Introduction
Although there has been a decrease in the mortality
rate due to improvements in clinical medicine, lung cancer has
caused >1,000,000 global mortalities annually since 2008
(1). In clinical practice, lung
cancer is divided into small cell lung carcinoma and non-small cell
lung carcinoma and the latter includes squamous cell carcinoma,
adenocarcinoma and large-cell carcinoma, according to the tumor
histological type (2). Over the
previous decade, the importance of acquired genetic or epigenetic
changes has been recognized in the development of lung malignancy,
in addition to other factors, including smoking (3). A previous study reported that a lung
cancer tumor suppressor region is typically deleted in premalignant
chromosomal aberrations that are associated with the development of
lung cancer (4). In the early stages
of lung cancer, chromosomal deletions frequently occur in tumor
suppressor genes (5,6).
RNA-binding motif (RBM) genes are ubiquitous genes
that encode RNA-binding proteins, which are a group of regulatory
proteins that interact with RNA (7).
RBM proteins are associated with a number of cellular activities,
including alternative splicing and RNA degradation (8–10). The
mutation of RNA-binding motif genes has been associated with cancer
development due to their ability to function in the regulation of
proteins at a post-transcriptional level (11–14).
Previous studies have established that RBM proteins may promote
cell apoptosis through a number of signaling pathways (15,16) and
that RBMs are widely dysregulated in numerous types of cancer
(17). The RNA-binding motif 10
(RBM10) gene possesses >50% conservation with the RNA binding
motif 5 (RBM5) gene, which may have a role in the proliferation of
cancer cells (18). RBM10 has also
been implicated to affect the proliferation of cancer cells
(19). In another previous study, a
variant of RBM10 was identified to be significantly associated with
the expression of wild type p53, which is a gene that serves an
important role in the caspase apoptotic signaling pathway (19). However, to the best of our knowledge,
the underlying mechanism of RBM10 in lung cancer has yet to be
elucidated.
In the present study, the expression levels of RBM10
in tissues from patients with lung cancer were investigated and it
reduced expression of the RBM10 gene in lung cancer cells was
detected. The pcDNA3.1 (pcDNA)-RBM10 vector was transfected into
the A549 human lung cancer line and used to investigate the
expression levels of pro-apoptotic proteins, including cleaved
caspase-3, caspase-9, poly(ADP-ribose) polymerase (PARP) proteins
and the anti-apoptotic protein B-cell lymphoma (Bcl)-2. The in
vivo anticancer effect of RBM10 was evaluated using a xenograft
Bagg albino coat (BALB/c) nude mouse model treated with
Salmonella enterica subspecies enterica serovar
Typhimurium containing an RBM10 or control DNA vector. The present
study may provide novel insights for the use of RBM10 in lung
cancer diagnosis and treatment.
Patients and methods
Patients, cells and tissues
The present study was approved by the Institutional
Review Board of the Guangxi University of Chinese Medicine
(Nanning, China). The lung cancer tissues were obtained from 25
patients diagnosed with primary lung adenocarcinoma by surgical
resection. The paired normal lung tissues from the disease-free
margins were also obtained from the same patients as controls. This
study was concordant with the 1964 Helsinki declaration as well as
its later amendments or comparable ethical standards. Formal
written consent was obtained from each patient involved in the
current study. Patient tissue samples were stored in liquid
nitrogen until RNA or protein was extracted. The A549 cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cultured in RPMI 1640 (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in an incubator containing 5%
CO2.
Transfection
The cells were transfected with pcDNA3.1 or
pcDNA3.1-RBM10 plasmids using Lipofectamine® 2000
reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. Briefly, 2 µg DNA
(pcDNA3.1 vector control or pcDNA3.1-RBM10) or 5 µl
Lipofectamine® 2000 was diluted in 200 µl Opti-MEM
(Thermo Fisher Scientific, Inc.) and incubated at room temperature
for 5 min. the diluted DNA and Lipofectamine® were
combined and incubated for another 20 min at room temperature prior
to their addition to 6-well plates seeded with 4×105
cells/well. Cells were maintained in 37°C in an incubator
containing 5% CO2 for 48 h prior to additional
experiments.
Proliferation assay
An MTT assay was used to assess the proliferation of
cells according to the manufacturer's protocol (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Briefly, the cells that were
transfected with plasmid DNA were subsequently seeded in 96 well
plates (5×104 cells/well). A total of 20 µl of 5 mg/ml
MTT in PBS was added to each well and incubated at 37°C for 4 h.
The cells were treated with 150 µl DMSO (Merck Millipore,
Darmstadt, Germany) and agitated at room temperature for 5 min. A
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
was used to measure the absorbance of cells in each well at a
wavelength of 570 nm.
Reverse transcription-polymerase chain
reaction (RT-PCR)
TRIzol (Thermo Fisher Scientific, Inc., USA) was
used to extract RNA following the manufacturer's protocol. For
reverse transcription, the components in a total volume of 10 µl
were used as follows: 3 µg total RNA; 10 mM deoxyribonucleotide
triphosphate; 0.5 µg oligo deoxythymine; 20 U RNasin®;
200 U Maloney murine leukemia virus reverse transcriptase (Thermo
Fisher Scientific, Inc., USA). The primer sequences were as
follows: RBM10 sense, 5′-GCACGACTATAGGCATGACAT-3′; antisense,
5′-AGTCAAACTTGTCTGCTCCA-3′; GAPDH sense, 5′-GAAGGTGAAGGTCGGAGTC3′;
antisense, 5′-GAAGATGGTGATGGGATTTC-3′. PCR was performed with 25–30
cycles as follows: 95°C for 30 sec; 55°C for 30 sec; 72°C for 1
min. Densitometry analysis was performed with ImageMaster VDS-CL
Image Master 1.0.3.7 software (GE Healthcare Bio-Sciences,
Pittsburg, PA, USA).
Protein extraction and western blot
analysis
The extraction of proteins from lung adenocarcinoma
tissue samples and A549 cells was performed as reported previously
(20,21). The protein concentrations were
determined using the BCA (Thermo Fisher Scientific, Inc., USA)
method according to the manufacturer's protocol. A total of 20 µg
of each extracted proteins were separated by 10% SDS-PAGE and
transferred onto a PVDF membrane (Merck Millipore). The membrane
was blocked with 5% bovine serum albumin (BSA; Santa Cruz
Biotechnology, Dallas, TX, USA) in TBS buffer with 0.01% Tween-20
(TBST) for 1 h at room temperature, followed by incubation with
primary antibodies. The following primary antibodies were used at
1:500 dilution in 5% BSA and incubated at 4°C overnight: Rabbit
anti-human RBM10 (Abcam, Cambridge, MA, USA; cat. no. ab26046);
Bcl-2; PARP (Cell Signaling Technology, Inc.; Danvers, MA, USA;
cat. no. 9532); cleaved-PARP (Cell Signaling Technology, Inc.; cat.
no. 5625); caspase-3 (Cell Signaling Technology, Inc.; cat. no.
9662); cleaved caspase-3 (Cell Signaling Technology, Inc.; cat. no.
9661); β-actin antibodies (Abcam; cat. no. ab8227). Membranes were
then incubated with the secondary antibody Goat anti-rabbit
immunoglobulin G-horseradish peroxidase (dilution, 1:100,000 in
TBST; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at
room temperature. The protein bands were analyzed with SuperSignal
West Pico Chemiluminescent Substrate (Pierce; Thermo Fisher
Scientific, Inc.). The intensity of the protein bands was analyzed
with Quantity One software (v1709600, Bio-Rad Laboratories,
Inc.).
Flow cytometry
Flow cytometry was used to analyze the number of
apoptotic cells following transfection with the described plasmid
DNA vectors. Briefly, 1×106 A549 cells were harvested
and resuspended in PBS. The total of 5 µl Annexin V (1 µg/ml)
(Beckman Coulter Inc., Brea, CA, USA) was added to cells and
incubated at room temperature for 15 min. Subsequently, propidium
iodide (1 µg/ml) was added to the cells for 5 min at room
temperature. All staining incubation steps were performed in
dark.
In vivo tumor growth
A total of 16 BALB/c nude mice (Shanghai Laboratory
Animal Center, Chinese Academy of Sciences, Shanghai, China) were
used. All mice used were female, aged 4–6 weeks and weight >20 g
prior to initiation of experiments. Mice were randomly assigned to
experimental groups. All mice were kept up to 4 mice per cage and
maintained in the vivarium room of Guangxi University of Chinese
Medicine (Nanning, China) with free access to water and food. Mice
were injected with 3×105 A549 cells subcutaneously.
Tumor diameters were measured with digital calipers starting from
the 8th day following injection. The tumor bearing cells were
treated with pcDNA as the control group or pcDNA-RBM10. The
plasmids were carried by an attenuated Salmonella enterica
subsp. enterica, serovar Typhimurium strain using
electroporation as reported previously (22,23).
Briefly, S. enterica subsp. enterica, serovar
Typhimurium cells (1×108 colony-forming units per 50 µl)
transfected with pcDNA or pcDNA-RBM10 plasmids were injected into
mice via the tail vein on day 7, 28 and 35 following subcutaneous
injection. The diameter of the tumors was measured every 3 days for
42 days with digital calipers. The weight of tumors was assessed
following the sacrifice of the mice by gradual CO2
asphyxiation. A secondary physical method, cervical dislocation,
was used to assure death. All the animal experiments were performed
under the regulation of the Animal Care Committee in Guangxi
University of Chinese Medicine.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA,
USA). The specific test used for each experiment to determine
significance is indicated in the fig. legends. Data are
representative of results obtained in at least 3 independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of RBM10 are
significantly reduced in lung cancer tissue
The expression levels of RBM10 mRNA and protein in
lung cancer tissues was analyzed using RT-PCR and western blot
analysis in 5 pairs of lung tumor tissues and non-tumor tissues.
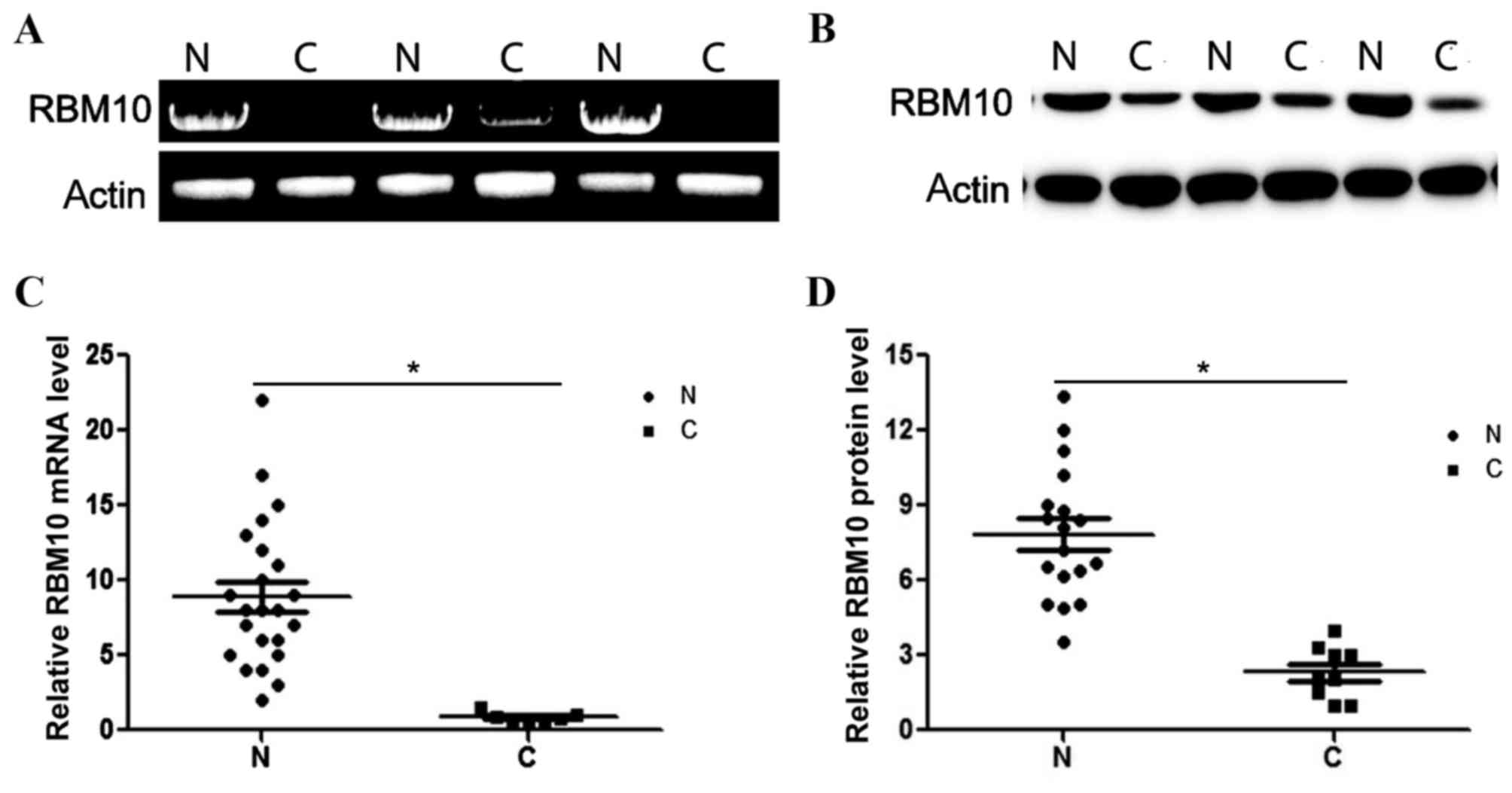
Fig. 1A and B present the expression
levels of RBM10 mRNA and protein in the analyzed control and tumor
tissues. The relative expression levels of RBM10 mRNA and protein
in tumor and control tissues were determined, indicating that the
levels of RBM10 mRNA and protein were reduced in the tumor tissues
(P<0.05; Fig. 1C and D). The
relative expression levels of RBM10 mRNA in the tumor tissues
demonstrated a 4.3-fold decrease compared with the RBM10 mRNA
levels in the normal tissues (P<0.05; Fig. 1C). The results of the western blot
analysis indicated that the level of RBM10 protein was decreased by
3.2-fold in the tumor tissues compared with the control tissues
(P<0.05; Fig. 1D).
A549 cells transfected with
pcDNA-RBM10 exhibit reduced cell proliferation and increased levels
of apoptosis
To further investigate the role of RBM10 in the
proliferation of tumor cells, pcDNA or pcDNA-RBM10 were transfected
into A549 cells for 48 h prior to evaluating the expression levels
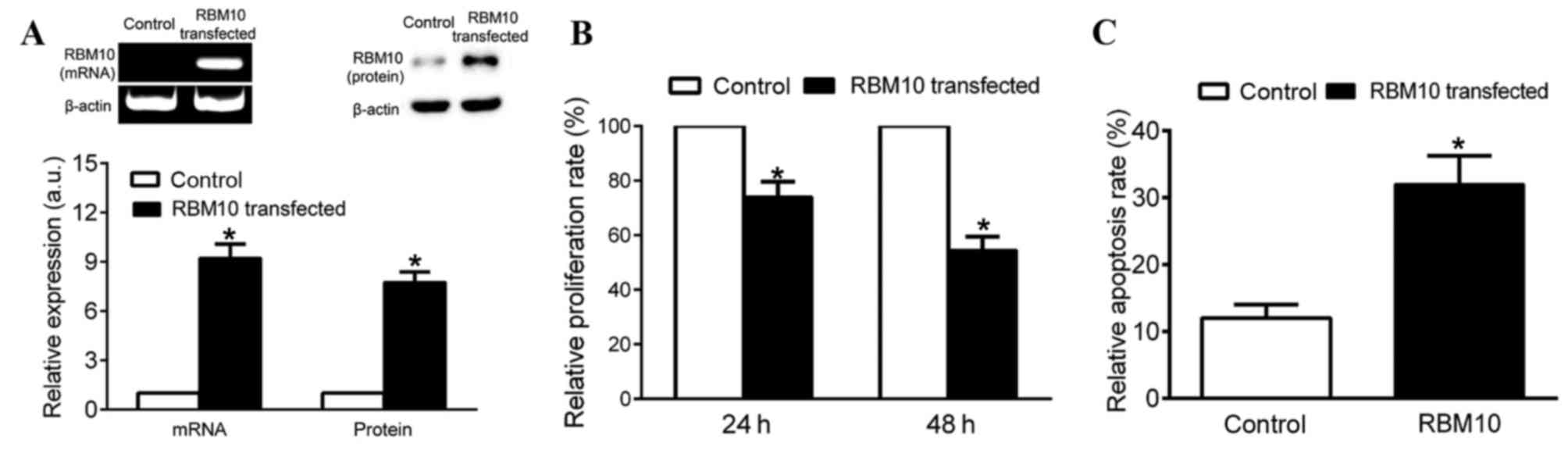
of RBM10. Fig. 2A presents the
expression levels of RBM10 mRNA and protein in cells transfected
with the control vector pcDNA or pcDNA-RBM10 and the increased
expression levels of RBM10 mRNA and protein indicated the
transfection and expression of the pcDNA vector. The expression
levels of RBM10 mRNA and protein in cells effective transfected
with pcDNA-RBM10 was significantly increased compared with the
control cells (P<0.05; Fig. 2A).
The proliferation of A549 cells transfected with pcDNA-RBM10 was
reduced compared with cells transfected with pcDNA (P<0.05;
Fig. 2B). The cells transfected with
pcDNA-RBM10 demonstrated an increased level of apoptosis compared
with cells transfected with pcDNA, which indicates that RBM10 may
serve an important role in inducing cancer cell apoptosis
(P<0.05; Fig. 2C).
RBM10 enhances cell apoptosis by
modulating the expression levels of apoptotic genes
The expression levels of apoptosis associated genes,
including Bcl-2, caspase-3, caspase-9 and PARP, were detected in
cells transfected with pcDNA or pcDNA-RBM10. Bcl-2 is one of the
key members of the Bcl-2 family of regulator proteins that are
involved in the regulation of cell apoptosis (20). The expression levels of Bcl-2 protein
were decreased significantly in cells transfected with RBM10
(P<0.05; Fig. 3A and B). Cleaved
caspase-3, cleaved-9 and PARP expression levels were significantly
increased in cells transfected with pcDNA-RBM10 compared with cells
transfected with pcDNA (P<0.05; Fig.
3A and B). These results indicate that RBM10 may promote
apoptosis via inducing the expression of caspase-3, caspase-9 and
PARP and decreasing Bcl-2 expression levels.
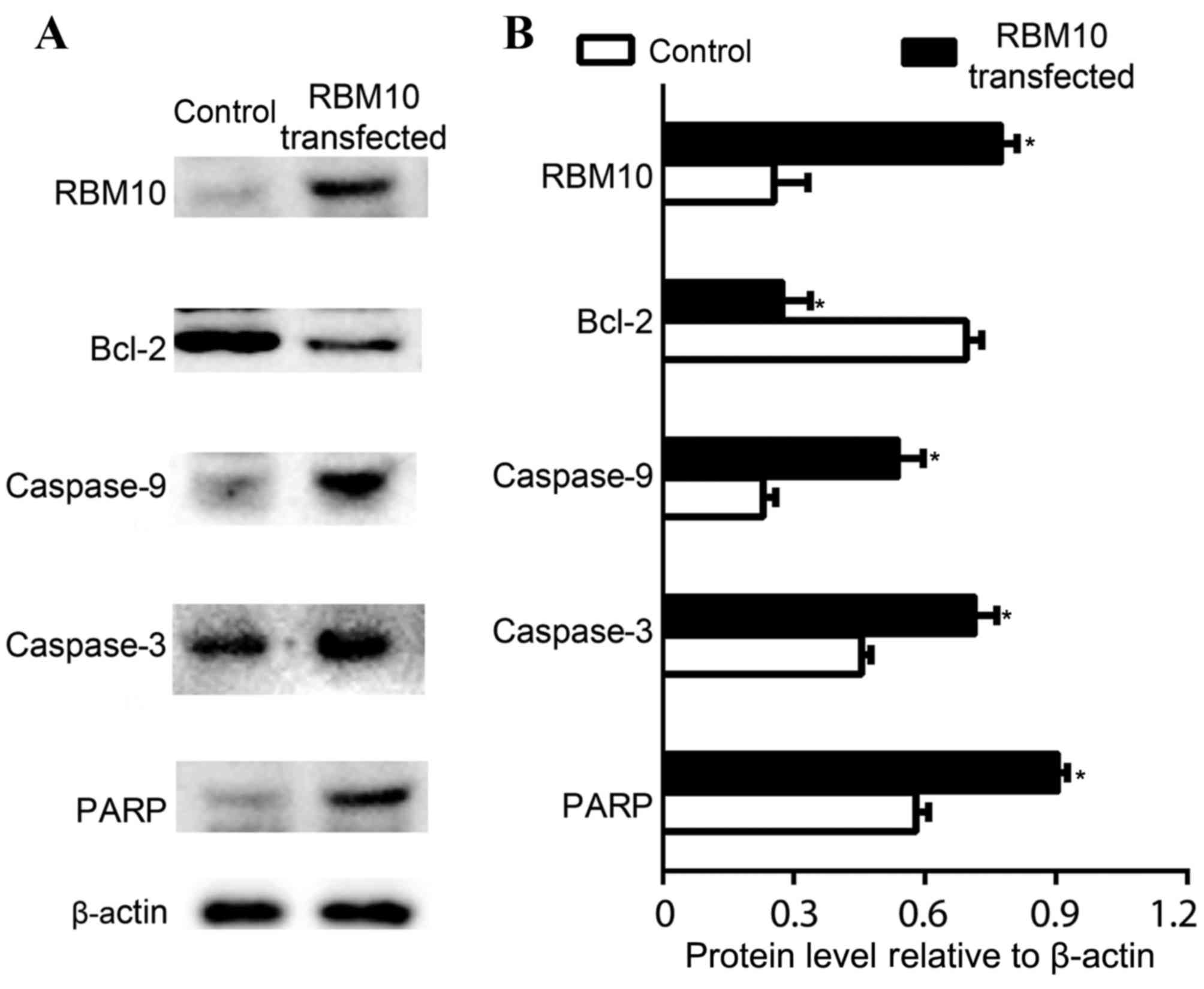 | Figure 3.Evaluation of apoptosis-associated
genes in cells transfected with pcDNA3.1 and pcDNA3.1-RBM10. (A)
mRNA expression levels of RBM10, Bcl-2, cleaved caspase-9, cleaved
caspase-3, PARP in CTRL cells and cells transfected with RBM10. The
transfection of RBM10 was validated by the over expression of RBM10
gene. (B) Quantitative analysis of the expression of
apoptosis-associated genes, including Bcl-2, caspase-9, caspase-3
and PARP. The expression levels of β-actin were used as a control.
Cleaved caspase-9 and cleaved caspase-3 are labeled as caspase-9
and caspase-3. Data are presented as means ± standard deviation
from three independent experiments. *P<0.05. RBM10, RNA-binding
motif protein 10; Bcl-2, B cell lymphoma 2; PARP, poly (ADP-ribose)
polymerase. |
RBM10 decreases tumor size in
tumor-bearing mice
The in vivo antitumor effect of RBM10 in mice
was investigated by transplanting tumor cells into mice. The mice
were then injected with S. enterica subsp. enterica,
serovar Typhimurium carrying RBM10 at day 7, 28 and 35 following
the transplantation of the tumor cells, and the tumor size was
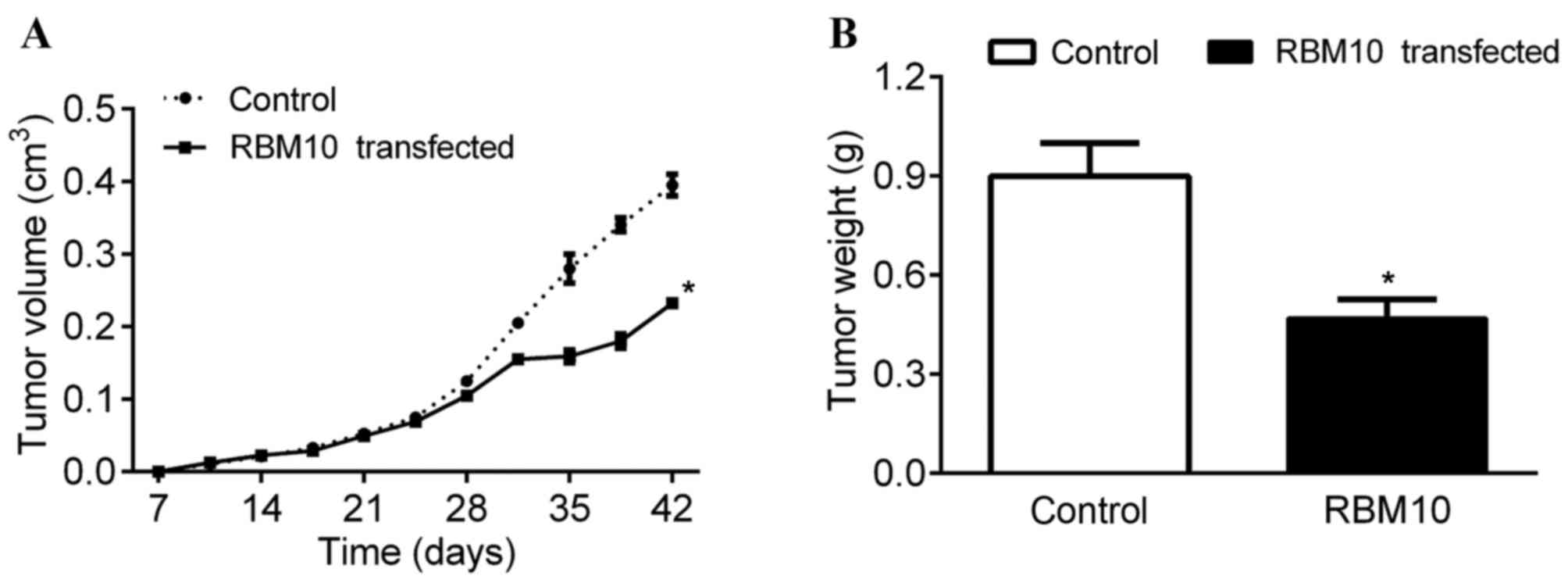
monitored. The present study identified that the two groups of mice
possessed tumors of a similar size until day 24 (Fig. 4A). However, the tumors from the mice
treated with S. enterica subsp. enterica, serovar
Typhimurium carrying pcDNA-RBM10 were smaller in size after day 24
compared with the tumors from the control mice (P<0.05; Fig. 4A). In addition, the mice treated with
S. enterica subsp. enterica, serovar Typhimurium
carrying pcDNA-RBM10 presented with tumors of a lighter weight
compared with the tumors from control mice that were treated with
the control pcDNA vector (P<0.05; Fig.
4B). These results indicate that the in vivo
accumulation and expression levels of RBM10 may reduce the growth
rate of the tumors.
Discussion
Lung cancer is one of the predominant causes of
cancer-associated mortality globally at present (21). Although previous studies have focused
on the diagnosis and treatment of lung cancer, the 5-year survival
rate for patients requires improvement (2,22,23). Therefore, further studies are required
into the underlying molecular mechanisms of lung cancer using in
vitro cellular and in vivo animal models. Previous
studies (24,25) have demonstrated that RBM10 serves an
important role in promoting cell apoptosis as well as being an RNA
binding protein that is involved in the regulation of
co-transcriptional modification of pre-mRNA (19). Certain studies have established a
positive correlation between the expression of RBM10 and
pro-apoptotic factors in breast tumor samples (19,24).
Another previous study demonstrated that there was co-expression of
RBM10 and caspase-3 in breast cancer specimens (24). Notably, RBM10 and RBM5 are paralogues
and RBM10 shares ~50% identity with RBM5, which is important as
RBM5 has been established to be involved in cancer suppression
(19). This indicates that RBM5 and
RBM10 may possess similar abilities or overlapping functions. The
underlying mechanisms of RBM5 are yet to be elucidated and there
are a number of previous studies (24,25)
investigating the role of RBM10.
In the current study, the expression of RBM10 mRNA
and protein was examined in clinical lung cancer tissues and this
indicated that the expression levels of RBM10 mRNA and protein are
significantly decreased in the tumor tissues (Fig. 1A and B). These results indicate that
RBM10 mRNA may serve an important role in lung cancer. To further
evaluate the suppressive role of RBM10 in cancer development,
RBM10-containing plasmid DNA was transfected into A549 cells and
this demonstrated that the proliferation and growth rate of A549
cells were significantly inhibited when RBM10 was overexpressed
(Fig. 2A). Additionally, there was an
inhibition of proliferation and an increase in the levels of
apoptosis in cells transfected with pcDNA-RBM10 (Fig. 2B and C).
To investigate the underlying molecular mechanisms
of the antitumor effect of RBM10, the expression levels of
antitumor associated genes were analyzed in cells transfected with
RBM10. The current study effectively overexpressed RBM10 by
transfecting lung cancer cells with pcDNA-RBM10 (Fig. 3A). Subsequently, the expression levels
of Bcl-2 were investigated. The abnormal expression of Bcl-2 has
been associated with various forms of cancer, including lung cancer
(26). The present study demonstrated
that the overexpression of RBM10 in A549 cells reduced the
expression levels of Bcl-2 (Fig. 3A)
and this was also associated with an enhanced level of apoptosis in
the cancer cells (Fig. 2C). These
results are concordant with previous studies that demonstrate the
reduction of Bcl-2 may facilitate the apoptosis of small-cell lung
cancer cells (27). The expression
levels of caspase-9, caspase-3 and PARP were analyzed in A549 cells
with endogenous or overexpressed levels of RBM10 mRNA (Fig. 3). The current study demonstrated an
increased expression of caspase-9, caspase-3 and PARP in cells upon
overexpression of RBM10 mRNA. This is concordant with a previous
study that demonstrates that the decrease in expression levels of
RBM10 increased the expression of caspase-3 and caspase-9 (28). Concordant with previous studies, the
results in the current study indicate that the downregulation of
Bcl-2 and upregulation of cleaved caspase-3, caspase-9 and PARP are
associated with an increase in the level of apoptosis in cancer
cells (6,29).
Based on the aforementioned clinical examination and
in vitro studies (24,25), this study focused on the in
vivo anticancer effect of the overexpression of RBM10 by
treating mice with attenuated Salmonella carrying pcDNA3.1
(control group) or pcDNA3.1-RBM10. Salmonella is a
facultative anaerobe that is able to grow in aerobic and anaerobic
conditions and, therefore, may be used to deliver numerous types of
therapeutic agents (30). This
technique has been used to provide tumor-targeting bacteria that
are able to deliver genes that encode pro-drug-converting enzymes,
angiogenic inhibitors or cytokines (31–33).
Primarily, this has been investigated for use in clinical trials
for gene therapy (34) and the
current study demonstrates its use as a carrier for delivering the
RBM10 gene into mice. The present in vivo study identified
that mice treated with pcDNA and pcDNA-RBM10 possess tumors of a
similar size until day 24 following tumor implantation. After day
24, mice treated with pcDNA-RBM10 exhibited a reduced tumor growth
rate and a reduction in tumor weight (Fig. 4A and B). However, the current study
did not assess the in vivo transfection efficiency of the
vector or the optimal dosage for in vivo use and this
remains to be investigated.
In conclusion, the current study established that
tumor tissues from patients with lung cancer have decreased
expression levels of RBM10 mRNA and protein. To further investigate
the role of RBM10 mRNA in lung cancer, the in vitro
anticancer effects of RBM10 mRNA were evaluated by transfecting the
lung cancer cells with a vector containing the RBM10 gene. The
current in vitro study demonstrated that cells transfected
with RBM10 DNA vector have a reduction in cell proliferation and an
increased level of apoptosis. The present study also established
that cells transfected with RBM10 have decreased expression of
Bcl-2 and increased expression of caspase-9, caspase-3 and PARP,
which are genes that are established to enhance cancer cell
apoptosis (35–37). Additionally, the present study
identified that tumor bearing mice that were treated with
Salmonella carrying pcDNA3.1-RBM10 presented with a
decreased tumor growth rate and reduced tumor weight compared with
control mice, which suggests that RBM10 may be involved in cancer
suppression. The current study may provide a novel marker for the
diagnosis of lung cancer and a potential chemotherapeutic
treatment.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Decroisette C, Galerneau LM, Hominal S and
Chouaid C: Epidemiology, management and cost of bone metastases
from lung cancer. Rev Mal Respir. 30:309–315. 2013.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romeo MS, Sokolova IA, Morrison LE, Zeng
C, Barón AE, Hirsch FR, Miller YE, Franklin WA and Varella-Garcia
M: Chromosomal abnormalities in non-small cell lung carcinomas and
in bronchial epithelia of high-risk smokers detected by
multi-target interphase fluorescence in situ hybridization. J Mol
Diagn. 5:103–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Massion PP and Carbone DP: The molecular
basis of lung cancer: Molecular abnormalities and therapeutic
implications. Respir Res. 4:122003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masuda A and Takahashi T: Chromosome
instability in human lung cancers: Possible underlying mechanisms
and potential consequences in the pathogenesis. Oncogene.
21:6884–6897. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glisovic T, Bachorik JL, Yong J and
Dreyfuss G: RNA-binding proteins and post-transcriptional gene
regulation. FEBS Lett. 582:1977–1986. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sutherland LC, Rintala-Maki ND, White RD
and Morin CD: RNA binding motif (RBM) proteins: A novel family of
apoptosis modulators? J Cell Biochem. 94:5–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang JY, Chan EK, Peng XX and Tan EM: A
novel cytoplasmic protein with RNA-binding motifs is an autoantigen
in human hepatocellular carcinoma. J Exp Med. 189:1101–1110. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xin H, Deng K and Fu M:
Post-transcriptional gene regulation by RNA-binding proteins in
vascular endothelial dysfunction. Sci China Life Sci. 57:836–844.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerstberger S, Hafner M, Ascano M and
Tuschl T: Evolutionary conservation and expression of human
RNA-binding proteins and their role in human genetic disease. Adv
Exp Med Biol. 825:1–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim MY, Hur J and Jeong S: Emerging roles
of RNA and RNA-binding protein network in cancer cells. BMB Rep.
42:125–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lukong KE, Chang KW, Khandjian EW and
Richard S: RNA-binding proteins in human genetic disease. Trends
Genet. 24:416–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Musunuru K: Cell-specific RNA-binding
proteins in human disease. Trends Cardiovasc Med. 13:188–195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar R, Vadlamudi RK and Adam L:
Apoptosis in mammary gland and cancer. Endocr Relat Cancer.
7:257–269. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motyl T, Gajkowska B, Zarzynska J,
Gajewska M and Lamparska-Przybysz M: Apoptosis and autophagy in
mammary gland remodeling and breast cancer chemotherapy. J Physiol
Pharmacol. 57 Suppl 7:S17–S32. 2006.
|
|
17
|
Kechavarzi B and Janga SC: Dissecting the
expression landscape of RNA-binding proteins in human cancers.
Genome Biol. 15:R142014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao C, Zhao L, Wang K, Xu W, Zhang J and
Yang B: The tumor suppressor gene RBM5 inhibits lung adenocarcinoma
cell growth and induces apoptosis. World J Surg Oncol. 10:1602012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martinez-Arribas F, Agudo D, Pollán M,
Gómez-Esquer F, Díaz-Gil G, Lucas R and Schneider J: Positive
correlation between the expression of X-chromosome RBM genes (RBMX,
RBM3, RBM10) and the proapoptotic Bax gene in human breast cancer.
J Cell Biochem. 97:1275–1282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tzifi F, Economopoulou C, Gourgiotis D,
Ardavanis A, Papageorgiou S and Scorilas A: The role of BCL2 family
of apoptosis regulator proteins in acute and chronic leukemias. Adv
Hematol. 2012:5243082012.PubMed/NCBI
|
|
21
|
Alberg AJ and Nonemaker J: Who is at high
risk for lung cancer? Population-level and individual-level
perspectives. Semin Respir Crit Care Med. 29:223–232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buyukcelik A, Yalcin B and Utkan G:
Multidisciplinary management of lung cancer. N Engl J Med.
350:2008–2010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin-Garabato E, Martinez-Arribas F,
Pollán M, Lucas AR, Sánchez J and Schneider J: The small variant of
the apoptosis-associated X-chromosome RBM10 gene is co-expressed
with caspase-3 in breast cancer. Cancer Genomics Proteomics.
5:169–173. 2008.PubMed/NCBI
|
|
25
|
Wang K, Bacon ML, Tessier JJ, Rintala-Maki
ND, Tang V and Sutherland LC: RBM10 modulates apoptosis and
influences TNF-α gene expression. J Cell Death. 5:1–19.
2012.PubMed/NCBI
|
|
26
|
Delbridge AR and Strasser A: The BCL-2
protein family, BH3-mimetics and cancer therapy. Cell Death Differ.
22:1071–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ziegler A, Luedke GH, Fabbro D, Altmann
KH, Stahel RA and Zangemeister-Wittke U: Induction of apoptosis in
small-cell lung cancer cells by an antisense oligodeoxynucleotide
targeting the Bcl-2 coding sequence. J Natl Cancer Inst.
89:1027–1036. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Glantz LA, Gilmore JH, Lieberman JA and
Jarskog LF: Apoptotic mechanisms and the synaptic pathology of
schizophrenia. Schizophr Res. 81:47–63. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pang H, Flinn R, Patsialou A, Wyckoff J,
Roussos ET, Wu H, Pozzuto M, Goswami S, Condeelis JS, Bresnick AR,
et al: Differential enhancement of breast cancer cell motility and
metastasis by helical and kinase domain mutations of class IA
phosphoinositide 3-kinase. Cancer Res. 69:8868–8876. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Camacho EM, Mesa-Pereira B, Medina C,
Flores A and Santero E: Engineering Salmonella as
intracellular factory for effective killing of tumour cells. Sci
Rep. 6:305912016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Fu GF, Fan YR, Liu WH, Liu XJ, Wang
JJ and Xu GX: Bifidobacterium adolescentis as a delivery system of
endostatin for cancer gene therapy: Selective inhibitor of
angiogenesis and hypoxic tumor growth. Cancer Gene Ther.
10:105–111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Low KB, Ittensohn M, Le T, Platt J, Sodi
S, Amoss M, Ash O, Carmichael E, Chakraborty A, Fischer J, et al:
Lipid A mutant Salmonella with suppressed virulence and
TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol.
17:37–41. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Theys J, Landuyt W, Nuyts S, Van Mellaert
L, van Oosterom A, Lambin P and Anné J: Specific targeting of
cytosine deaminase to solid tumors by engineered Clostridium
acetobutylicum. Cancer Gene Ther. 8:294–297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lerman MI and Minna JD: The 630-kb lung
cancer homozygous deletion region on human chromosome 3p21.3:
Identification and evaluation of the resident candidate tumor
suppressor genes. The international lung cancer chromosome 3p21.3
tumor suppressor gene consortium. Cancer Res. 60:6116–6133.
2000.PubMed/NCBI
|
|
35
|
Swanton E, Savory P, Cosulich S, Clarke P
and Woodman P: Bcl-2 regulates a caspase-3/caspase-2 apoptotic
cascade in cytosolic extracts. Oncogene. 18:1781–1787. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu GS and Ding Z: Caspase 9 is required
for p53-dependent apoptosis and chemosensitivity in a human ovarian
cancer cell line. Oncogene. 21:1–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|