Introduction
Ganglioneuromas (GNs) are rare, slow-growing benign
tumors arising from the sympatho-adrenal neuroendocrine system
(1,2).
GNs typically occur in children and adolescents, with up to 60% of
patients <20 years old at the time of diagnosis. Additionally,
females are more likely to be affected than males (3). GNs are the benign ends of a wide
spectrum of peripheral neuroblastic tumors, which also include
neuroblastoma, ganglioneuroblastoma and ganglioneuroblastoma
intermixed (2,4). GN can be diagnosed de novo in
healthy patients or in specific cases result from spontaneous or
chemo- or radiotherapy induced maturation of less-differentiated
neuroblastic tumors (neuroblastoma and ganglioneuroblastoma)
(1,5).
In addition, certain studies have presented an association between
GNs and specific familial diseases including neurofibromatosis type
2 and multiple endocrine neoplasia type 2 (6,7). While GNs
can occur anywhere along the sympathetic nervous system, the two
most common locations of occurrence are the posterior mediastinum
and retroperitoneum (6).
Retroperitoneal tumors may originate from the adrenal glands or
extra-adrenal tissues (6). There are
a limited number of studies reporting tumor occurrence in uncommon
locations, including the tongue, bladder, uterus and skin (8–10).
Although complete surgical excision is considered to
be the treatment of choice in the management of these tumors,
occasionally it can be challenging and in specific cases (such as
when large tumors are involving the surrounding vital organs) it is
not a feasible option. The current case report presents an
innovative surgical approach to the management of a large
retroperitoneal GN that was initially considered inoperable due to
its encasement of major visceral vasculature. To the best of our
knowledge, this is the first report of an abdominal cavity tumor
managed by ex vivo tumor resection and bilateral renal
auto-transplantation.
Case report
Patient
A previously healthy 21-year-old male was referred
to the Columbia Presbyterian Hospital medical center (New York, NY,
USA) with a large retroperitoneal tumor. The tumor was diagnosed
almost a year prior to referral, following imaging studies,
including computed tomography (CT) and magnetic resonance imaging
(MRI), which were performed to investigate abdominal pain.
Image-guided biopsy confirmed the diagnosis of GN; however, the
date of surgical resection was deferred due to encasement of major
visceral vessels. At the time of referral to the Columbian
Presbyterian Hospital medical center, the patient was experiencing
worsening epigastric pain accompanied by nausea. General laboratory
work-up results were within normal limits (white blood cells,
7,000/l; hemoglobin, 11.1 g/dl; serum creatinine, 0.74 mg/dl).
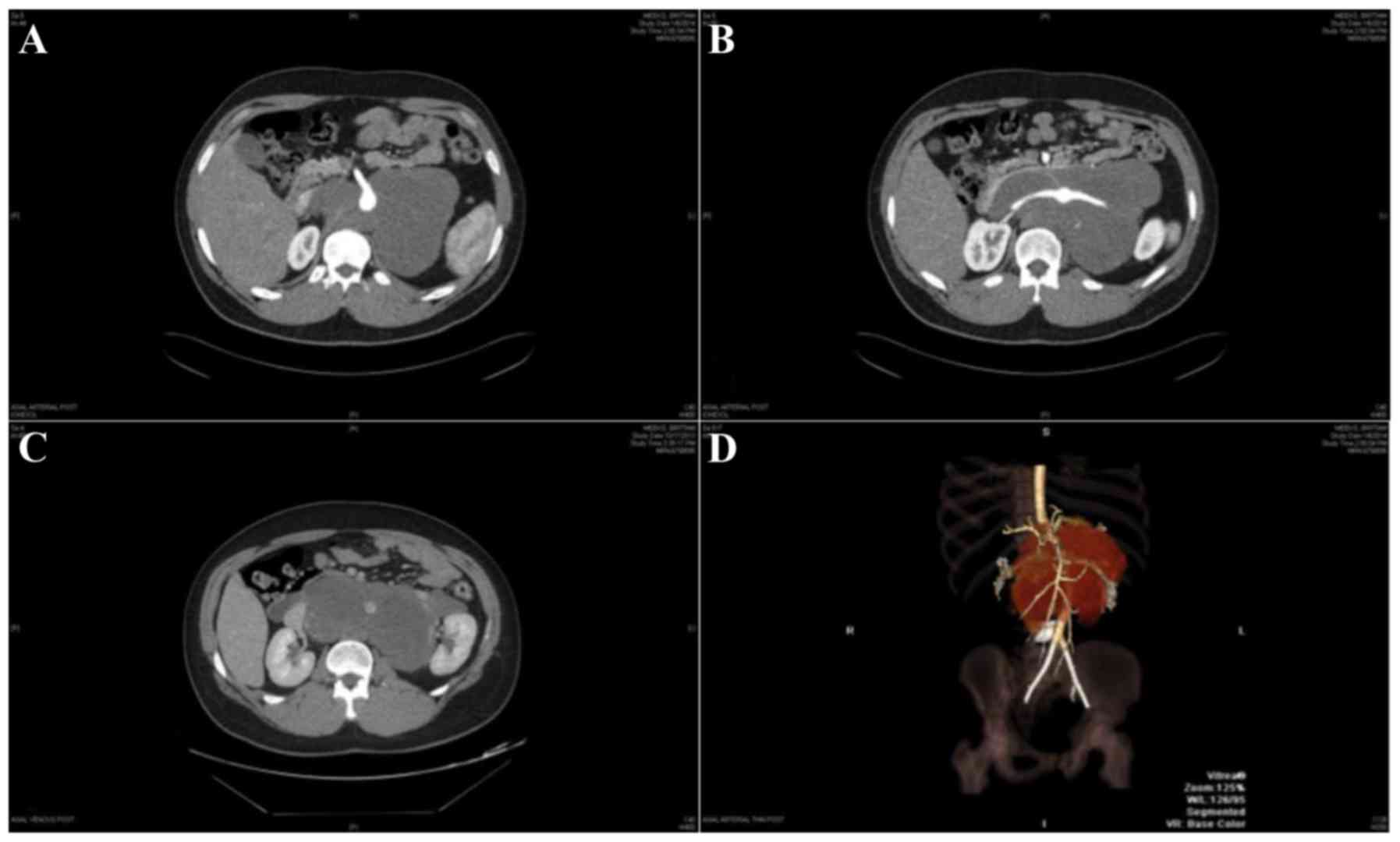
Contrast-enhanced CT scans revealed a large (11.5×19.4×16.0 cm)
homogeneously hypo-dense retroperitoneal mass, occupying almost the
entire abdominal cavity, with a lateral displacement of the
bilateral kidneys (Fig. 1). The tumor
size was significantly larger compared with the patient's previous
MRI scan, which was performed approximately a year earlier. CT
angiography revealed that the aorta was encircled by the tumor
without any obvious invasion, that the inferior vena cava (IVC)
exhibited tumor involvement; the common hepatic artery was totally
replaced at the origin from the superior mesenteric artery (SMA);
there was a replaced left hepatic artery at the origin from the
left gastric artery; and the origin of the SMA and both renal
arteries were encased by the tumor (Fig.
1). A positron emission tomography-CT scan did not reveal any
signs of metastasis. Considering the worsening clinical symptoms
and growing size of the tumor, the patient underwent surgical
resection.
Surgical technique
The abdominal cavity was accessed through a midline
incision with bilateral subcostal extensions. The tumor was visible
in the middle of abdomen. Subsequent to mobilizing the duodenum and
right hemi-colon, the distal portion of the SMA was exposed and the
splenic artery was isolated. The common hepatic artery was
completely replaced with origin arising from the SMA. The SMA was
dissected down to the aorta. Since the SMA origin was encased by
the tumor, the distal section of the SMA (with a completely
replaced common hepatic artery) was anastomosed to the splenic
artery (a branch of the celiac artery) subsequent to ligating the
distal splenic artery. Subsequently, the tumor and the bilateral
kidneys were further mobilized from the retroperitoneum. The right
adrenal gland was carefully preserved; however, the left adrenal
gland was mostly removed with the tumor specimen. The aorta and IVC
were then cross-clamped above and below the tumor and divided, as
both structures were encased by the tumor. Following the dissection
of the tumor from additional retroperitoneal attachments and
division of the two ureters, the tumor and bilateral kidneys were
removed to the back-table.
The kidneys were flushed with Ringer's lactate
solution at the back table and the two kidneys were removed from
the tumor. While the kidneys were being prepared for
auto-transplantation, the aorta and IVC were replaced by a 19 mm
Dacron IMPRA® F3519 graft (BARD Peripheral Vascular,
Inc., Tempe, AZ, USA) and a 20 mm ringed-Gortex MAQUET 095220 graft
(Boston Scientific, Marlborough, MA, USA), respectively. Following
the restoration of flow through the aorta and the vena cava, the
right kidney was auto-transplanted to the right groin in a standard
manner and successfully reperfused (cold ischemia time, 3 h 33 min)
(11). The left kidney was
subsequently implanted to the left groin and reperfused
uneventfully (cold ischemia time, 4 h 57 min). The blood flow to
the two kidneys was examined using Doppler ultrasound, which
revealed arterial and venous flows and waveforms to be normal.
Subsequent to the completion of hemostasis, ureteroureterostomy
anastomosis was performed over double J stents in both sides. The
surgery was uneventful and the patient was transferred to surgical
intensive care unit for monitoring. Continuous veno-venous
hemodialysis was continued until post-operative day five and
subsequently stopped as the serum creatinine level normalized. The
post-operative course was complicated by prolonged ileus possibly
attributed to narcotic use. The patient was discharged home 19 days
after surgery. At the one-year follow-up appointment, the patient
was doing well and a follow-up CT scan did not reveal any signs of
recurrence.
Specimen
The tumor presented as a semi-firm multi-lobulated
tan-white mass measuring 21.5×18.1×7.8 cm. Samples (4 µm thickness)
were fixed with 10% neutral-buffered formalin solution for 6 h at
room temperature, stained for S-100 protein and examined with a
light microscope [for staining, Rabbit anti-S100 (cat. no.
760-2523) was used as primary and iVIEW DAB Detection kit (cat. no.
760-091) as secondary antibody (both Ventana Medical Systems, Inc.,
Tucson, AZ, USA) incubated for 30 min at 37°C]. Upon
histopathological examination, the tumor was observed to be
composed of interwoven bundles of neurofilaments (stained strongly
positive for the S-100 protein) and Schwannian-like spindle cells
on a loose and edematous background without signs of nuclear atypia
or mitoses. This was consistent with the diagnosis of
ganglioneuroma. Surgical margins were free of the tumor.
Discussion
GNs originate from neural crest cells (12). GNs are clinically silent tumors and
are usually identified incidentally during routine imaging studies.
When GNs become symptomatic, it is mainly due to the effect of the
tumor mass on neighboring organs (12). Furthermore, these tumors are capable
of excreting a wide variety of neuropeptides, and clinical
presentation with signs and symptoms owing to hormone excess
including hypertension, diarrhea and sweating is infrequent
(2,13). Despite the availability of different
non-invasive radiologic modalities e.g., CT scans and MRI scans, a
definitive pathological diagnosis of these tumors is dependent on
the post-operative histopathological findings and in certain
instances pre-operative image-guided biopsies (6,14,15).
The prognosis of patients following complete
surgical removal of the tumor is excellent, without any need for
adjuvant or neoadjuvant radio- or chemotherapy (2,6). However,
surgical excision in the case of the tumors involving surrounding
anatomic structures, particularly visceral vessels, can be
demanding and occasionally not feasible. Besides a high rate of
reported surgery-related complications (including neurological
dysfunctions) in the literature, in a number of studies patients
underwent elective nephrectomy to make the tumor resectable
(16–18). In the presented case, surgical
resection was initially deferred due to encasement of the aorta,
IVC, origins of the superior mesenteric artery and bilateral renal
arteries. Previously, complete resection of the tumor with
reconstruction of major vascular structures was shown to be a safe
method with acceptable outcome for complete resection of large
retroperitoneal tumors including sarcoma (19–22).
However, even in these studies, nephrectomy appeared to be
inevitable in order to meet the oncological standards of complete
resection (20,22). In the case of the present study,
considering the bilateral involvement of the origin of renal
arteries, nephrectomy would have left the patient requiring
lifetime dialysis. The shorter cold ischemia time may result in a
faster post-implantation renal function recovery. Wan et al
introduced fractionated resection as a novel method for completing
the resection of retroperitoneal tumors surrounding major vessels
(23). Considering the slow-growth
pattern of the tumor and the possibility of late recurrence,
long-term follow-up of the patients including vigilant physical
examination and repeated imaging studies is an important part of
the post-operative management (23).
In conclusion, although routine surgical excision is
the treatment of choice in the management of retroperitoneal GNs,
occasionally complete in vivo resection is deemed to be
impossible due to extent of involvement of surrounding vital
structures. In such cases, ex vivo resection with
auto-transplantation of the explanted organs may offer the most
effective method for improving patient prognosis.
References
|
1
|
Shimada H, Ambros IM, Dehner LP, Hata J,
Joshi VV and Roald B: Terminology and morphologic criteria of
neuroblastic tumors: Recommendations by the International
Neuroblastoma Pathology Committee. Cancer. 86:349–363. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geoerger B, Hero B, Harms D, Grebe J,
Scheidhauer K and Berthold F: Metabolic activity and clinical
features of primary ganglioneuromas. Cancer. 91:1905–1913. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moriwaki Y, Miyake M, Yamamoto T, Tsuchida
T, Takahashi S, Hada T, Nishigami T and Higashino K:
Retroperitoneal ganglioneuroma: A case report and review of the
Japanese literature. Intern Med. 31:82–85. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimada H, Ambros IM, Dehner LP, Hata J,
Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK and
Castleberry RP: The international neuroblastoma pathology
classification (the Shimada system). Cancer. 86:364–372. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zugor V, Amann K, Schrott KM and Schott
GE: Retroperitoneal ganglioneuroma. Aktuelle Urol. 36:349–352.
2005.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain M, Shubha BS, Sethi S, Banga V and
Bagga D: Retroperitoneal ganglioneuroma: Report of a case diagnosed
by fine-needle aspiration cytology, with review of the literature.
Diagn Cytopathol. 21:194–196. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lora MS, Waguespack SG, Moley JF and
Walvoord EC: Adrenal ganglioneuromas in children with multiple
endocrine neoplasia type 2: A report of two cases. J Clin
Endocrinol Metab. 90:4383–4387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papavramidis TS, Michalopoulos N, Georgia
K, Kesisoglou I, Valentini T, Georgia R and Papavramidis ST:
Retroperitoneal ganglioneuroma in an adult patient: A case report
and literature review of the last decade. South Med J.
102:1065–1067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gary C, Robertson H, Ruiz B, Zuzukin V and
Walvekar RR: Retropharyngeal ganglioneuroma presenting with neck
stiffness: Report of a case and review of literature. Skull Base.
20:371–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mithofer K, Grabowski EF, Rosenberg AE,
Ryan DP and Mankin HJ: Symptomatic ganglioneuroma of bone. A case
report. J Bone Joint Surg Am. 81:1589–1595. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barry JM: Renal transplant-recipient
surgery. BJU Int. 99:701–717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayes FA, Green AA and Rao BN: Clinical
manifestations of ganglioneuroma. Cancer. 63:1211–1214. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kogner P: Neuropeptides in neuroblastomas
and ganglioneuromas. Prog Brain Res. 104:325–338. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG
and Lee JM: Neurogenic tumors in the abdomen: Tumor types and
imaging characteristics. Radiographics. 23:29–43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lonergan GJ, Schwab CM, Suarez ES and
Carlson CL: Neuroblastoma, ganglioneuroblastoma, and
ganglioneuroma: Radiologic-pathologic correlation. Radiographics.
22:911–934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Retrosi G, Bishay M, Kiely EM, Sebire NJ,
Anderson J, Elliott M, Drake DP, Coppi PD, Eaton S and Pierro A:
Morbidity after ganglioneuroma excision: Is surgery necessary? Eur
J Pediatr Surg. 21:33–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Bernardi B, Gambini C, Haupt R, Granata
C, Rizzo A, Conte M, Tonini GP, Bianchi M, Giuliano M, Luksch R, et
al: Retrospective study of childhood ganglioneuroma. J Clin Oncol.
26:1710–1716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strauss DC, Hayes AJ and Thomas JM:
Retroperitoneal tumours: Review of management. Ann R Coll Surg
Engl. 93:275–280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwarzbach MH, Hormann Y, Hinz U,
Leowardi C, Böckler D, Mechtersheimer G, Friess H, Büchler MW and
Allenberg JR: Clinical results of surgery for retroperitoneal
sarcoma with major blood vessel involvement. J Vasc Surg. 44:46–55.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tseng WW, Wang SC, Eichler CM, Warren RS
and Nakakura EK: Complete and safe resection of challenging
retroperitoneal tumors: Anticipation of multi-organ and major
vascular resection and use of adjunct procedures. World J Surg
Oncol. 9:1432011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quinones-Baldrich W, Alktaifi A and Eilber
F and Eilber F: Inferior vena cava resection and reconstruction for
retroperitoneal tumor excision. J Vasc Surg. 55:1386–1393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu YH, Guo KJ, Guo RX, Ge CL, Tian YL and
He SG: Surgical management of 143 patients with adult primary
retroperitoneal tumor. World J Gastroenterol. 13:2619–2621. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan Z, Yin T, Chen H and Li D: Surgical
treatment of a retroperitoneal benign tumor surrounding important
blood vessels by fractionated resection: A case report and review
of the literature. Oncol Lett. 11:3259–3264. 2016.PubMed/NCBI
|