroxidation. The
inflammatory factors also activated TGFβ that exacerbates liver
fibrosis. It is known that TGFβ can transduce proteins by
regulating downstream signaling factors such as smad family
proteins (25,26). Therefore, intestinal flora imbalance
increases alcohol induced liver fibrosis in mice by upregulating
the TGFβ/smad signaling pathway, which is shown by the
downregulation of smad3 and smad4 in HSCs of the group III
mice.
In summary, liver fibrosis and intestinal flora
imbalance influence each other, leading to a cycle in which liver
fibrosis produces intestinal flora imbalance, which in turn
aggravates liver fibrosis. The present study demonstrated that
correcting intestinal flora imbalance is necessary to break this
cycle and maintain liver homeostasis. Subsequent to correcting
intestinal flora imbalance, the proliferation and activation of
HSCs was attenuated, which reduced excessive production of
extracellular matrixes by downregulating the TGFβ/smad signaling
pathway. Therefore, correcting intestinal flora imbalance is
crucial and can be an effective method in the treatment of liver
fibrosis.
Acknowledgements
The present study was supported by the Medical
College of Qingdao University (Qingdao, China) and the Department
of Gastroenterology in Qingdao Center Hospital (Qingdao,
China).
References
|
1
|
Blachier M, Leleu H, Peck-Radosavljevic M,
Valla DC and Roudot-Thoraval F: The burden of liver disease in
Europe: A review of available epidemiological data. J Hepatol.
58:593–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szabo G and Bala S: Alcoholic liver
disease and the gut-liver axis. World J Gastroenterol.
16:1321–1329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu ZW, Ling ZX, Lu HF, Zuo J, Sheng JF,
Zheng SS and Li LJ: Changes of gut bacteria and immune parameters
in liver transplant recipients. Hepatobiliary Pancreat Dis Int.
11:40–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, Kim B, Park YK, Koo SI and Lee JY:
Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression
by inhibiting Smad3 activation in hepatic stellate cells. Biochim
Biophys Acta. 1850:178–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Q, Duan ZP, Ha DK, Bengmark S,
Kurtovic J and Riordan SM: Synbiotic modulation of gut flora:
Effect on minimal hepatic encephalopathy in patients with
cirrhosis. Hepatology. 39:1441–1449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lata J, Novotný I, Príbramská V, Juránková
J, Fric P, Kroupa R and Stibůrek O: The effect of probiotics on gut
flora, level of endotoxin and child-pugh score in cirrhotic
patients: Results of a double-blind randomized study. Eur J
Gastroenterol Hepatol. 19:1111–1113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shukla S, Shukla A, Mehboob S and Guha S:
Meta-analysis: The effects of gut flora modulation using
prebiotics, probiotics and synbiotics on minimal hepatic
encephalopathy. Aliment Pharmacol Ther. 33:662–671. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bauer TM, Schwacha H, Steinbrückner B,
Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M and
Blum HE: Small intestinal bacterial overgrowth in human cirrhosis
is associated with systemic endotoxemia. Am J Gastroenterol.
97:2364–2370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maddrey WC: Alcohol-induced liver disease.
Clin Liver Dis. 4:115–131, vii. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan G, Li B, Xin X, Xu M, Ji G and Yu H:
MicroRNA-34a promotes hepatic stellate cell activation via
targeting ACSL1. Med Sci Monit. 21:3008–3015. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jarzembowski T, Daca A, Bryl E, Wiśniewska
K, Gołębiewska J, Dębska-Ślizień A, Rutkowski B and Witkowski J:
Increased pheromone cCF10 expression in Enterococcus faecalis
biofilm formed by isolates from renal transplant patients. Curr
Microbiol. 65:656–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Wu Z, Ma W, Yu Y and Chen Y: Changes
in intestinal microflora in patients with chronic severe hepatitis.
Chin Med J (Engl). 114:869–872. 2001.PubMed/NCBI
|
|
14
|
Schreiber S, Rosenstiel P, Albrecht M,
Hampe J and Krawczak M: Genetics of Crohn disease, an archetypal
inflammatory barrier disease. Nat Rev Genet. 6:376–388. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Housset C and Guéchot J: Hepatic fibrosis:
Physiopathology and biological diagnosis. Pathol Biol (Paris).
47:886–894. 1999.(In French). PubMed/NCBI
|
|
16
|
Liao SL, Kao TK, Chen WY, Lin YS, Chen SY,
Raung SL, Wu CW, Lu HC and Chen CJ: Tetramethylpyrazine reduces
ischemic brain injury in rats. Neurosci Lett. 372:40–45. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nozaki Y, Fujita K, Wada K, Yoneda M,
Kessoku T, Shinohara Y, Imajo K, Ogawa Y, Nakamuta M, Saito S, et
al: Deficiency of iNOS-derived NO accelerates lipid
accumulation-independent liver fibrosis in non-alcoholic
steatohepatitis mouse model. BMC Gastroenterol. 15:422015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reyes-Gordillo K, Shah R,
Arellanes-Robledo J, Hernández-Nazara Z, Rincón-Sánchez AR, Inagaki
Y, Rojkind M and Lakshman MR: Mechanisms of action of acetaldehyde
in the up-regulation of the human α2(I) collagen gene in hepatic
stellate cells: Key roles of Ski, SMAD3, SMAD4, and SMAD7. Am J
Pathol. 184:1458–1467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Reilly S, Ciechomska M, Cant R and van
Laar JM: Interleukin-6 (IL-6) trans signaling drives a
STAT3-dependent pathway that leads to hyperactive transforming
growth factor-β (TGF-β) signaling promoting SMAD3 activation and
fibrosis via Gremlin protein. J Biol Chem. 289:9952–9960. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun YB, Qu X, Li X, Nikolic-Paterson DJ
and Li J: Endothelial dysfunction exacerbates renal interstitial
fibrosis through enhancing fibroblast Smad3 linker phosphorylation
in the mouse obstructed kidney. PLoS One. 8:e840632013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang XZ, Wen D, Zhang M, Xie Q, Ma L,
Guan Y, Ren Y, Chen J and Hao CM: Sirt1 activation ameliorates
renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J Cell
Biochem. 115:996–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwartze JT, Becker S, Sakkas E, Wujak
ŁA, Niess G, Usemann J, Reichenberger F, Herold S, Vadász I, Mayer
K, et al: Glucocorticoids recruit Tgfbr3 and Smad1 to shift
transforming growth factor-β signaling from the Tgfbr1/Smad2/3 axis
to the Acvrl1/Smad1 axis in lung fibroblasts. J Biol Chem.
289:3262–3275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu JN, Chen R, Fu YH, Lin QX, Huang S,
Guo LL, Zhang MZ, Deng CY, Zou X, Zhong SL, et al: Smad3
inactivation and MiR-29b upregulation mediate the effect of
carvedilol on attenuating the acute myocardium infarction-induced
myocardial fibrosis in rat. PLoS One. 8:e755572013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv KY, Zhong QS, Liu XF, Zhu SH, Xiao SC,
Wang GY, Ma B and Xia ZF: Deficiency of Smad3 results in enhanced
inducible nitric oxide synthase-mediated hypotension in
lipopolysaccharide-induced endotoxemia. J Surg Res. 187:640–645.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji F, Fu SJ, Shen SL, Zhang LJ, Cao QH, Li
SQ, Peng BG, Liang LJ and Hua YP: The prognostic value of combined
TGF-β1 and ELF in hepatocellular carcinoma. BMC Cancer. 15:1162015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamato D, Burch ML, Piva TJ, Rezaei HB,
Rostam MA, Xu S, Zheng W, Little PJ and Osman N: Transforming
growth factor-β signalling: Role and consequences of Smad linker
region phosphorylation. Cell Signal. 25:2017–2024. 2013. View Article : Google Scholar : PubMed/NCBI
|