Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of malignancy and is the third leading cause of
cancer-associated mortality worldwide (1). There are ~500,000 cases diagnosed as HCC
each year, which represents >5% of all cancer cases (2). The incidence of HCC has a considerable
geographic variation, with the majority of the cases occurring in
developing countries, including Southeast Asia and sub-Saharan
Africa (3). However, in recent
decades, epidemiological studies have also indicated a rising trend
in the incidence and mortality of HCC in Western countries
(4). Despite substantial and
accelerated studies focused on the treatment of HCC, the five-year
survival rate of advanced HCC remains poor (5–7). Thus, the
development of novel and efficient therapy strategies for HCC are
warranted.
Long non-coding RNA (lncRNA) is a group of noncoding
RNAs that are >200 nucleotides in length, and serve regulatory
roles in different physiological processes, including growth,
differentiation, senescence and apoptosis (8). Recently, increasing evidence suggest
that the dysregulation of lncRNAs are involved in diverse
pathological conditions, in particular in various types of cancer
(9–11). BRAF-activated non-coding RNA (BANCR),
a 693-bp lncRNA on chromosome 9, was first identified to be
overexpressed in melanoma cells and serve as a regulator in the
migration of melanoma cells (12,13).
Reportedly, BANCR is abnormally expressed in gastric tumor
(14), papillary thyroid carcinoma
(15), colorectal cancer (16), retinoblastoma (17), papillary thyroid carcinoma (18) and non-small cell lung cancer (19). Despite the majority of these studies
indicating an oncogenic property of BANCR, Sun et al
(19) and Shi et al (16) reported tumor suppressive activity of
BANCR in non-small cell lung cancer, and colorectal carcinoma,
respectively.
In the present study, the expression of BANCR in
Huh7 cells was downregulated using short hairpin (sh)RNA, and the
effect of BANCR on proliferation, apoptosis, migration and invasion
of HCC cells was investigated in vitro. Furthermore, the
underlying mechanisms of BANCR in HCC were explored.
Materials and methods
Cell lines, culture condition and
treatment
The human hepatocellular carcinoma cell line Huh7
was purchased from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). Human normal liver L-02
cells were purchased from Zhongqiaoxinzhou Biotechnology Co., Ltd
(Shanghai, China). Cells were maintained in Dulbecco's modified
Eagle's medium or RPMI-1640 (both from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and supplemented with 10% fetal
bovine serum (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
in a humidified atmosphere of 5% CO2 at 37°C. Cells was
transfected with BANCR shRNA or negative control (NC) shRNA using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The target sequences were
as follows: BANCR shRNA, 5′-GGAGTGGCGACTATAGCAA-3′ and NC shRNA,
5′-TTCTCCGAACGTGTCACGT-3′.
Reverse transcription-quantitative
polymerase chain reaction (PCR)
Total RNA was extracted using the RNApure total RNA
extraction kit and reverse transcribed to cDNA using Super M-MLV
reverse transcriptase at 25°C for 10 min followed by 42°C for 50
min. (both from Bioteke Corporation, Beijing, China). SYBR
Green-based PCR was performed using an Exicycler™ 96 real-time PCR
system (Bioneer Corporation, Daejeon, Korea) with 2X Power Taq PCR
Master mix (Bioteke Corporation), the thermocycling conditions was
as follows: 95°C for 10 min followed by 40 cycles of 95°C for 10
sec, 60°C for 20 sec and 72°C for 30 sec. The specific primers for
BANCR were as follows: Forward, 5′-TCAGAAGAAACAAGAGGGAGG-3′ and
reverse, 5′-AGCAGCATGAACTGGGAAAC-3′. The specific primers of
β-actin were as follows: Forward, 5′-CTTAGTTGCGTTACACCCTTTCTTG-3′
and reverse, 5′-CTGTCACCTTCACCGTTCCAGTTT-3′. Each experiment was
repeated for three times. The relative BANCR mRNA level was
calculated using the 2−ΔΔCq method (20) and normalized to β-actin
expression.
Western blot analysis
Protein from each group was extracted using the
Whole Cell Lysis kit (Wanleibio, Shenyang, China). A total of 40 µg
protein per lane were electrophoresed using 8, 10 or 13% SDS-PAGE
and subsequently electrotransfered to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). After blocking in 5%
non-fat milk at room temperature for 1 h, the membranes were
incubated at 4°C overnight with the following primary antibodies:
Bcl-2 (1:400 dilution, cat. no. BA0412), Bax (1:400 dilution, cat.
no. BA0315) (both from Wuhan Boster Biological Technology, Ltd.,
Wuhan, China), cleaved caspase-3 (1:1,000 dilution; cat. no.
ab2302, Abcam, Cambridge, MA, USA), phosphorylated
(p)-extracellular signal-regulated kinase (p-ERK; 1:500 dilution;
cat. no. bs-1522R), ERK (1:500 dilution; cat. no. bs-2637R),
p-janus kinase (p-JNK; 1:500 dilution; cat. no. bs-1640R), JNK
(1:500 dilution; cat. no. bs-10562R), p-P38 (1:500 dilution; cat.
no. bs-5477R), P38 (1:500 dilution; cat. no. bs-0637R) (all from
BIOSS, Beijing, China), p-MAPK/ERK kinase (p-MEK; 1:200 dilution;
cat. no. sc-271914), MEK (1:200 dilution; cat. no. sc-219), and
β-actin (1:1,000 dilution; cat. no. sc-47778) (all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Subsequently, the membranes
were incubated with horseradish peroxidase-conjugated goat anti
rabbit (cat. no. WLA023) or goat anti mouse (cat. no. WLA024)
secondary antibodies (Wanleibio) at 37°C for 45 min. The specific
bands were visualized with the Immu-Plus ECL system (Wanleibio),
and the protein levels were semi-quantified using densitometry
analysis that performed by Gel-Pro Analyzer Version 3.0 (Media
Cybernetics, Silver Spring, MD, USA). β-actin served as an internal
control.
Cell viability assay
Cells were seeded in a 96-well plate at a density of
3×103 cells/well and cultivated at 37°C for 24 h.
Subsequently, cells were transfected with BANCR shRNA or NC shRNA,
and further incubated for 24, 48, 72 or 96 h. Cells were then
incubated with 0.5 mg/ml MTT for 4 h, followed by the addition of
200 µl dimethyl sulfoxide. Lastly, the absorbance at 490 nm was
measured using the ELX-800 microplate spectrophotometer (Biotek
Instruments, Inc., Winooski, VT, USA). For colony formation, cells
were seeded in a 35-mm culture dish (300 cells/dish), and
maintained in culture media supplemented with 10% fetal bovine
serum for two weeks. The cell clones were fixed with 4%
paraformaldehyde at room temperature for 20 min, then stained with
Wright-Giemsa dye at room temperature for 5 min. Colony formation
rate was calculated as follows: (colony number/seeding number)
×100%.
Flow cytometry
Cells were collected 48 h post-transfection for cell
cycle analysis, cells were incubated with 25 µl propidium iodide
(Beyotime Institute of Biotechnology, Haimen, China) for 30 min at
37°C in the dark. For apoptosis detection, cells were incubated
with 5 µl Annexin V-FITC and 5 µl propidium iodide following the
manufacturer's protocol (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). Following staining, the cell cycle or apoptosis status was
analyzed using flow cytometry (BD Accuri C6; BD Biosciences,
Franklin Lakes, NJ, USA).
Hoechst staining
Cells were seeded on 12-well plates at a density of
5×104 cells/well, transfected with BANCR shRNA or NC
shRNA. A total of 48 h after transfection, cells were fixed and
stained with 2 µg/ml Hoeschst staining solution (Beyotime Institute
of Biotechnology) according to the manufacturer's protocol.
Apoptotic nuclei were observed under a fluorescent microscope
(BX53; Olympus Corporation, Tokyo, Japan).
Wound healing assay
Cells were allowed to grow until 80–90% confluence
was achieved on 6-well plates, then transfected with BANCR shRNA or
NC shRNA. A total of 48 h after transfection, cells were incubated
with 1 µg/ml mitomycin C (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 1 h, and a wound was created with a 200-µl pipette
tip. Subsequently, cells were cultured in a humidified atmosphere
of 5% CO2 at 37°C for 24 h. The migration distance was
measured under an inverted phase contrast microscope (AE31; Motic
Instruments, Richmond, BC, Canada).
Transwell assays
Transwell chambers (Corning Incorporated, Corning,
NY, USA) were pre-coated with 40 µl Matrigel (BD Biosciences) and
placed on to 24-well plates. Cells from each group were seeded in
the top chamber, 2×104 cells/well. A total of 800 µl
cell culture medium supplemented with 20% fetal bovine serum was
added to the lower chamber as a chemoattractant. The non-invading
cells on the upper-side of the membrane were removed with cotton
swabs 24 after incubation. The invasive cells were fixed with 4%
paraformaldehyde at room temperature for 20 min and stained with
0.5% crystal violet solution at room temperature for 5 min. The
number of invasive cells was counted under inverted phase-contrast
microscope in a blinded manner.
Statistical analysis
The cell viability assay was repeated five times;
all other experiments were repeated three times. All values are
expressed as the mean ± standard deviation. Differences between
groups were analyzed using one-way analysis of variance followed by
Bonferroni's multiple comarisons test with SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 were considered to
indicate a statistically significant difference.
Results
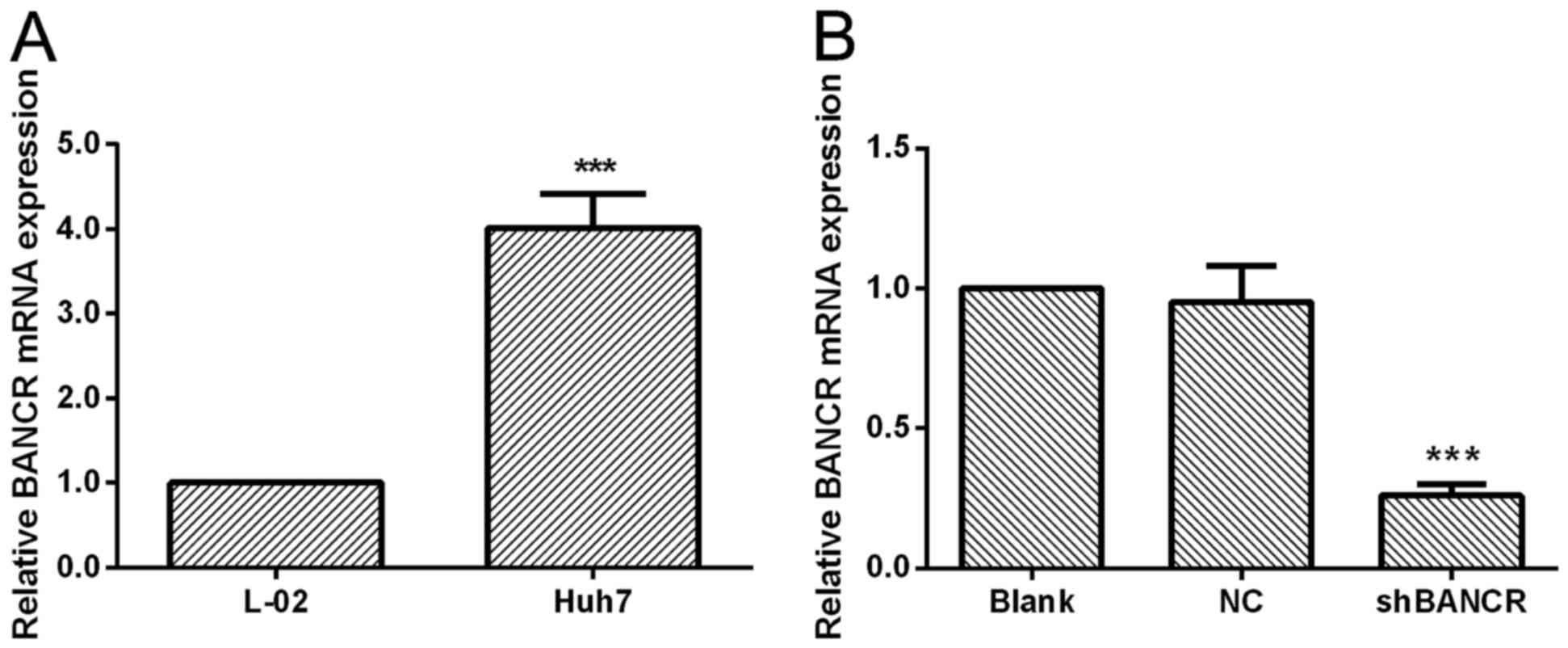
BANCR is overexpressed in HCC
cells
To assess the biological role of BANCR in the
progression of HCC, the expression level of BANCR in Huh7 HCC cells
and normal liver cells was measured by using RT-PCR. As presented
in Fig. 1A, BANCR was significantly
overexpressed in the human HCC cell line Huh7 when compared with
normal liver cells (P<0.001). Thus, Huh7 cells were transfected
with BANCR shRNA or NC shRNA, the expression level of BANCR in
transfected cells was also determined (Fig. 1B). The results revealed that
transfection with BANCR shRNA resulted in a significant decrease
(72.59±1.59%) in BANCR levels compared with NC shRNA-transfected
Huh7 cells (P<0.001), suggesting that the expression of BANCR
was significantly inhibited by BANCR shRNA.
Downregulation of BANCR suppresses the
viability of HCC cells
To investigate whether BANCR was functionally
involved in HCC cell viability, the proliferation of HCC cells was
detected using an MTT assay. The results demonstrated that the
proliferation of Huh7 cells transfected with BANCR shRNA were
significantly decreased compared with NC shRNA-transfected cells
(Fig. 2A; P<0.01). Simultaneously,
the clonogenicity tests revealed that downregulation of BANCR
resulted in a significantly decreased colony formation rate
compared with the NC shRNA group (Fig.
2B; P<0.001). Furthermore, cell cycle analysis was performed
to assess the role of BANCR in the regulation of cell
proliferation, as presented in Fig.
2C, the G0/G1 phase cell proportion was
increased significantly in shRNA BANCR-transfected cells compared
with NC shRNA-transfected cells (P<0.05). Thus, the
downregulation of BANCR may inhibit HCC cell proliferation by
induction of cell cycle arrest.
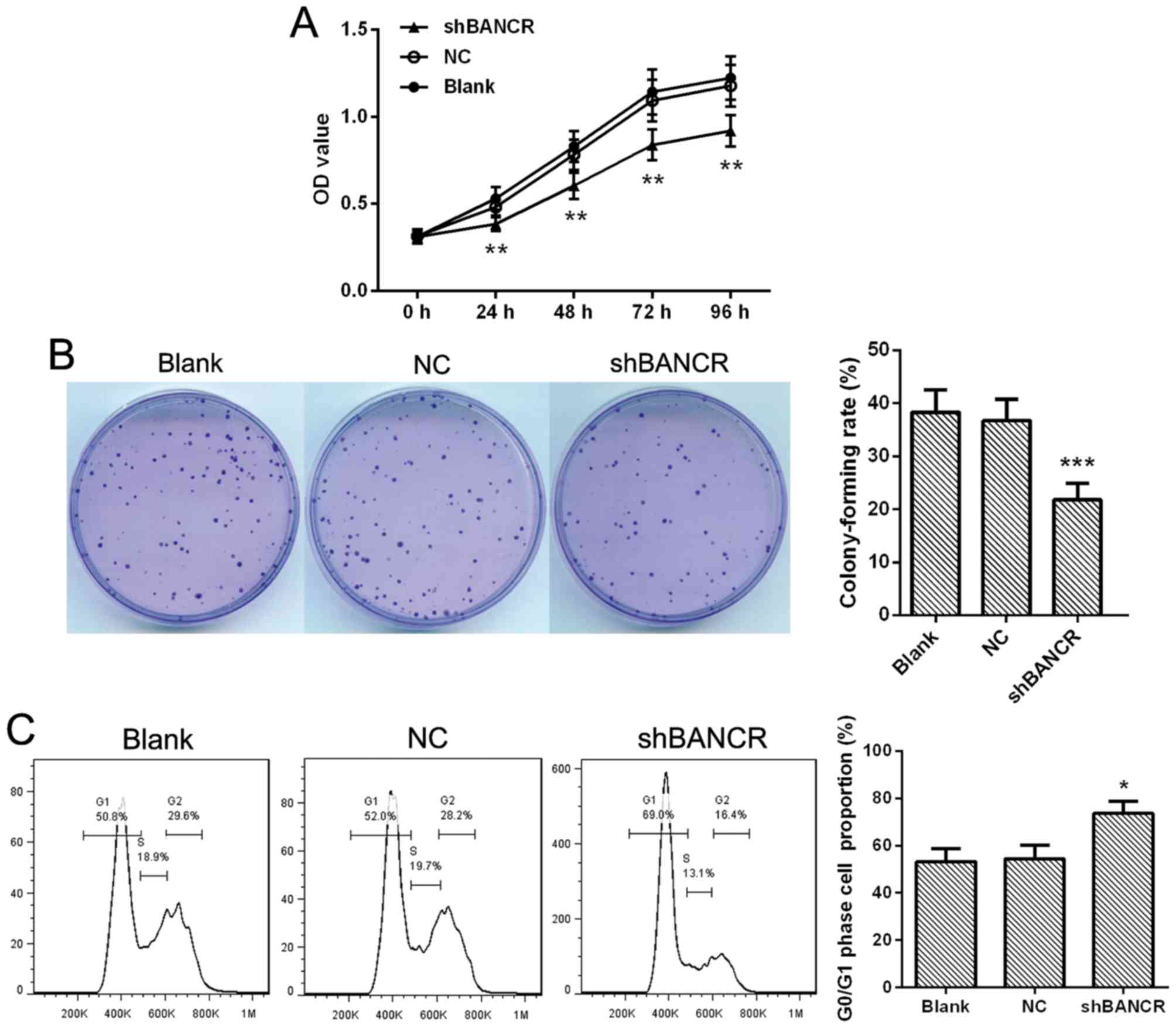 | Figure 2.Downregulation of BANCR suppresses the
viability of Huh7 cells. (A) Huh7 cells were cultured in 96-well
plates, the proliferation at 24, 48, 72 and 96 h were determined by
MTT assay, n=5. (B) Cells were cultured at 35-mm culture dish for
two weeks, stained with Wright-Giemsa, and the colony formation
rate was calculated, n=3. (C) A total of 48 h after transfection,
the cells were stained with propidium iodide, cell cycle was
detected by flow cytometry, n=3. Representative images are
presented and data are expressed as the mean ± standard deviation,
*P<0.05, **P<0.01, ***P<0.001 compared with the NC group.
BANCR, BRAF-activated non-coding RNA; NC, negative control;
shRNA/sh, short hairpin RNA. |
Downregulation of BANCR induces the
apoptosis of HCC cells
The apoptosis status of BANCR shRNA-transfected
cells were determined using flow cytometry (Fig. 3A), it was demonstrated that the
apoptosis cell proportion in Huh7 cells was significantly higher in
BANCR shRNA-transfected cells compared with NC shRNA-transfected
cells (P<0.001), and this was also confirmed by Hoechst
staining. As presented in Fig. 3B,
typical apoptotic bodies were notably observed in BANCR
shRNA-transfected cells. In addition, the expression of
apoptosis-associated proteins was measured (Fig. 3C). As expected, when compared with the
NC group, downregulation of BANCR significantly inhibited the
expression of anti-apoptotic protein Bcl-2, and this was
accompanied by an increase in proapoptotic protein Bax and cleaved
caspase-3. Taken together, downregulation of BANCR promoted
apoptosis of HCC cells.
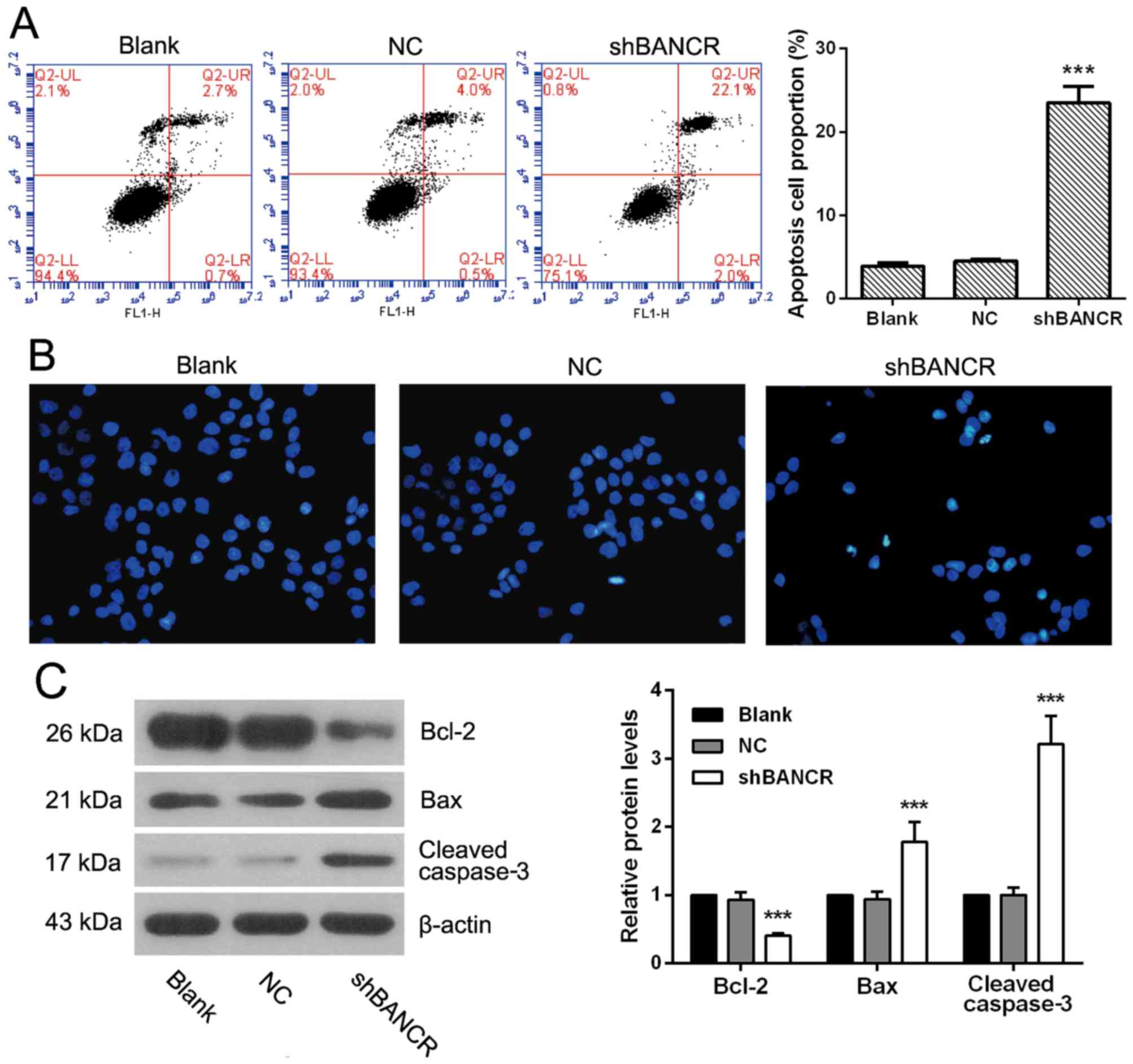 | Figure 3.Downregulation of BANCR induces
apoptosis of hepatocellular carcinoma cells. (A) Cells were stained
with Annexin V-FITC and propidium iodide, apoptosis cells were
detected by flow cytometry, n=3. (B) Cells were cultured on 12-well
plates, stained with Hoeschst solution, apoptosis nucleus were
observed under fluorescent microscope, n=3. Scale bar, 50 µm. (C)
The protein level of Bcl-2, Bax and cleaved caspase-3 were
determined by western blotting, n=3, representative bands are
presented. Data are expressed as mean ± standard deviation,
***P<0.001 compared with the NC group. lncRNA, long non-coding
RNA; BANCR, BRAF-activated non-coding RNA; NC, negative control;
shRNA, short hairpin RNA. |
Downregulation of BANCR inhibits the
migration and invasion of HCC cells
To clarify the effect of BANCR on migration and
invasion of HCC cells, wound healing and Transwell assays were
performed. As presented in Fig. 4A and
B, when compared with the NC group, the migration rate, and
invasive cells in the BANCR shRNA-transfected group were
significantly decreased (P<0.05), indicating that downregulation
of BANCR can inhibit the migration and invasion of HCC cells.
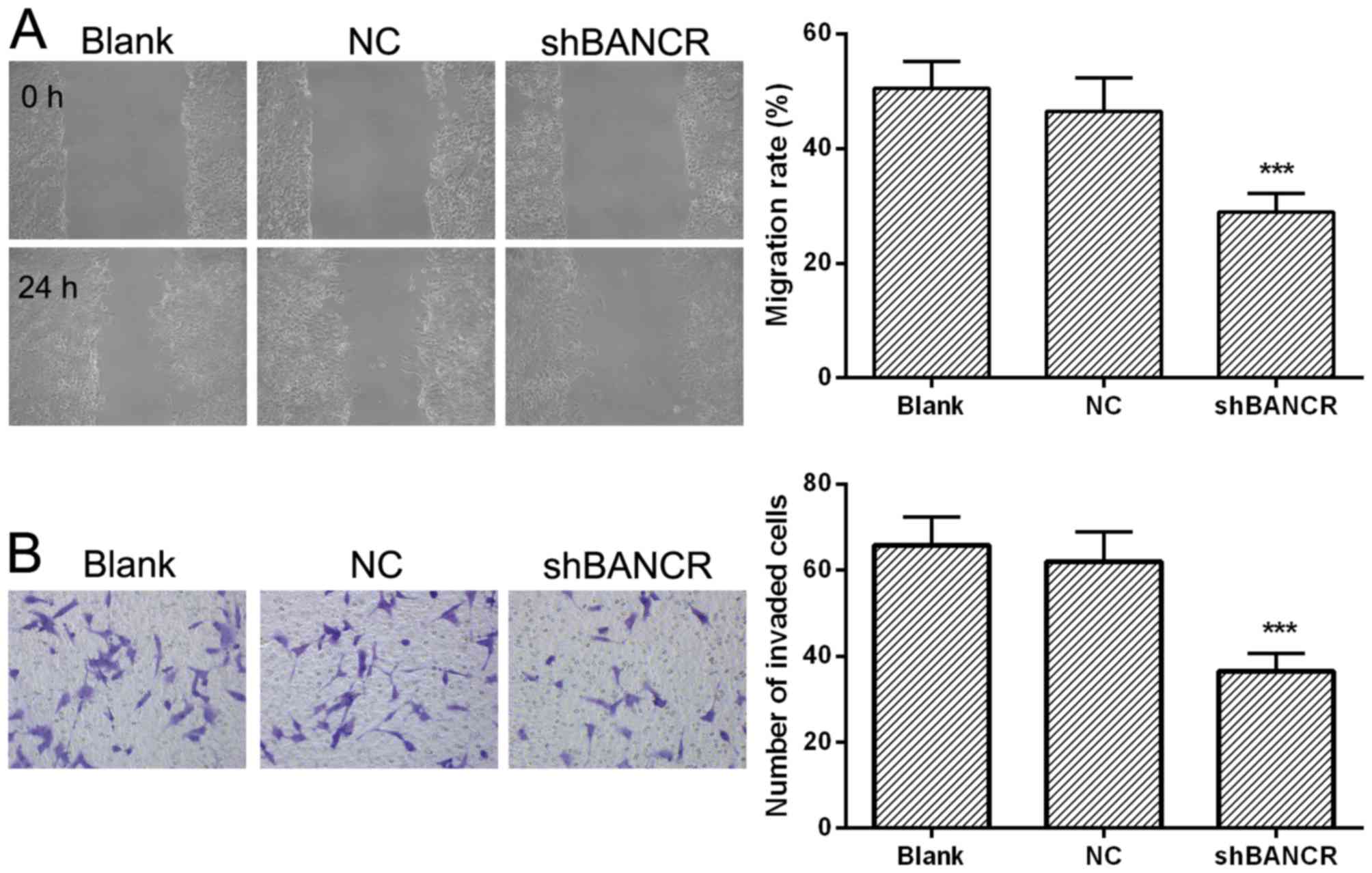 | Figure 4.Downregulation of BANCR inhibits the
migration and invasion of Huh7 cells. (A) Cells were transfected
with BANCR shRNA or NC shRNA, grown to 80–90% confluence on 6-well
plates, wounded with a 200-µl pipette tip, and the migration
distance at 12 and 24 h was measured, n=3. Scale bar, 200 µm. (B)
Cells were seeded on Matrigel-coated 24-well plates, the
invasiveness of cells was determined using a Transwell assay, n=3.
Scale bar, 100 µm. Representative images are presented and data are
expressed as the mean ± standard deviation, ***P<0.001 compared
with the NC group. lncRNA, long non-coding RNA; BANCR,
BRAF-activated non-coding RNA; NC, negative control; shRNA, short
hairpin RNA. |
Downregulation of BANCR inactivates
MEK, ERK and JNK, but does not affect the activity of P38 MAPK
signaling pathway
The activation of MAPK and MEK pathway were examined
using western blot analysis, to explore the potential underlying
mechanisms of BANCR in the progress of HCC. It was revealed that
the MEK, ERK and JNK signaling pathways were inactivated
significantly in BANCR shRNA-transfected cells when compared with
NC shRNA-transfected cells (Fig.
5A-C). However, the activity of the P38 pathway was not
affected by the downregulation of BANCR (Fig. 5D). Thus, these results suggest that
the MEK, ERK and JNK signaling pathways were involved in the
BANC-associated malignance of HCC cells.
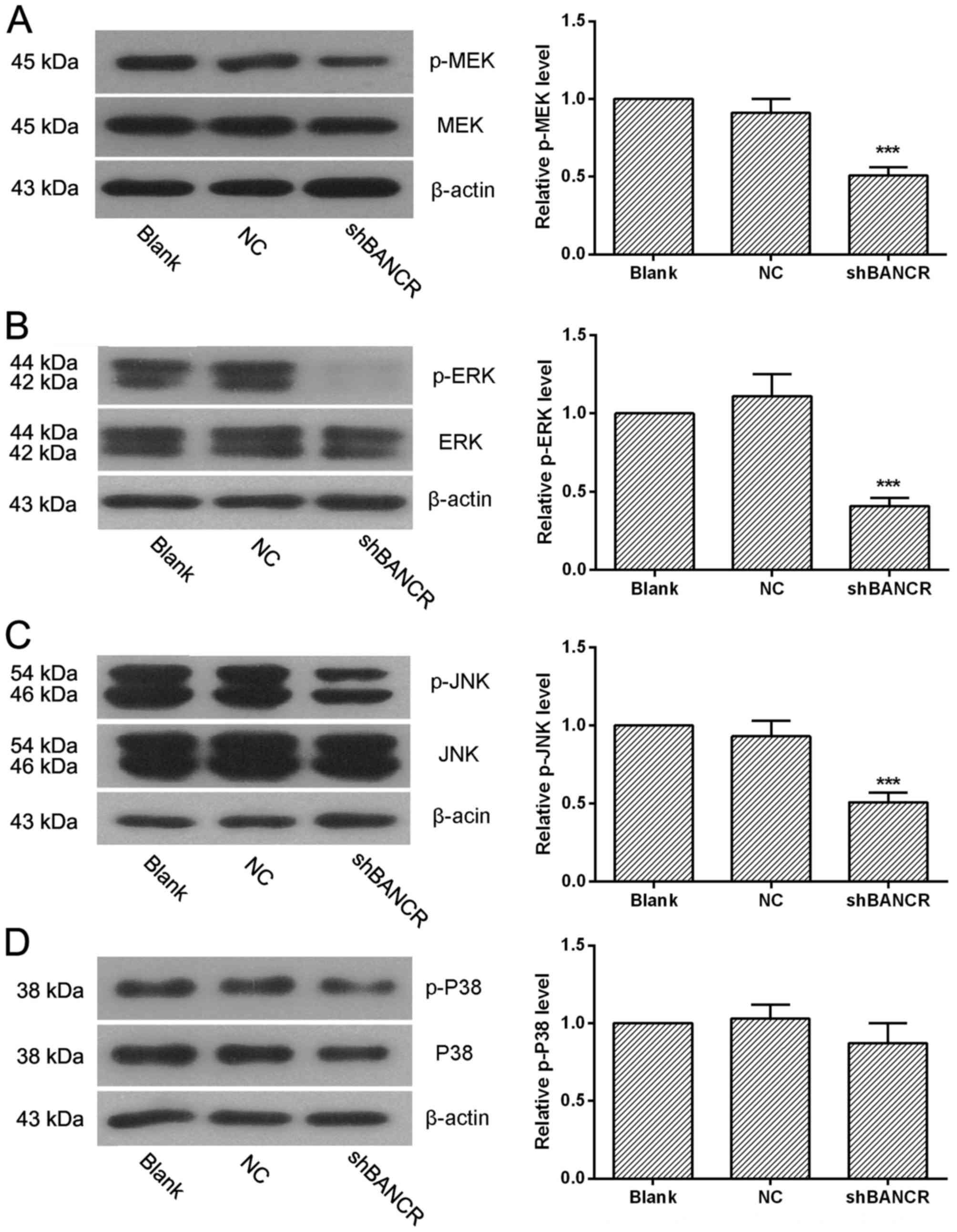 | Figure 5.Downregulation of BANCR inactivates
MEK, ERK and JNK signaling pathways, but does not affect the p38
mitogen-activated protein kinase pathway. The activity of (A) MEK,
(B) ERK, (C) JNK and (D) P38 pathways were determined by western
blotting, n=3. Representative images are presented and data are
expressed as the mean ± standard deviation, ***P<0.001 compared
with the NC group. lncRNA, long non-coding RNA; BANCR,
BRAF-activated non-coding RNA; NC, negative control; shRNA, short
hairpin RNA; MEK, MAPK/ERK kinase; ERK, extracellular
signal-regulated kinase; JNK, janus kinase. |
Discussion
LncRNAs are newly recognized RNAs, which may serve
important roles in the development of cancer. Previous studies have
reported that the lncRNA BANCR is abnormally expressed in various
malignant tumors and participates in tumor development (14–19). In
the present study, it was demonstrated that the lncRNA BANCR was
overexpressed in the HCC cell line Huh7 when compared with the
normal liver cell line L-02. The downregulation of BANCR by shRNA
significantly suppressed the proliferative capacity, clonogenicity
and induced apoptosis of Huh7 cells. The migratory and invasive
ability of Huh7 cells were also inhibited by BANCR shRNA.
Furthermore, these tumor inhibitory effects appear to be associated
with the repression of MEK, ERK and JNK signaling pathways. These
findings demonstrated an essential role for BANCR in the regulation
of proliferation, apoptosis, migration and invasion in HCC
cells.
BANCR has been identified to be significantly
upregulated in human malignant melanoma (21), papillary thyroid carcinoma (18), gastric tumor (14,22) and
retinoblastoma tissues (23).
Previous studies revealed that the knockdown of BANCR resulted in
cell growth inhibition and cell cycle arrest in human thyroid
cancer cells (15), and human
melanoma cells (21). The
aforementioned studies described a tumor promotional role of BANCR.
However, other studies have reported that BANCR was expressed at a
lower level in various cancer types, and its expression was
inversely correlated with tumor malignancy, meanwhile, in
vitro experiments on colorectal cancer, non-small cell lung
cancer and lung carcinomas cells suggested that BANCR serves as an
cancer suppressor gene (16,19,24).
Thereby, BANCR serves distinct roles in different tumor types,
which may be attributed to tumor heterogenicity. The present study
demonstrated that BANCR was significantly overexpressed in Huh7 HCC
cells when compared with normal liver cells, and the downregulation
of BANCR significantly inhibited proliferation, colony formation
and induced cell cycle arrest in Huh7 cells, suggesting a tumor
promoter property of BANCR in HCC cells.
It was also demonstrated that the repression of
BANCR significantly induced apoptosis of Huh7 cells. Given the
important roles of apoptosis-associated proteins in the cell
apoptosis signaling pathway (25,26), the
expression of Bcl-2, Bax and cleaved caspase-3 was determined using
western blotting. The results revealed that transfection with BANCR
shRNA significantly inhibited the expression of Bcl-2, increased
the level of Bax and cleaved caspase-3, further elucidating the
role of BANCR in the regulation of apoptosis.
Flockhart et al (12) reported that BANCR was recurrently
overexpressed in melanomas tissues, and knockdown of BANCR in
melanoma cells significantly reduced cell migration. In addition,
Guo et al (27) confirmed that
BANCR contributes to the migration of colorectal cancer cells by
the promotion of epithelial-mesenchymal transition. In the present
study, it was revealed that the migration rate and invasive cell
number of Huh7 cells were significantly reduced following BANCR
shRNA transfection, these results consistently suggest that the
downregulation of BANCR can suppress the migration and invasion of
HCC cells.
It is accepted that the abnormal activation of
numerous cellular and molecular signaling pathways are involved in
the biological process of hepatocarcinogenesis (28–32). MEK
and MAPK signaling pathways are key pathways that participate in
the regulation of cell proliferation, apoptosis, and
differentiation (33,34). The suppression of MEK/ERK and MAPK
signaling pathways using chemotherapeutic drugs has yielded major
improvements in the management of HCC (35,36). In
the present study, the downregulation of BANCR significantly
inhibited the activity of MEK, ERK and JNK signaling pathways in
Huh7 cells, but had no effect on P38 MAPK signaling. Thus, implying
that MEK, ERK and JNK pathways may be implicated in BANCR-mediated
tumor development, but not the P38 MAPK pathway. These findings are
in line with previous studies demonstrating that the activation of
MEK and MAPK signaling pathways are positively associated with the
expression of BANCR (21,24,27).
Although the downregulation of lncRNA BANCR
demonstrated a significant cancer inhibitory effect in the HCC cell
line Huh7, the role of lncRNA BANCR on other HCC cell lines remains
unclear. A limitation of the present study is that a single
hepatocellular carcinoma cell line was used to investigate the
effect of lncRNA BANCR. Thus, further studies are warranted to
verify the function role of lncRNA BANCR in the development of
HCC.
In conclusion, the results of the present study
demonstrated that lncRNA BANCR was overexpressed in Huh7 cells when
compared with normal liver cells L-02. Downregulation of BANCR
significantly inhibited the proliferation, migration, invasion and
aggravated apoptosis of Huh7 cells. Parallel to these biological
changes, the downregulation of BANCR also suppressed the activity
of MEK, ERK and JNK signaling pathways in HCC cells. The present
study described a cancer promotion property of BANCR and provided a
potential molecular target for the treatment of HCC.
References
|
1
|
Knudsen ES, Gopal P and Singal AG: The
changing landscape of hepatocellular carcinoma: Etiology, genetics
and therapy. Am J Pathol. 184:574–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahn J and Flamm SL: Hepatocellular
carcinoma. Dis Mon. 50:556–573. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Everson GT: Increasing incidence and
pretransplantation screening of hepatocellular carcinoma. Liver
Transpl. 6 (6 Suppl 2):S2–S10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruix J and Llovet JM: Major achievements
in hepatocellular carcinoma. Lancet. 373:614–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olsen SK, Brown RS and Siegel AB:
Hepatocellular carcinoma: Review of current treatment with a focus
on targeted molecular therapies. Therap Adv Gastroenterol. 3:55–66.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forner A and Bruix J: Biomarkers for early
diagnosis of hepatocellular carcinoma. Lancet Oncol. 13:750–751.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meseure D, Alsibai Drak K, Nicolas A,
Bieche I and Morillon A: Long noncoding RNAs as new architects in
cancer epigenetics, prognostic biomarkers, and potential
therapeutic targets. Biomed Res Int. 2015:3202142015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai MC, Spitale RC and Chang HY: Long
intergenic noncoding RNAs: New links in cancer progression. Cancer
Res. 71:3–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flockhart RJ, Webster DE, Qu K,
Mascarenhas N, Kovalski J, Kretz M and Khavari PA: BRAFV600E
remodels the melanocyte transcriptome and induces BANCR to regulate
melanoma cell migration. Genome Res. 22:1006–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCarthy N: Epigenetics. Going places with
BANCR. Nat Rev Cancer. 12:4512012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang ZX, Liu ZQ, Jiang B, Lu XY, Ning XF,
Yuan CT and Wang AL: BRAF activated non-coding RNA (BANCR)
promoting gastric cancer cells proliferation via regulation of
NF-κB1. Biochem Biophys Res Commun. 465:225–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng H, Wang M, Jiang L, Chu H, Hu J,
Ning J, Li B, Wang D and Xu J: BRAF-activated long non-coding RNA
modulates papillary thyroid carcinoma cell proliferation through
regulating thyroid stimulating hormone receptor. Cancer Res Treat.
48:698–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi Y, Liu Y, Wang J, Jie D, Yun T, Li W,
Yan L, Wang K and Feng J: Downregulated long noncoding RNA BANCR
promotes the proliferation of colorectal cancer cells via
downregulation of p21 expression. PLoS One. 10:e01226792015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su S, Gao J, Wang T, Wang J, Li H and Wang
Z: Long non-coding RNA BANCR regulates growth and metastasis and is
associated with poor prognosis in retinoblastoma. Tumour Biol.
36:7205–7211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Guo Q, Zhao Y, Chen J, Wang S, Hu
J and Sun Y: BRAF-activated long non-coding RNA contributes to cell
proliferation and activates autophagy in papillary thyroid
carcinoma. Oncol Lett. 8:1947–1952. 2014.PubMed/NCBI
|
|
19
|
Sun M, Liu XH, Wang KM, Nie FQ, Kong R,
Yang JS, Xia R, Xu TP, Jin FY, Liu ZJ, et al: Downregulation of
BRAF activated non-coding RNA is associated with poor prognosis for
non-small cell lung cancer and promotes metastasis by affecting
epithelial-mesenchymal transition. Mol Cancer. 13:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L
and Sha N: Long non-coding RNA BANCR promotes proliferation in
malignant melanoma by regulating MAPK pathway activation. PLoS One.
9:e1008932014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L, Zhang L, Zhang Y and Zhou F:
Increased expression of LncRNA BANCR is associated with clinical
progression and poor prognosis in gastric cancer. Biomed
Pharmacother. 72:109–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su S, Gao J, Wang T, Wang J, Li H and Wang
Z: Long non-coding RNA BANCR regulates growth and metastasis and is
associated with poor prognosis in retinoblastoma. Tumour Biol.
36:7205–7211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang W, Zhang D, Xu B, Wu Z, Liu S, Zhang
L, Tian Y, Han X and Tian D: Long non-coding RNA BANCR promotes
proliferation and migration of lung carcinoma via MAPK pathways.
Biomed Pharmacother. 69:90–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng JH, Follis Viacava A, Kriwacki RW
and Moldoveanu T: Discoveries and controversies in BCL-2
protein-mediated apoptosis. FEBS J. 283:2690–2700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo Q, Zhao Y, Chen J, Hu J, Wang S, Zhang
D and Sun Y: BRAF-activated long non-coding RNA contributes to
colorectal cancer migration by inducing epithelial-mesenchymal
transition. Oncol Lett. 8:869–875. 2014.PubMed/NCBI
|
|
28
|
Galuppo R, Ramaiah D, Ponte OM and Gedaly
R: Molecular therapies in hepatocellular carcinoma: What can we
target? Dig Dis Sci. 59:1688–1697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Q, Lui VW and Yeo W: Targeting the
PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol.
7:1149–1167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sicklick JK, Li YX, Jayaraman A, Kannangai
R, Qi Y, Vivekanandan P, Ludlow JW, Owzar K, Chen W, Torbenson MS
and Diehl AM: Dysregulation of the Hedgehog pathway in human
hepatocarcinogenesis. Carcinogenesis. 27:748–757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Delire B and Stärkel P: The Ras/MAPK
pathway and hepatocarcinoma: Pathogenesis and therapeutic
implications. Eur J Clin Invest. 45:609–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neuzillet C, Tijeras-Raballand A, de
Mestier L, Cros J, Faivre S and Raymond E: MEK in cancer and cancer
therapy. Pharmacol Ther. 141:160–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao JJ, Shi ZY, Xia JF, Inagaki Y and Tang
W: Sorafenib-based combined molecule targeting in treatment of
hepatocellular carcinoma. World J Gastroenterol. 21:12059–12070.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen C and Wang G: Mechanisms of
hepatocellular carcinoma and challenges and opportunities for
molecular targeted therapy. World J Hepatol. 7:1964–1970. 2015.
View Article : Google Scholar : PubMed/NCBI
|