Introduction
Esophageal cancer is a common malignancy in China,
and surgery is currently the primary treatment modality. The
efficacy of surgery is associated with the tumor-node-metastasis
staging of the tumor. For patients with early-stage esophageal
cancer, surgery may provide strong local control; however, in
patients with locally advanced cancer, the benefits of surgery are
limited (1). In previous studies,
comprehensive treatments, particularly those including neoadjuvant
therapy, have been identified to improve the prognosis of patients
with locally advanced cancer (2,3). However,
previous studies have demonstrated that the improved efficacy
associated with neoadjuvant therapy is limited to patients who
respond to chemotherapy, and the prognosis of non-responders is
worse compared with that of patients who received surgery alone
(2,4).
Thus, prediction of the efficacy of neoadjuvant chemotherapy prior
to treatment and identifying useful predictive biomarkers is
required.
The highly conserved homeobox (HOX) gene
family serves an important function in embryonic development; it is
responsible for encoding transcription factors that regulate cell
proliferation and differentiation. The expression of HOX
genes strictly follows the principle of temporal and spatial
collinearity. Therefore, the expression patterns of HOX
genes vary according to the type of tissue and the developmental
stage (5,6). Alterations in the expression patterns of
HOX gene family members cause dysregulation of HOX protein
function, further leading to unbalanced cell proliferation and
differentiation; this is hypothesized to contribute to
tumorigenesis. In the last two decades, studies have identified
that the expression patterns of HOX genes are abnormal in a variety
of tumors, including esophageal cancer (7–11). Our
previous studies (8,10) demonstrated that HOXC6 serves a key
function in esophageal squamous cell carcinoma (ESCC) and is
abnormally expressed in ESCC tissues. Single-surgery patients with
increased expression of HOXC6 exhibited a significantly shorter
median survival time compared with patients with decreased
expression of HOXC6. Additionally, we previously demonstrated with
in vitro study that cell strains with decreased expression
of HOXC6 exhibit markedly lower proliferation capacity, and higher
apoptosis rates, compared with cell lines with increased expression
of HOXC6 (unpublished data). We previously applied gene expression
profiling to analyze the gene expression difference between
chemosensitive and chemoresistant cell lines and found that cells
exhibiting high HOXC6 expression are more sensitive to cisplatin
and paclitaxel (12). Therefore, it
is hypothesized that HOXC6 may be involved in mediating
chemosensitivity in patients with ESCC. In the present study, the
association between HOXC6 expression in ESCC tissues from patients
who received neoadjuvant chemotherapy and the pathological
indicator of tumor regression grade (TRG), which reflects
chemoresponsiveness, were investigated. The applicability of HOXC6
as a biomarker for the prediction of neoadjuvant chemotherapy
outcomes was also assessed and the results were validated in
vitro.
Materials and methods
Patients and specimens
In total 224 patients who underwent neoadjuvant
chemotherapy and esophagectomy by a single-surgeon team between
January 2000 and December 2012, at the Department of Thoracic
Surgery, Peking University Cancer Hospital (Beijing, China) were
enrolled in the present study. Of the 224 patients, 176 were male,
48 were female and the mean age was 59.7 years. A total of 51 cases
of formalin-fixed paraffin-embedded blocks of pretreatment biopsies
and 186 cases of resected specimens were collected. The present
study was approved by the Ethics and the Academic Committees of
Peking University School of Oncology (Beijing, China) and informed
verbal consent was obtained from all patients.
Neoadjuvant chemotherapy
A platinum-based two-drug combination, primarily
paclitaxel/cisplatin at a proportion of 95 and 5% was
5-FU/cisplatin, was used in neoadjuvant chemotherapy. On day 1,
paclitaxel, at a dose of 175 mg/m2 of body surface area,
was administered intravenously. On days 1–3, cisplatin, at a dose
of 25 mg/m2 of body surface area, was administered
intravenously and a single cycle of treatment lasted 21 days.
Enhanced chest computed tomography (CT) and esophagography were
used to evaluate the curative effects of the treatment. Between 1
and 4 cycles of neoadjuvant chemotherapy were administered prior to
surgery.
Tumor regression grade assessment
All enrolled subjects were reviewed again by two
experienced pathologists who were blinded to the clinical
information and gene expression. Tumors were graded using the TRG;
a four-point scale based on the histological tumor response
assessment, described by Mandard et al (13). This assessment was defined as: Grade
I, no residual tumor cells; grade II, almost complete response with
<10% vital residual tumor cells (VRTCs); grade III, 10–50%
VRTCs; and grade IV, >50% VRTCs. In the present study,
histological tumor response was classified as major (TRG1/2/3) or
minor (TRG4) histopathological tumor response.
Cell lines and cell culture
Human ESCC EC109 cells were obtained from the Cancer
Hospital of the Chinese Academy of Medical Science (Beijing,
China). Human ESCC KYSE 150, KYSE410, KYSE450 and KYSE510 cells
were purchased from the Japanese Collection of Research
Bioresources cell bank (Osaka, Japan). Cells were cultured in
RPMI-1640 medium (Hyclone; GE Healthcare, Logan, UT, USA) with 10%
heat-inactivated fetal bovine serum at 37°C in a humidified
atmosphere containing 5% CO2.
A cellular model for stable HOXC6 knockdown was
established in KYSE450 cells. A total of 3×105 KYSE450
cells per well were plated in a 6-well plate and were grown
overnight, in the conditions previously described, to 80%
confluence. Then the cells were transfected with 100 µl medium
containing either short hairpin RNA (shRNA;
lentivirus-plasmid-mediated) against HOXC6 (sequence:
GGACATAACACCAGACC TCA) or scrambled shRNA, in the Pglv3/GFP+puro
vector (all from Shanghai GenePharma Co., Ltd., Shanghai, China).
Stably transfected cells were selected with 2 µg/ml puromycin
(Amresco, LLC, Solon, OH, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from EC109, KYSE150, KYSE410, KYSE450 and
KYSE510 cells was extracted with Trizol (Invitrogen; Thermo Fisher
Scientific Inc., Waltham, MA, USA) according to the manufacture's
protocol and 1 µg RNA was reverse-transcribed using the RevertAid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific Inc.).
The reverse transcription reaction was performed sequentially for
60 min at 42°C and for 5 min at 70°C. qPCR was performed using
Power SYBR-Green PCR Master Mix (Thermo Fisher Scientific Inc.).
PCR runs and fluorescence detection were performed in a Rotor-Gene
6000 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The sequences of the PCR primers were as
follows: HOXC6 forward, 5′-CACCGCCTATGATCCAGTGAGGCA-3′ and reverse,
5′-GCTGGAACTGAACACGACATTCTC-3′; GAPDH forward,
5′-CTCTGACTTCAACAGCGACACC-3′; and reverse,
5′-CTGTTGCTGTAGCCAAATTCGTT-3′. The cycling conditions were as
follows: 95°C for 10 min followed by 40 cycles of 95°C for 20 sec,
58°C for 20 sec and 70°C for 20 sec. GAPDH was used as an internal
control. The 2−ΔΔCq method was used to determine the RNA
expression level (14). All
experiments were performed in triplicate.
Western blotting
The proteins were extracted by using
radioimmunoprecipitation lysis buffer and separated by 10% SDS-PAGE
with 30 µg protein per lane and transferred onto a polyvinylidene
fluoride membrane, followed by western blot analysis. The membrane
was blocked using 5% bovine serum albinum at room temperature for 1
h. It was then immunoreacted with rabbit anti-HOXC6 polyclonal
antibody (dilution, 1:300; #ab151575; Abcam, Cambridge, UK) as
primary antibody. Goat anti-rabbit polyclonal horseradish
peroxidase-conjugated IgG was used as a secondary antibody.
Immunoreactivity was detected using an enhanced chemiluminescence
reaction kit. As a loading control, GADPH was detected using a goat
polyclonal antibody (dilution, 1:2,000; #ab9845; Abcam). All
experiments were performed in triplicate.
Cell Counting Kit-8 (CCK-8) assay
Cells were plated in 96-well plates at a density of
10,000 cells/well in 100 µl complete medium and incubated overnight
at 37°C. The cells were then treated with gradient dilution of
cisplatin (0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12.8 and 25.6 µg/ml) and
paclitaxel (0.002, 0.008, 0.032, 0.128, 0.512, 2.048, 8.192 and
32.768 µM) in 100 µl complete medium. After 48 h of incubation, 100
µl CCK-8 reagent (Dojindo Molecular Technologies Inc., Kumamoto,
Japan) was added to each well. After 4 h of incubation at 37°C, the
absorbance of each well was measured at 450 nm using a VersaMax
microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Immunohistochemistry (IHC)
The sections were deparaffinized in xylene and
rehydrated using decreasing concentrations of ethanol (100, 95, 85
and 75% ethanol). Following routine deparaffinization and
rehydration, tissue sections were treated with 3%
H2O2 and then heated in citrate buffer (pH
6.0) for antigen retrieval. The HOXC6 antigen-antibody reaction
occurred overnight at 4°C, following blocking with goat serum
(ZSGB-Bio, Beijing, China). The streptavidin/peroxidase
amplification kit (Zymed; Thermo Fisher Scientific, Inc.) was used
to detect the signal of the HOXC6 antigen-antibody reaction.
Peroxidase activity was developed with diaminobenzidine. All
sections were counterstained with hematoxylin. Purified rabbit
polyclonal antibody against human HOXC6 (cat no. #ab151575; Abcam)
was used at a dilution of 1:200, and biotin-conjugated goat
anti-rabbit IgG was used as secondary antibody (dilution, 1:300;
cat no. SPN-9001; ZSGB-Bio). Immunohistochemical signals were
scored by two independent observers. The scores were calculated by
multiplying the staining intensity and extent. The staining
intensity was categorized by relative intensity as follows: 0,
negative; 1, weak; 2, moderate; and 3, strong. Scores <3 were
considered as low-level expression, whereas scores 3 were
considered as high-level expression.
Statistical analysis
SPSS software (version 19.0; IBM SPSS, Armonk, NY,
USA) was used to perform the statistical analysis. All in
vitro experiments were performed at least three times with
triplicates. When the data from different groups were compared,
normal analysis and homogeneity of variance were checked first, and
then an unpaired two-tailed Student's t-test analysis was used.
Results are presented as the mean ± standard deviation. The
association between gene expression and TRG were evaluated using a
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Increased HOXC6 expression is
associated with tumor regression in patients with ESCC
To investigate the association between HOXC6
expression and chemoresistance, IHC was performed in 51 cases of
patients with complete pretreatment biopsies and matched resected
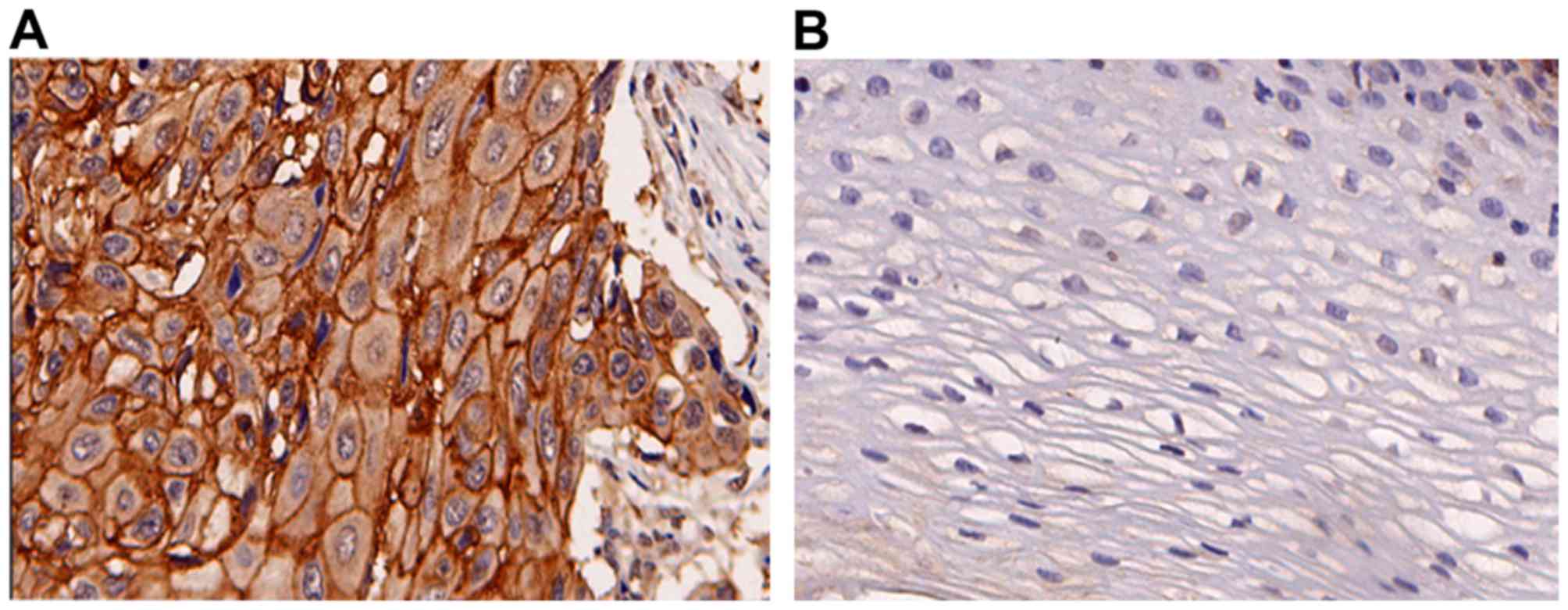
specimens. HOXC6 was overexpressed in ESCC cells compared with the
normal epithelium (Fig. 1). HOXC6
expression in the pretreatment biopsies was associated with the
response to chemotherapy in this set of patients with ESCC, but not
resected specimens. The patients with high expression of HOXC6 in
pretreatment biopsies achieved improved objective response to
chemotherapy (TRG1/2/3). A total of 26 samples from patients
sensitive to chemotherapeutic drugs exhibited significantly
increased HOXC6 expression, and 9 cases from patients resistant to
anticancer drugs exhibited decreased HOXC6 expression (Table I). To further investigate the
association between HOXC6 expression in resected specimens and TRG,
HOXC6 expression was examined in a larger cohort. The results
revealed that in 164 cases of resected specimens (samples with TRG1
were not included, as TRG1 was defined as ‘no residual tumor
cells’), the patients who exhibited an improved response to
chemotherapy were associated with decreased expression of HOXC6
(Table II).
 | Table I.Association of HOXC6 expression in
pretreatment biopsies TRG (n=51). |
Table I.
Association of HOXC6 expression in
pretreatment biopsies TRG (n=51).
| HOXC6 expression
(endoscope) | TRG1/2/3, n (%) | TRG4, n (%) | P-value |
|---|
| Low | 6
(18.8) | 9
(47.4) | 0.030 |
| High | 26 (81.3) | 10 (52.6) |
|
 | Table II.Association of HOXC6 expression in
postoperative specimens and TRG (n=164). |
Table II.
Association of HOXC6 expression in
postoperative specimens and TRG (n=164).
| HOXC6 expression
(endoscope) | TRG2/3, n (%) | TRG4, n (%) | P-value |
|---|
| Low | 41 (62.1) | 25 (37.9) | 0.005 |
| High | 39 (39.8) | 59 (60.2) |
|
Downregulation of the HOXC6 gene
increases ESCC cell chemoresistance
In our previous study (12), the inhibition of cell proliferation
induced by chemotherapy agents cisplatin and paclitaxel was
determined in a panel of 5 ESCC cell lines (EC109, KYSE450,
KYSE510, KYSE410 and KYSE510). It has been demonstrated that EC109
was relatively sensitive, KYSE 410 and KYSE510 were relatively
resistant, and KYSE450 and KYSE150 exhibited intermediate
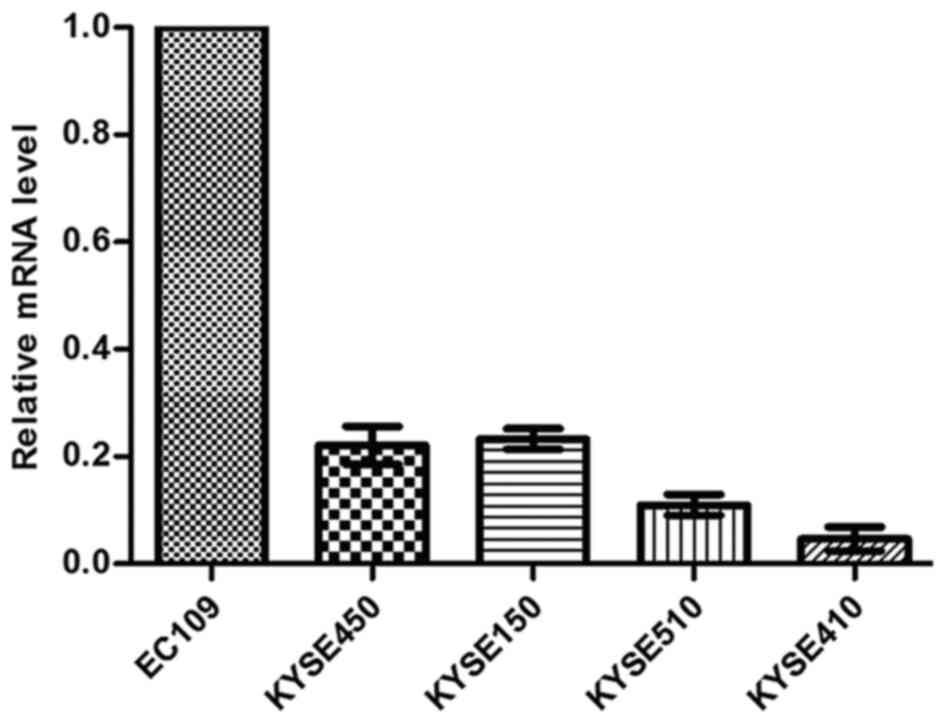
sensitivity. Using microarray gene expression profile analysis on
these cell lines, a 2.6-fold upregulation of HOXC6 was identified
in relatively sensitive ESCC cells, compared with the relatively
resistant ESCC cells. The result was confirmed at the mRNA level
using qPCR (Fig. 2), and was
consistent with results from gene expression profiling. Since HOXC6
was upregulated in relatively sensitive cells, it was hypothesized
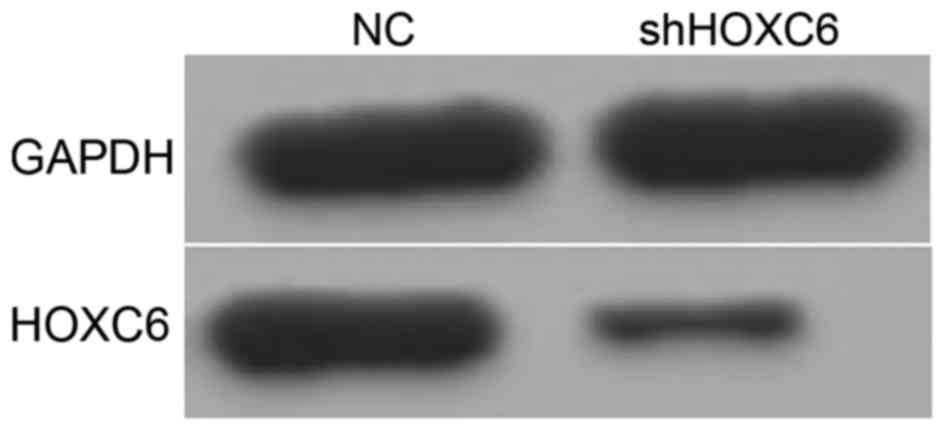
that HOXC6 may be involved in chemosensitivity. Previously, three
small interfering RNAs against HOXC6 were designed to inhibit HOXC6
expression in the intermediately sensitive parental ESCC cell line
KYSE450, and stable knockdown cell lines were established (Fig. 3; data not shown). To investigate the
effects of HOXC6 on the chemosensitivity of ESCC, this cell model
was used to determine the effect of HOXC6 on cell apoptosis induced
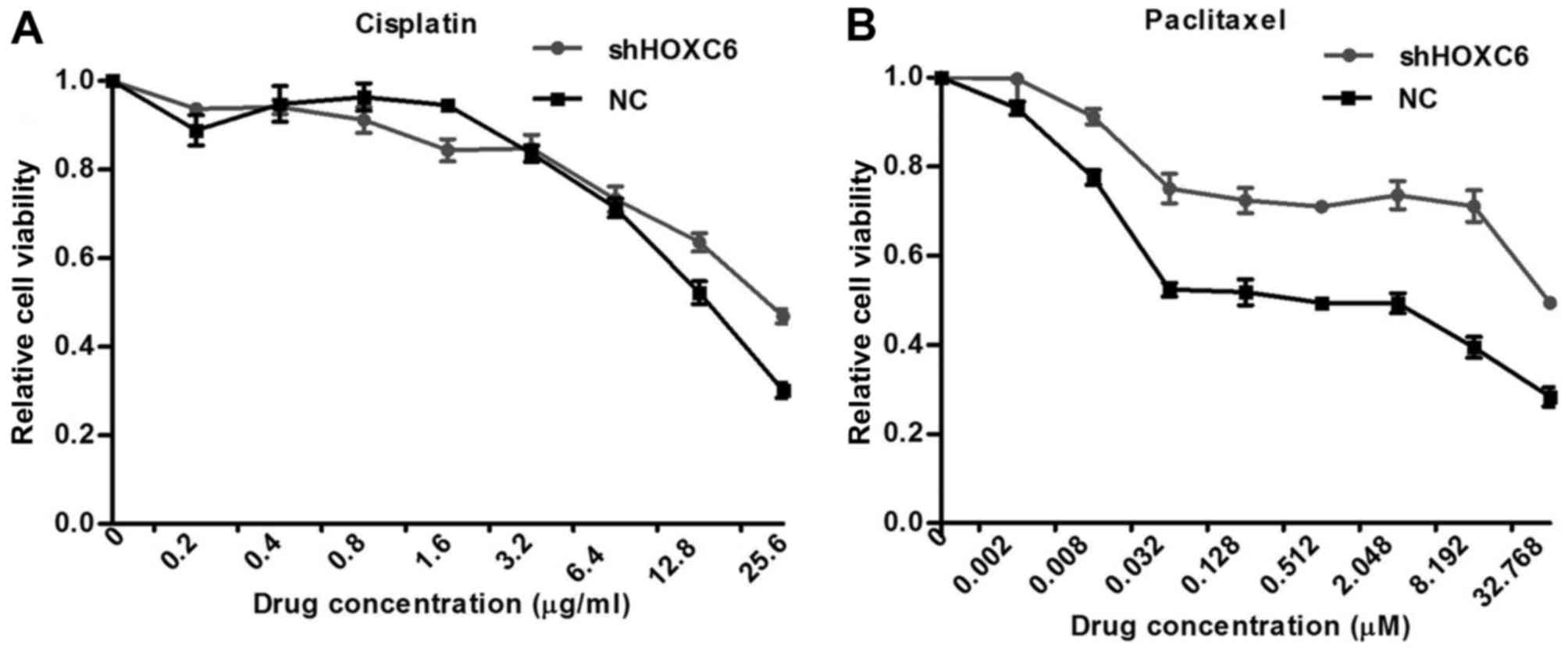
by chemotherapy agents. Inhibiting HOXC6 also partially inhibited
cisplatin and paclitaxel-induced apoptosis. Treatment for 48 h with
cisplatin and paclitaxel resulted in increased the half-maximal
inhibitory concentration (IC50) of HOXC6 short hairpin
RNA (shRNA)-transfected cells compared with scrambled
shRNA-transfected cells (Fig. 4).
Discussion
In the present study, the expression of HOXC6 was
determined using cytology and IHC, and the association between
HOXC6 expression and chemosensitivity in ESCC was investigated. The
results demonstrated that HOXC6 expression was associated with
chemosensitivity in ESCC tissues. Patients with increased
expression of HOXC6 prior to treatment were more sensitive to
chemotherapy, and knockdown of HOXC6 decreased chemosensitivity to
cisplatin and paclitaxel in ESCC cell lines. Thus, determining
HOXC6 expression levels prior to surgery may be a predictor of the
response to chemotherapy in patients with ESCC.
Oncology, also known as ‘disorganized embryology’,
is associated with the disorder of cell proliferation, death,
differentiation, recognition and migration, all of which are
involved in embryonic development and cancer. The identification
and application of α-fetoprotein (AFP) and carcinoembryonic antigen
(CEA) confirmed this view, and suggested that studies of genes and
proteins related to embryonic development may be useful for the
identification of clinically applicable tumor biomarkers.
As key factors controlling embryonic development,
HOX genes have been demonstrated to be abnormally expressed
in a number of types of cancer and are involved in tumor cell
proliferation, differentiation and apoptosis. Numerous studies
(15–18) have also identified the association
between HOX genes and chemosensitivity of tumors. A previous
study (19) using gene expression
profiling revealed that HOX family members, including HOXA9,
HOXA10, HOXB13, HOXC4, HOXC10, HOXC11, HOXC13 and HOXD1, are
activated in the temozolomide-resistant pediatric glioblastoma cell
line KNS42. Among these activated HOX family members, HOXA9 and
HOXA10 are key factors for predicting chemoresistance. Similarly,
Tang et al (20) identified
that HOXA7, HOXA9, HOXA13, HOXB2, HOXB5, HOXB6, HOXB7, HOXB8, HOXB9
and HOXC9 are overexpressed in the carboplatin-resistant small cell
lung cancer cell line DMS53. The expression of HOXA1 in small cell
lung cancer tissues is associated with chemoresponsiveness;
patients with increased expression of HOXA1 are more sensitive to
chemotherapy and thus have improved prognoses compared with
patients with decreased expression of HOXA1 (16). Consistently, downregulation of HOXA5
expression in breast cancer cells partly blocks apoptosis induced
by retinoic acid-like drugs (21). In
chronic myeloid leukemia cells treated with an Abl kinase inhibitor
(AMN107) and phosphoinositide 3-kinase inhibitor (LY294002) HOXA10
expression is upregulated, and inhibition of HOXA10 expression,
using RNA interference, decreases the sensitivity of chronic
myeloid leukemia cells to these two drugs (22). Similarly, HOXB13 overexpression
suppresses the synthesis of estrogen receptor (ER) and activates
the transcription of interleukin 6, resulting in resistance to
tamoxifen, a commonly used chemotherapeutic drug in patients with
ER(+) breast cancer (23). Therefore,
these studies provide important insights into the associations
between HOX gene expression and chemoresponsiveness.
In our previous study (12), it was identified that HOXC6 was
overexpressed in chemosensitive ESCC cell lines and downregulated
in chemoresistant cell lines using a gene expression profiling
approach. In the present study, these results were confirmed using
qPCR. The influence of HOXC6 on chemosensitivity was further
determined using cell lines exhibiting stable knockdown of HOXC6.
The results demonstrated that inhibition of HOXC6 expression
decreased chemosensitivity and increased the corresponding
IC50 values. Therefore, HOXC6 may be associated with
chemoresponsiveness in ESCC. To confirm this hypothesis, the
expression of HOXC6 was assessed in pretreatment biopsy specimens
and matched resected specimens from 51 patients with ESCC who
received neoadjuvant chemotherapy. The results demonstrated that
patients with higher TRGs exhibited increased expression of HOXC6
in pretreatment biopsy specimens, but no association between TRG
and HOXC6 expression was identified in resected specimens. The
sample size of resected specimens from patients with neoadjuvant
chemotherapy was expanded, and it was revealed that HOXC6 was
expressed at decreased levels in postoperative samples from
patients with higher TRGs. Whether HOXC6 may be identified as a
potential predictor for evaluating TRG in patients with ESCC
therefore remains unclear; however, the results of the present
study provide meaningful indications.
There are limitations to the present study. First,
the retrospective nature of the study made it difficult to collect
pretreatment biopsy specimens and postoperative samples from all
patients; therefore, it was not possible to analyze matched tissues
in a large sample size. In other words, since it was only possible
to analyze the samples that were available, it was not possible to
conclusively define the role of HOXC6 in predicting the
chemoresponsiveness of ESCC tumors. Larger prospective studies are
required to validate the results of the present study.
Acknowledgements
The authors thank Dr Liang Dai, Dr Xiao-Zheng Kang,
Dr Zhen Liang, Dr Hong-Chao Xiong, Dr He-Li Yang and Dr Hao Fu for
patient provision and contribution to care. The present study was
supported financially by the National Natural Science Foundation of
China (grant no. 81301748), the National High Technology Research
and Development Program of China (grant no. 2015AA020403), the
Beijing Municipal Administration of Hospitals Clinical Medicine
Development of Special Funding Support (grant no. ZYLX201509), the
National Basic Research Program of China (grant no. 2011CB504300),
the Specialized Research Fund for the Doctoral Program of Higher
Education (grant no. 20130001110108) and the Science Fund for
Creative Research Groups of the Ministry Education of China (grant
no. IRT13003).
References
|
1
|
Kelsen DP, Ginsberg R, Pajak TF, Sheahan
DG, Gunderson L, Mortimer J, Estes N, Haller DG, Ajani J, Kocha W,
et al: Chemotherapy followed by surgery compared with surgery alone
for localized esophageal cancer. N Engl J Med. 339:1979–1984. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medical Research Council Oesophageal
Cancer Working Group: Surgical resection with or without
preoperative chemotherapy in oesophageal cancer: A randomised
controlled trial. Lancet. 359:1727–1733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davies AR, Gossage JA, Zylstra J, Mattsson
F, Lagergren J, Maisey N, Smyth EC, Cunningham D, Allum WH and
Mason RC: Tumor stage after neoadjuvant chemotherapy determines
survival after surgery for adenocarcinoma of the esophagus and
esophagogastric junction. J Clin Oncol. 32:2983–2990. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abate-Shen C: Deregulated homeobox gene
expression in cancer: Cause or consequence? Nat Rev Cancer.
2:777–785. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beck F: Homeobox genes in gut development.
Gut. 51:450–454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grier DG, Thompson A, Kwasniewska A,
McGonigle GJ, Halliday HL and Lappin TR: The pathophysiology of HOX
genes and their role in cancer. J Pathol. 205:154–171. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen KN, Gu ZD, Ke Y, Li JY, Shi XT and Xu
GW: Expression of 11 HOX genes is deregulated in esophageal
squamous cell carcinoma. Clin Cancer Res. 11:1044–1049.
2005.PubMed/NCBI
|
|
9
|
Gu ZD, Shen LY, Wang H, Chen XM, Li Y,
Ning T and Chen KN: HOXA13 promotes cancer cell growth and predicts
poor survival of patients with esophageal squamous cell carcinoma.
Cancer Res. 69:4969–4973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du YB, Dong B, Shen LY, Yan WP, Dai L,
Xiong HC, Liang Z, Kang XZ, Qin B and Chen KN: The survival
predictive significance of HOXC6 and HOXC8 in esophageal squamous
cell carcinoma. J Surg Res. 188:442–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Shen LY, Yan WP, Dong B, Kang XZ,
Dai L, Yang YB, Fu H, Yang HL, Zhou HT, et al: Deregulated HOXB7
expression predicts poor prognosis of patients with esophageal
squamous cell carcinoma and regulates cancer cell proliferation in
vitro and in vivo. PLoS One. 10:e01305512015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen LY, Wang H, Dong B, Yan WP, Lin Y,
Shi Q and Chen KN: Possible prediction of the response of
esophageal squamous cell carcinoma to neoadjuvant chemotherapy
based on gene expression profiling. Oncotarget. 7:4531–4541. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mandard AM, Dalibard F, Mandard JC, Marnay
J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G,
et al: Pathologic assessment of tumor regression after preoperative
chemoradiotherapy of esophageal carcinoma. Clinicopathologic
correlations. Cancer. 73:26801994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamada S, Satoh K, Hirota M, Kanno A,
Umino J, Ito H, Masamune A, Kikuta K, Kume K and Shimosegawa T: The
homeobox gene MSX2 determines chemosensitivity of pancreatic cancer
cells via the regulation of transporter gene ABCG2. J Cell Physiol.
227:729–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao F, Bai Y, Chen Z, Li Y, Luo L, Huang
J, Yang J, Liao H and Guo L: Downregulation of HOXA1 gene affects
small cell lung cancer cell survival and chemoresistance under the
regulation of miR-100. Eur J Cancer. 50:1541–1554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu W, Deng L, Song Y and Redell M: DOT1L
inhibition sensitizes MLL-rearranged AML to chemotherapy. PLoS One.
9:e982702014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li N, Jia X, Wang J, Li Y and Xie S:
Knockdown of homeobox A5 by small hairpin RNA inhibits
proliferation and enhances cytarabine chemosensitivity of acute
myeloid leukemia cells. Mol Med Rep. 12:6861–6866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaspar N, Marshall L, Perryman L, Bax DA,
Little SE, Viana-Pereira M, Sharp SY, Vassal G, Pearson AD, Reis
RM, et al: MGMT-independent temozolomide resistance in pediatric
glioblastoma cells associated with a PI3-kinase-mediated HOX/stem
cell gene signature. Cancer Res. 70:9243–9252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang CH, Parham C, Shocron E, McMahon G
and Patel N: Picoplatin overcomes resistance to cell toxicity in
small-cell lung cancer cells previously treated with cisplatin and
carboplatin. Cancer Chemother Pharmacol. 67:1389–1400. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Zhang H, Lee J, Liang X, Wu X, Zhu
T, Lo PK, Zhang X and Sukumar S: HOXA5 acts directly downstream of
retinoic acid receptor beta and contributes to retinoic
acid-induced apoptosis and growth inhibition. Cancer Res.
67:8007–8013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sugimoto Y, Nakamura S, Okinaka K, Hirano
I, Ono T, Shigeno K, Shinjo K and Ohnishi K: HOXA10 expression
induced by Abl kinase inhibitors enhanced apoptosis through PI3K
pathway in CML cells. Leuk Res. 32:962–971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shah N, Jin K, Cruz LA, Park S, Sadik H,
Cho S, Goswami CP, Nakshatri H, Gupta R, Chang HY, et al: HOXB13
mediates tamoxifen resistance and invasiveness in human breast
cancer by suppressing ERα and inducing IL-6 expression. Cancer Res.
73:5449–5458. 2013. View Article : Google Scholar : PubMed/NCBI
|