Introduction
To date, 5-fluorouracil (5-FU)-based concurrent
chemoradiotherapy (CRT) followed by total mesorectal excision (TME)
has been the standard treatment for locally advanced rectal cancer
(LARC) (1,2). Although local tumor regression was
distinctly observed in the majority of patients following CRT,
distant metastases remained the main cause for failure, possibly
due to the insufficient control of systemic micro-metastasis by CRT
(3,4).
To enhance local and systemic control, our previous study applied
oxaliplatin into capecitabine-based (XELOX regimen) preoperative
CRT, which was demonstrated to be feasible and well tolerated
(5). The short-term result of this
study demonstrated a favorable pathological complete response (pCR;
22%, 9/41), which was consistent with previous studies in the same
setting (6–8).
Several phase II studies using the XELOX regimen
combined with radiotherapy presented pCR as the primary endpoint
(9,10). However, the early surrogate endpoint
of pCR may not completely reflect the authentic clinical efficacy
of CRT for LARC (11). It is
well-known that overall survival (OS) is the determinant endpoint
in a clinical study (12,13). Thus, long-term follow-up data are
required to draw specific conclusions with respect to the rates of
local recurrence and distant metastases. With a median follow-up
time of 84 months, the present study investigated the 5-year OS and
disease-free survival (DFS) rates, the cumulative incidence of
local and distant recurrences, and the long-term complications
found in patients with LARC who underwent preoperative
chemoradiotherapy with the XELOX regimen in our previous phase II
study (5).
Patients and methods
Patients and methods
The prospective, single arm phase II study (clinical
trial number ChiCTR-OIC-17011632) was conducted between March 2007
and June 2008 at the Cancer Center of Sun Yat-sen University
(Guangzhou, China). We have previously reported details of the
study, including eligibility criteria, evaluation method, treatment
model, pathological analysis and short-term endpoints (pCR rate),
CRT-associated toxicities, R0 resection rates, sphincter-sparing
rates and 1-month surgical complications (5). Patients with stage II and III (T3-T4
and/or N+) histologically confirmed rectal
adenocarcinomas received radiotherapy (46 Gy in 23 fractions) in
combination with capecitabine (1,000 mg/m2, twice daily,
on days 1–14 and 22–35) and oxaliplatin (130 mg/m2 on
days 1 and 22). TME surgery was scheduled to take place 4–6 weeks
after completion of preoperative CRT. Either six cycles of XELOX
regimen or four cycles of XELOX regimen plus two cycles of
capecitabine, was recommended for patients 4 weeks after surgery.
All patients provided written informed consent and the Ethical
Committee of Sun Yat-sen University Cancer Center approved the
study protocol. The study was performed in accordance with the
ethical standards of the World Medical Association Declaration of
Helsinki.
Follow-up
All patients were observed through subsequent visits
every 3 months for 2 years, and then semi-annually until 5 years
post-surgery. Evaluation included clinical examination,
carcinoembryonic antigen level, abdominal ultrasonography and chest
radiography. Chest computed tomography, abdominal/pelvic magnetic
resonance imaging and colonoscopy were performed annually.
Recurrence in the pelvis was defined as local recurrence, and
recurrence outside the pelvis was considered as distant
metastasis.
Study endpoints
The primary endpoints of the XELOX phase II study,
including tumor regression and toxicities, were reported previously
(5). Long-term secondary endpoints
included OS, DFS and the cumulative incidence of recurrences. OS
time was calculated from the beginning of surgery to rectal
cancer-associated mortality or the time of last follow-up. DFS time
was measured between the date of radical surgery and the date of
diagnosis of local recurrence and distant metastasis from rectal
cancer. Local and distant recurrence analyses were performed on all
eligible patients who underwent a complete local resection
(patients only with R0 resection of the primary tumor were
included, whereas patients with R1 and R2 resection of the primary
tumor were excluded). Long-term complication was defined according
to the literature as occurring or persisting 6 months after surgery
for primary rectal cancer (14).
Statistical analysis
Statistical analysis was performed with the
SPSS® statistical package for Windows (version 17.0;
SPSS, Inc., Chicago, IL, USA). Continuous variables are summarized
as the median (range) and categorical variables are presented as
percentages. Analyses for recurrences were reported as cumulative
incidence rates. Kaplan-Meier methodology was applied to calculate
OS, DFS and cumulative incidence rates by performing survival
curves. All tests were two-tailed, in which P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
In total, 47 patients were enrolled in the present
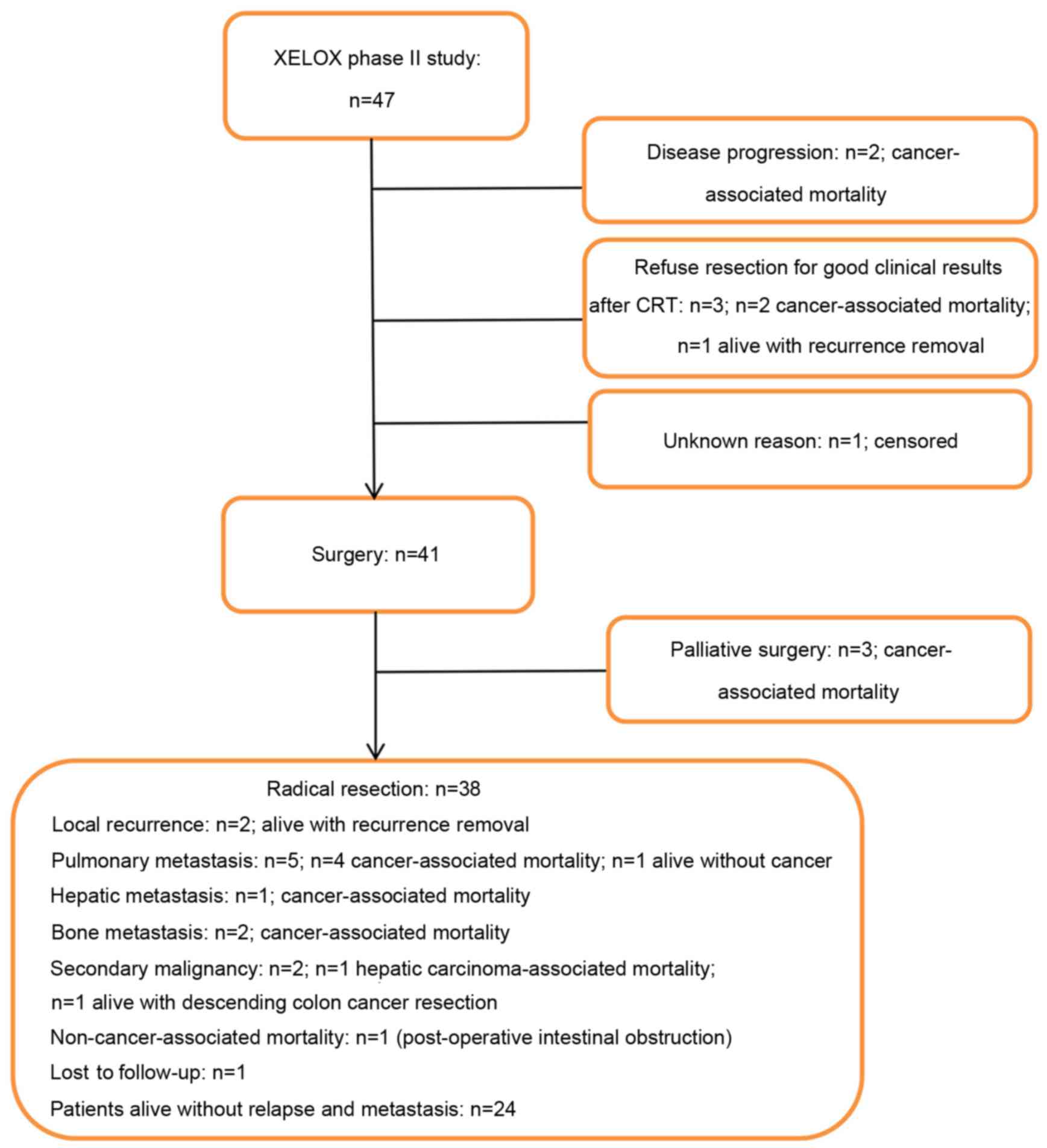
study and received preoperative CRT as planned. As shown in
Fig. 1, 6 patients did not undergo
surgery; 2 patients were diagnosed with an unresectable tumor with
hepatic metastases following preoperative CRT and then administered
palliative chemotherapy, 3 patients refused to receive surgery due
to good tumor regression after CRT and 1 patient did not undergo
surgery for an unknown reason. As a result, 41 patients (87.2%)
received surgery following preoperative CRT. The clinical baseline
characteristics of those patients are shown in Table I. Primary tumor palliative resection
was performed in 3 patients: 1 patient was diagnosed with hepatic
metastasis following CRT, and pelvic metastases were detected
intraoperatively in the other 2 patients. In total, radical
resection was performed on 38 patients (92.7%). Clinicopathological
parameters subsequent to treatments are presented in Table II. T stage downstaging was observed
in 24 out of 41 patients (58.5%) and Union for International Cancer
Control downstaging was observed in 25 of 41 patients (61.0%)
(15). A pCR was achieved in 9 out of
41 patients (22.0%) (5). In total, 33
patients (80.5%) subsequently received post-surgery adjuvant
chemotherapy, with a median of 6 cycles (1–6 cycles), 60.6% (20/33)
of whom underwent complete adjuvant chemotherapy as planned.
 | Table I.Baseline characteristics of patients
undergoing surgery. |
Table I.
Baseline characteristics of patients
undergoing surgery.
| Characteristics | Patient values
(n=41) |
|---|
| Median age (range),
years | 53 (26–75) |
| Sex, n (%) |
|
| Male | 26 (63.4) |
|
Female | 15 (36.6) |
| Tumor distance from
anal verge, n (%) |
|
| <6
cm | 27 (65.9) |
| 6–10
cm | 14 (34.1) |
| Median
primary tumor size (range), cm | 4.5 (2–8) |
| Clinical TNM stage,
n (%) |
|
| II | 13 (31.7) |
|
III | 28 (68.3) |
| cT stage, n
(%) |
|
| T3 | 25 (61.0) |
| T4 | 16 (39.0) |
| cN stage, n
(%) |
|
| N0 | 13 (31.7) |
| N1 | 16 (39.0) |
| N2 | 12 (29.3) |
 | Table II.Clinicopathological parameters after
preoperative chemoradiotherapy followed by surgery. |
Table II.
Clinicopathological parameters after
preoperative chemoradiotherapy followed by surgery.
| Patients
parameters | Patient values
(n=41) |
|---|
| Type of surgery, n
(%) |
|
|
Anterior resection | 21 (51.2) |
|
Abdominal perineal
resection | 17 (41.5) |
|
Hartmann's operation | 2 (4.9) |
| Resection of
adjacent organsa, n
(%) | 1 (2.4) |
| Resection status, n
(%) |
|
| R0 | 38 (92.7) |
| R1 | 0 (0.0) |
| R2 | 3 (7.3) |
| Median number of
investigated lymph nodes (range) | 7 (1–27) |
| Pathological ypTNM
stages, n (%) |
|
|
ypT0N0M0 | 9 (22.0) |
|
ypT1-2N0M0 | 7 (17.1) |
|
ypT3-4N0M0 | 12 (29.3) |
|
ypT1-4N1-2M0 | 10 (24.4) |
|
ypTxNxM1 | 3 (7.3) |
| Tumor regression
grading, n (%) |
|
| 4
(complete regression) | 9 (22.0) |
| 3
(>50% of tumor mass) | 10 (24.4) |
| 2
(25–50% of tumor mass) | 10 (24.4) |
| 1
(<25% of tumor mass) | 9 (22.0) |
| 0 (no
regression) | 3 (7.3) |
| Post-operative
chemotherapy |
|
|
None | 8 (19.5) |
| XELOX
regimen | 15 (36.6) |
| XELOX +
capecitabine | 14 (34.1) |
|
Capecitabine regimen | 3 (7.3) |
| FOLFOX
regimen | 1 (2.4) |
Long-term postoperative
complications
A total of 41 patients who underwent surgery
following preoperative CRT were evaluated for long-term
postoperative complications. Long-term complications were observed
in 14 of 41 patients (34.1%). As shown in Table III, 4 patients (9.8%) experienced
sexual dysfunction, 3 patients (7.3%) suffered a severe defecation
disorder, 3 patients (7.3%) had sequential acroanesthesia due to
peripheral nerve toxicity for 5 years, 2 patients (4.9%) were found
to have anastomotic stenosis through colonoscopy following 6 months
of postoperative conservative treatment, 2 patients (4.9%) were
diagnosed with hepatic carcinoma and descending colon cancer after
treatment, and 1 patient (2.4%) succumbed to reiterant intestinal
obstruction with uropoiesis dysfunction.
 | Table III.Long-term complications after
preoperative chemoradiotherapy followed by surgery in patients with
local advanced rectal cancer. |
Table III.
Long-term complications after
preoperative chemoradiotherapy followed by surgery in patients with
local advanced rectal cancer.
| Complications | Patient values, n
(%) |
|---|
| Defecation
disorder | 3 (7.3) |
| Uropoiesis
dysfunction | 1 (2.4) |
| Anastomotic
stenosis | 2 (4.9) |
| Postoperative
obstruction | 1 (2.4) |
| Femoral head
necrosis | 0 (0.0) |
| Sexual
dysfunction | 4 (9.8) |
| Second primary
malignancya | 2 (4.9) |
| Peripheral nerve
toxicity | 3 (7.3) |
Recurrence and survival
parameters
During the median 84-month follow-up period (range,
1–99 months), the survival outcome of the 6 patients who failed to
receive surgery was as followed: 1 patient was lost follow-up, 2
patients succumbed at 1 and 13 months after CRT due to disease
progression, and of the 3 patients who refused surgery due to a
favorable CRT results, 2 succumbed at 8 and 14 months,
respectively, due to disease progression, and the other patient who
had local recurrence and then underwent lesion removal survived. Of
those patients who underwent surgery (n=41), the 3 patients who
underwent R2 resection succumbed to tumor progression after 6, 15
and 17 months, respectively. Ultimately, 38 patients who underwent
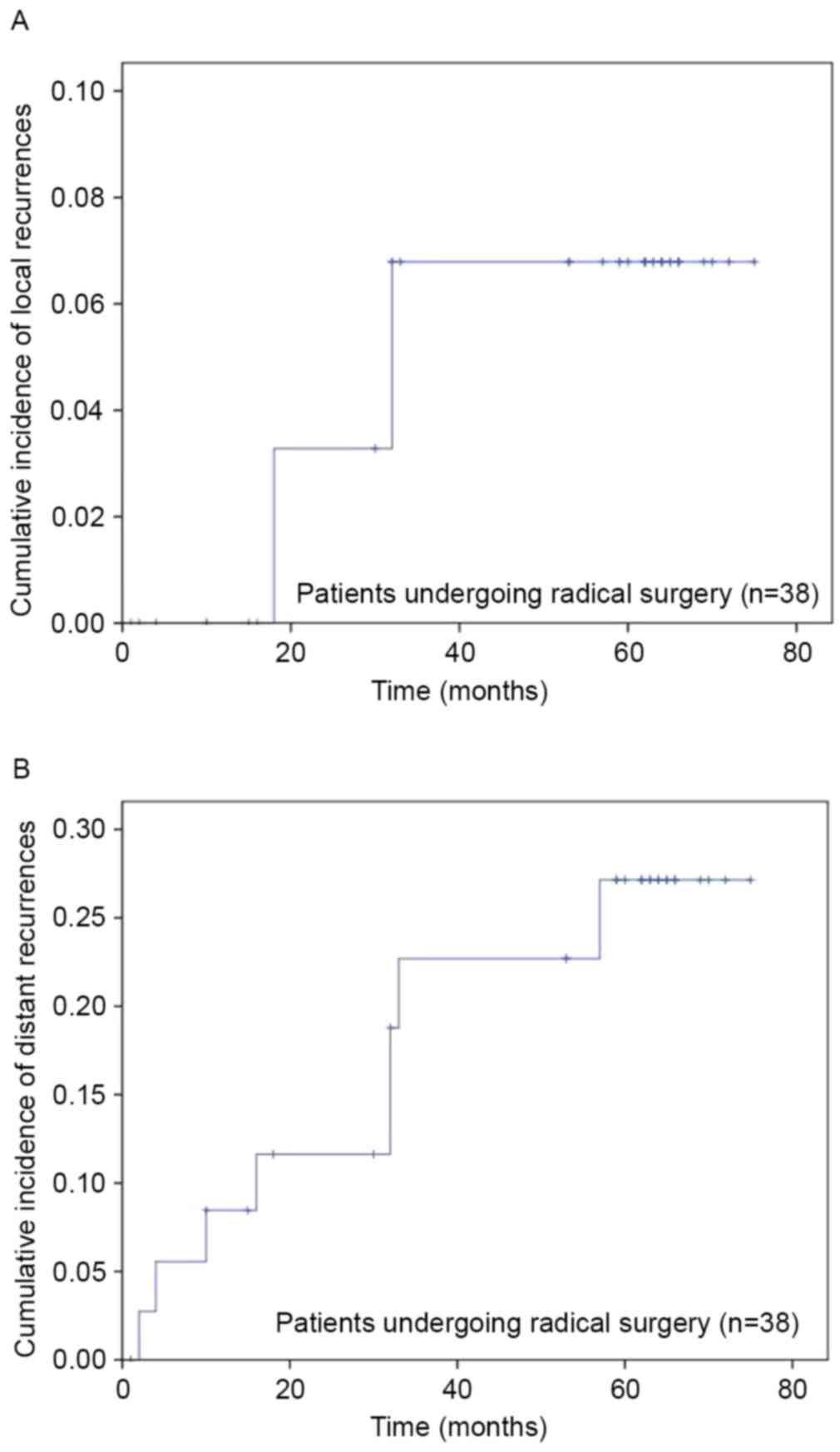
R0 were evaluated for recurrences (Fig.
1). In this cohort, 2 patients (5.3%) developed local
recurrences, with a 6.6% cumulative incidence rate of local
recurrences at 5 years (Fig. 2A). The
median local recurrence time was 25 months (range, 18–32 months).
The cumulative incidence rate of distant recurrence at 5 years was
28.2% (Fig. 2B). The median distant
recurrence time was 16 months (range, 2–57 months). In total, 8
(21.1%) patients developed distant metastases, including pulmonary
metastases (13.2%), hepatic metastases (2.6%) and bone metastases
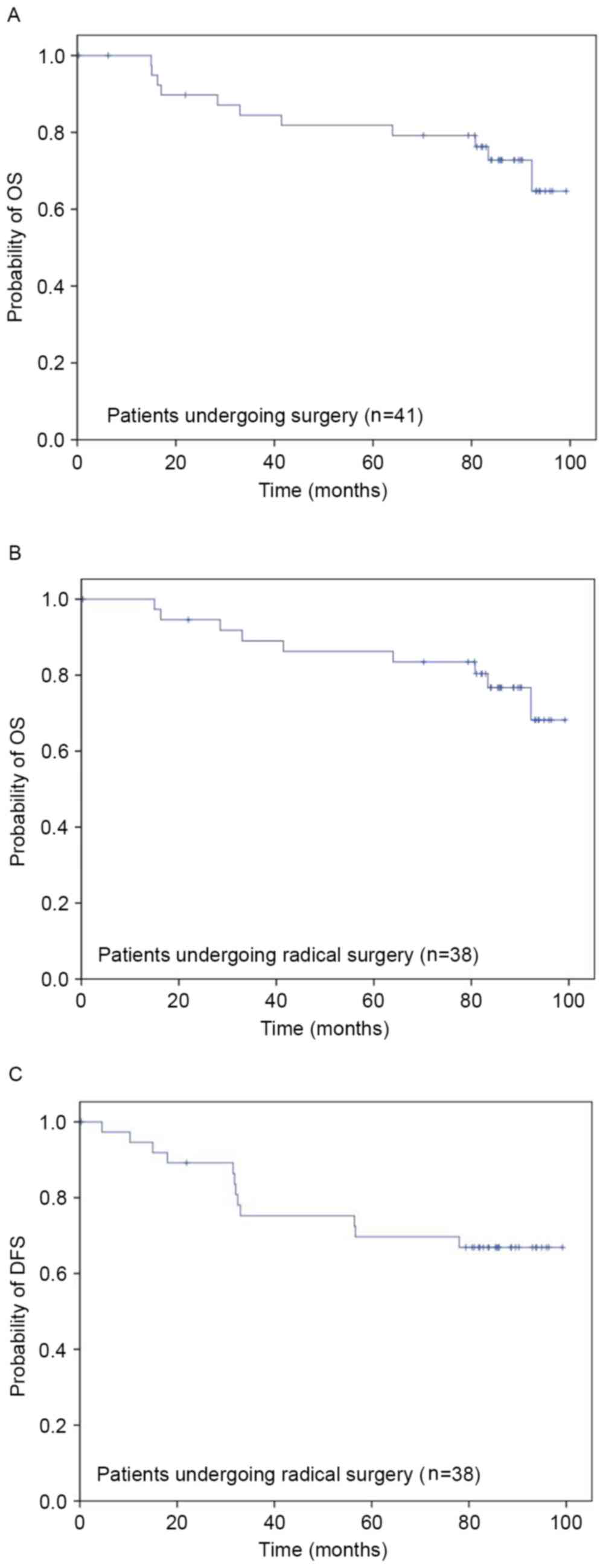
(5.3%). For patients receiving surgery (n=41), OS rates at 1, 3 and
5 years were 100.0, 84.5 and 81.8%, respectively (Fig. 3A). In the patients who received an R0
resection (n=38), OS rates at 1, 3 and 5 years were 100.0, 89.0,
86.2%, respectively (Fig. 3B), and
DFS rates at 1, 3 and 5 years were 94.6, 75.3, 69.7%, respectively
(Fig. 3C).
Discussion
The optimal chemotherapeutic selection for
preoperative CRT in LARC remains an ongoing issue (16). Although preoperative CRT with
5-FU-based regimen has substantially reduced the risk of local
recurrence, systemic failure remains the major challenge in the
management of LARC (17,18). Previous clinical trials have focused
on intensification of conventional chemotherapy by the addition of
a second cytotoxic drug to a fluorouracil backbone (19,20). As a
potent radiosensitizer, oxaliplatin has been experimentally shown
to enhance cytotoxicity and radiosensization for treating rectal
cancer, which have become important components in preoperative
chemotherapy regimens (21,22). In order to improve clearance of the
primary tumor and systemic micro-metastasis, and to ultimately
translate this into a survival improvement, oxaliplatin was added
to capecitabine-based preoperative CRT, and short-term and
long-term efficacies were then evaluated for this strategy. The
final results demonstrated that integration of oxaliplatin and
capecitabine to CRT followed by TME surgery was feasible, with good
compliance, acceptable toxicity and low surgical morbidity
(5). With a median follow-up time of
84 months, favorable 5-year DFS (69.7%) and OS (86.2%) rates were
obtained from the present study, which was consistent with previous
studies of oxaliplatin-based preoperative CRT for treating LARC
(23,24).
It has been shown that preoperative CRT with the
XELOX regimen, followed by optimized TME surgery, markedly achieved
a higher pCR rate and decreased local recurrence rate of 5–8%,
whereas the distant metastatic rate in LARC following TME surgery
was 3–6 times higher than the local recurrent rate in previous
clinical trials and in the present study (25,26).
However, the value of controlling distant metastasis by performing
oxaliplatin-based preoperative CRT remains uncertain. The
CAO/ARO/AIO-04 trial showed that the cumulative incidence of
distant recurrences at 3 years after R0/1 resection was 18.5% [95%
confidence interval (CI), 15.2–21.7] in an oxaliplatin group and
22.4% (95% CI, 19.1–25.8) in a non-oxaliplatin group. Although a
numerical difference could be observed, it failed to reach
statistical significance (27). The
STAR-01 trial reported that integrating oxaliplatin into a
capecitabine-based preoperative CRT regimen could contribute to a
reduced percentage of intra-abdominal metastases (0.5% with
oxaliplatin vs. 2.9% without; P=0.014) (28). However, the incidence of pulmonary
metastasis in the CAPEOX group in the ACCORD 12/0405 PRODIGE 2
trial was not less than that of the capecitabine group at 3 years
(11.0 vs. 10.4%) (29). Similar to
the uncertain impact of oxaliplatin in distant metastasis, the
benefit of adding oxaliplatin to preoperative CRT for a significant
improvement in long-term survival has also not been specifically
confirmed thus far. As shown in Table
IV, the addition of oxaliplatin delivered different 3-year DFS
rates (range, 60.0–78.6%) and 5-year OS rates (range, 54.7–92.0%)
(23–27,29–31). Among
those phase III studies, only the CAO/ARO/AIO-04 trial demonstrated
a survival benefit from adding oxaliplatin to preoperative
treatment (DFS at 3 years: 75.9% with oxaliplatin vs. 71.2%
without; P=0.03; hazard ratio, 0.79; 95% CI, 0.64–0.98) (27). By contrast, the long-term outcomes of
the ACCORD 12/0405-Prodige 2 trial and the NSABP R-04 trial did not
reach a statistically significant difference in terms of the 3- and
5-year DFS rates (29,31). All current studies failed to achieve a
significant benefit to overall survival by adding oxaliplatin to
preoperative CRT (27,29–31).
However, it must be noted that the CAO/ARO/AIO-04 trial
administered oxaliplatin in preoperative and post-operative
chemotherapy, while the ACCORD12/0405-Prodige 2 trial only
administered oxaliplatin during preoperative treatment.
Furthermore, with the exception of the CAO/ARO/AIO-04 trial, adding
oxaliplatin to the conventional CRT led to increased toxicity and
reduced tolerance, resulting in a lower dose of oxaliplatin in the
combination arm, which ultimately compromised the local and
systematic effect of CRT. Therefore, optimizing the dose and
schedule for administration of oxaliplatin possibly contributes to
good tolerance, which may finally result in a favorable long-term
outcome.
 | Table IV.Studies of oxaliplatin-based
preoperative chemoradiotherapy followed by total mesorectal
excision treating locally advanced rectal cancer. |
Table IV.
Studies of oxaliplatin-based
preoperative chemoradiotherapy followed by total mesorectal
excision treating locally advanced rectal cancer.
| Study | Phase | n | CT regimen | RT dose (Gy) | pCR, % | LR, % | DM, % | 3-year DFS, % | 5-year OS, % | (Refs.) |
|---|
| Pucciarelli et
al, 2006 | II | 23 | 5-FU + OX 25, 35,
45 and 60 mg/m2 on the first day of each
radiotherapy | 50.4 | 30.4 | 4.5 | 4.5 | 89.0a | 92.0 | (25) |
| Chitapanarux et
al, 2011 | II | 35 | 5-FU + OX 130
mg/m2 on week 1 and week 4 for 5 days | 50.0 | 16.7 | NR | NR | 60.0 | NR | (23) |
| Gérard et
al, 2012 | III | 299 | Cape + OX 50
mg/m2 once per week for 5 weeks | 50.0 | 19.2 | 4.4 | 22.1 | 72.7 | 88.3 | (29) |
| Chao et al,
2014 | II | 20 | UFT + OX 55
mg/m2 every 2 weeks for 5 weeks | 50.0 | 40.0 | 0 | 20.0 | 78.6 | 94.1b | (24) |
| Wong et al,
2015 | II | 52 | 5-FU + OX 50
mg/m2 once per week for 5 weeks | 50.4 | 20.8 | 18.0 | 30.0 | 65.0 | 75.0c | (30) |
| Liu et al,
2015 | II | 58 | Cape + OX 130
mg/m2 on day 1 and 28 | 46.0 | 20.8 | 12.1 | 51.3 | 47.2a | 54.7 | (26) |
| Allegra et
al, 2015 | III | 659 | 5-FU/Cape + OX 50
mg/m2 once per week for 5 weeks | 50.4 | 19.5 | 11.2 | NR | 69.2a | 81.3 | (31) |
| Rödel et al,
2015 | III | 613 | 5-FU + OX 50
mg/m2 on day 1, 8, 22 and 29 | 50.4 | 17.4 | 2.9 | 18.5 | 75.9 | 88.7b | (27) |
| Present study | II | 38 | Cape + OX 130
mg/m2 on day 1 and 22 | 46.0 | 21.1 | 6.6 | 28.2 | 69.7a | 86.2 |
|
A concern of adding oxaliplatin concomitantly to
conventional CRT is that it may significantly increase toxicity,
with ~50% of patients ending up not receiving the complete
chemotherapy (6,7) and 13–16% of patients not receiving the
complete dose of radiation (28,32). The
current strategy was well tolerated and all patients received the
preoperative CRT as planned. The low toxicities and high compliance
to treatment may be attributed to the strategy of drug delivery (as
aforementioned). In the present study, the XELOX regimen was
delivered with a 1-week chemotherapy intermission, which possibly
contributed to the good tolerance. In addition, unlike the poor
capecitabine tolerability found in the American population
(33,34), acceptable toxicities and compliance
were found in the Chinese patients in the current study, when
administered the standard dose of capecitabine (1,000
mg/m2, twice daily, on days 1–14 of a 3-week schedule).
With a median follow-up time of 84 months, the major long-term
complications, including sexual dysfunction (9.8%), defecation
disorders (7.3%) and peripheral nerve toxicity (7.3%), were
observed without lethal cause in the present study. The long-term
surgical complications were comparable to those in other studies
using conventional 5-FU-based CRT (1,4). With the
exception of peripheral nerve toxicity, it was considered that
long-term complications may be to a great extent relevant to the
postoperatively shortened intestinal length, diminished rectal
reservoir and damage to the sphincter complex or its innervation,
but not the toxicity of oxaliplatin.
There were several limitations in the present study,
including the small number of patients and selection bias.
Consequently, randomized control trials are required to determine
the definite role of the current strategy. In addition, 19.5% of
the patients in the present study failed to receive post-operative
chemotherapy, which may impact the long-term outcome to a large
extent (35). In addition, despite
the satisfactory median follow-up period of the present study (84
months), the follow-up time is insufficient to measure the 10-year
long-term outcome for patients with LARC. Longer outcome data are
required for this setting.
In conclusion, addition of oxaliplatin into
capecitabine-based preoperative radiotherapy could achieve
favorable OS and DFS rates without increasing long-term
oxaliplatin-associated complications in LARC. Distant recurrence
remains the predominant pattern of failure after preoperative CRT
followed by TME.
Acknowledgements
This study was supported by grants from the Sun
Yat-sen University Clinical Research 5010 Program (no. 2015024) and
a grant of Guangzhou Science and Technology Plan Projects (Health
Medical Collaborative Innovation Program of Guangzhou) (grant no.
201400000001-4).
References
|
1
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC: EORTC Radiotherapy Group Trial 22921: Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roh MS, Colangelo LH, O'Connell MJ,
Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L,
Ursiny CS, Petrelli NJ and Wolmark N: Preoperative multimodality
therapy improves disease-free survival in patients with carcinoma
of the rectum: NSABP R-03. J Clin Oncol. 27:5124–5130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanfilippo NJ, Crane CH, Skibber J, Feig
B, Abbruzzese JL, Curley S, Vauthey JN, Ellis LM, Hoff P, Wolff RA,
et al: T4 rectal cancer treated with preoperative chemoradiation to
the posterior pelvis followed by multivisceral resection: Patterns
of failure and limitations of treatment. Int J Radiat Oncol Biol
Phys. 51:176–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sauer R, Becker H, Hohenberger W, Rödel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative versus postoperative chemoradiotherapy for
rectal cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin JZ, Zeng ZF, Wu XJ, Wan DS, Chen G, Li
LR, Lu ZH, Ding PR and Pan ZZ: Phase II study of pre-operative
radiotherapy with capecitabine and oxaliplatin for rectal cancer
and carcinoembryonic antigen as a predictor of pathological tumour
response. J Int Med Res. 38:645–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao L, Bai C, Shao Y, Guan M, Jia N, Xiao
Y, Qiu H, Zhang F, Yang T, Zhong G and Chen S: A phase II study of
neoadjuvant chemoradiotherapy with oxaliplatin and capecitabine for
rectal cancer. Cancer Lett. 310:134–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koeberle D, Burkhard R, von Moos R,
Winterhalder R, Hess V, Heitzmann F, Ruhstaller T, Terraciano L,
Neuweiler J, Bieri G, et al: Phase II study of capecitabine and
oxaliplatin given prior to and concurrently with preoperative
pelvic radiotherapy in patients with locally advanced rectal
cancer. Br J Cancer. 98:1204–1209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aschele C, Friso ML, Pucciarelli S,
Lonardi S, Sartor L, Fabris G, Urso ED, Del Bianco P, Sotti G, Lise
M and Monfardini S: A phase I–II study of weekly oxaliplatin,
5-fluorouracil continuous infusion and preoperative radiotherapy in
locally advanced rectal cancer. Ann Oncol. 16:1140–1146. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carlomagno C, Farella A, Bucci L,
D'Armiento FP, Pesce G, Pepe S, Cannella L, Pacelli R, De Stefano
A, Solla R, et al: Neo-adjuvant treatment of rectal cancer with
capecitabine and oxaliplatin in combination with radiotherapy: A
phase II study. Ann Oncol. 20:906–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salazar R, Navarro M, Losa F, Alonso V,
Gallén M, Rivera F, Benavides M, Escudero P, González E, Massutí B,
et al: Phase II study of preoperative radiotherapy and concomitant
weekly intravenous oxaliplatin combined with oral capecitabine for
stages II–III rectal cancer. Clin Transl Oncol. 14:592–598. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonnetain F, Bosset JF, Gerard JP, Calais
G, Conroy T, Mineur L, Bouché O, Maingon P, Chapet O,
Radosevic-Jelic L, et al: What is the clinical benefit of
preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal
cancer in a pooled analysis of EORTC 22921 and FFCD 9203 trials:
Surrogacy in question? Eur J Cancer. 48:1781–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foster NR, Qi Y, Shi Q, Krook JE, Kugler
JW, Jett JR, Molina JR, Schild SE, Adjei AA and Mandrekar SJ: Tumor
response and progression-free survival as potential surrogate
endpoints for overall survival in extensive stage small-cell lung
cancer: Findings on the basis of North Central Cancer Treatment
Group trials. Cancer. 117:1262–1271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oba K, Paoletti X, Alberts S, Bang YJ,
Benedetti J, Bleiberg H, Catalano P, Lordick F, Michiels S, Morita
S, et al: Disease-free survival as a surrogate for overall survival
in adjuvant trials of gastric cancer: A meta-analysis. J Natl
Cancer Inst. 105:1600–1607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Birgisson H, Påhlman L, Gunnarsson U and
Glimelius B: Swedish Rectal Cancer Trial Group: Adverse effects of
preoperative radiation therapy for rectal cancer: Long-term
follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol.
23:8697–8705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobin LH: TNM, sixth edition: New
developments in general concepts and rules. Semin Surg Oncol.
21:19–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohiuddin M, Mohiuddin MM, Marks J and
Marks G: Future directions in neoadjuvant therapy of rectal cancer:
Maximizing pathological complete response rates. Cancer Treat Rev.
35:547–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, et al: Preoperative radiotherapy with or without
concurrent fluorouracil and leucovorin in T3-4 rectal cancers:
Results of FFCD 9203. J Clin Oncol. 24:4620–4625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Glynne-Jones R, Falk S, Maughan TS,
Meadows HM and Sebag-Montefiore D: A phase I/II study of irinotecan
when added to 5-fluorouracil and leucovorin and pelvic radiation in
locally advanced rectal cancer: A Colorectal Clinical Oncology
Group Study. Br J Cancer. 96:551–558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao YH, An X, Sun WJ, Cai J, Cai MY, Kong
LH, Lin JZ, Liu GC, Tang JH, Wu XJ, et al: Evaluation of
capecitabine and oxaliplatin administered prior to and then
concomitant to radiotherapy in high risk locally advanced rectal
cancer. J Surg Oncol. 109:478–482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hermann RM, Rave-Fränk M and Pradier O:
Combining radiation with oxaliplatin: A review of experimental
results. Cancer Radiother. 12:61–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin LK and Bekaii-Saab T: Optimizing
neoadjuvant therapy for rectal cancer with oxaliplatin. J Natl
Compr Canc Netw. 11:298–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chitapanarux I, Chitapanarux T,
Tharavichitkul E, Mayurasakorn S, Siriwittayakorn P, Yamada S and
Lorvidhaya V: A phase II study of oxaliplatin with 5-FU/folinic
acid and concomitant radiotherapy as a preoperative treatment in
patients with locally advanced rectal cancer. Biomed Imaging Interv
J. 7:e252011.doi: 10.2349/biij.7.4.e25. PubMed/NCBI
|
|
24
|
Chao JY, Wang HM, Chiang FF, Lin JC, Chang
CF, Lin JF and Yeh HL: Preoperative chemoradiotherapy with
oxaliplatin and tegafur-uracil in locally advanced rectal cancer:
Pathologic complete response rate and preliminary results of
overall and disease-free survival in a single institute in Taiwan.
J Chin Med Assoc. 77:128–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pucciarelli S, Urso E, DeSalvo GL, Aschele
C, Friso ML, Rugge M, Toppan P, Bruttocao A, Fabris G, Ferraro B,
et al: 5-fluorouracil and weekly oxaliplatin combined with
radiotherapy for locally advanced rectal cancer: Surgical
complications and long-term results. Arch Med Res. 37:860–865.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Cao C, Zhu Y, Li D, Feng H, Luo J,
Tang Z, Liu P, Lu K, Ju H and Zhang N: Preoperative
chemoradiotherapy with 5-fluorouracil and oxaliplatin for locally
advanced rectal cancer: Long-term results of a phase II trial. Med
Oncol. 32:702015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rödel C, Graeven U, Fietkau R, Hohenberger
W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA,
Lang-Welzenbach M, et al: Oxaliplatin added to fluorouracil-based
preoperative chemoradiotherapy and postoperative chemotherapy of
locally advanced rectal cancer (the German CAO/ARO/AIO-04 study):
Final results of the multicentre, open-label, randomised, phase 3
trial. Lancet Oncol. 16:979–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aschele C, Cionini L, Lonardi S, Pinto C,
Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti
P, et al: Primary tumor response to preoperative chemoradiation
with or without oxaliplatin in locally advanced rectal cancer:
Pathologic results of the STAR-01 randomized phase III trial. J
Clin Oncol. 29:2773–2780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gérard JP, Azria D, Gourgou-Bourgade S,
Martel-Lafay I, Hennequin C, Etienne PL, Vendrely V, François E, de
La Roche G, Bouché O, et al: Clinical outcome of the ACCORD 12/0405
PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol.
30:4558–4565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wong SJ, Moughan J, Meropol NJ, Anne PR,
Kachnic LA, Rashid A, Watson JC, Mitchell EP, Pollock J, Lee RJ, et
al: Efficacy endpoints of radiation therapy group protocol 0247: A
randomized, phase 2 study of neoadjuvant radiation therapy plus
concurrent capecitabine and irinotecan or capecitabine and
oxaliplatin for patients with locally advanced rectal cancer. Int J
Radiat Oncol Biol Phys. 91:116–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Allegra CJ, Yothers G, O'Connell MJ, Beart
RW, Wozniak TF, Pitot HC, Shields AF, Landry JC, Ryan DP, Arora A,
et al: Neoadjuvant 5-FU or capecitabine plus radiation with or
without oxaliplatin in rectal cancer patients: A phase III
randomized clinical trial. J Natl Cancer Inst. 107:djv2482015.doi:
10.1093/jnci/djv248. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gérard JP, Azria D, Gourgou-Bourgade S,
Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E,
de La Roche G, Bouché O, et al: Comparison of two neoadjuvant
chemoradiotherapy regimens for locally advanced rectal cancer:
Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin
Oncol. 28:1638–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grothey A: A comparison of XELOX with
FOLFOX-4 as first-line treatment for metastatic colorectal cancer.
Nat Clin Pract Oncol. 6:10–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hochster HS, Hart LL, Ramanathan RK,
Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr
Y, Saif MW, et al: Safety and efficacy of oxaliplatin and
fluoropyrimidine regimens with or without bevacizumab as first-line
treatment of metastatic colorectal cancer: Results of the TREE
study. J Clin Oncol. 26:3523–3529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ,
Park YS, Park JO, Kim SY, Kim TY, Kim JH, et al: Oxaliplatin,
fluorouracil, and leucovorin versus fluorouracil and leucovorin as
adjuvant chemotherapy for locally advanced rectal cancer after
preoperative chemoradiotherapy (ADORE): An open-label, multicentre,
phase 2, randomised controlled trial. Lancet Oncol. 15:1245–1253.
2014. View Article : Google Scholar : PubMed/NCBI
|