Introduction
Liver cancer is the sixth most common type of cancer
and the second leading cause of cancer-associated mortality
worldwide, with the highest incidence in Asia and sub-Saharan
Africa (1). Hepatocellular carcinoma
(HCC) is the most common form of liver cancer, accounting for
>90% of cases. HCC affects >700,000 patients per year
worldwide and is the most rapidly increasing cause of
cancer-associated mortality in developed nations (2). HCC is typically correlated with chronic
viral hepatitis infections, particularly hepatitis B or C,
aflatoxin B-contaminated dietary intake, alcoholism and metabolic
syndrome, including fatty liver disease (3,4). The
all-stage survival rate of HCC is 16% and the incidence of HCC is
on the rise every year; the incidence rate increased by ~12% from
2006 to 2010 (5). Developing
diagnostic and preventive strategies for HCC has been an attractive
area for researchers. However, HCC can only be diagnosed at a late
stage by currently available serum biomarkers, including
α-fetoprotein (AFP) (6),
des-γ-carboxy prothrombin and squamous cell carcinoma
antigen-immunoglobulin M complexes (7). With the late diagnosis, the five-year
survival rate of patients with HCC has been estimated to be very
low (5).
Establishing appropriate animal models for HCC is
required for basic and translational studies. Several rodent models
have been used to study HCC pathogenesis; one of the best
experimental systems is the laboratory mouse, owing to the
molecular, genetic and physiologic similarities to humans, its
breeding capacity, short lifespan and the unlimited options offered
by genetic engineering (8).
Since the 1960s, the genotoxic drug
diethylnitrosamine (DEN) has been used to induce HCC in rodents
(9) and is the most widely used
chemical to induce liver cancer in mice (8). DEN is the member of the N-nitroso
compounds (NOC) family, is considered highly carcinogenic, and has
been revealed as a contaminant of beverages, food, tobacco,
cosmetic and personal care products among others (8). DEN is a DNA alkylating agent, which can
lead to the formation of mutagenic DNA adducts (10). In addition, DEN can generate reactive
oxygen species (ROS) following activation by cytochrome P450
(10), which damages DNA, proteins
and lipids, and results in cell death. In hepatocytes, DEN is
activated by the cytochrome P450 family enzymes (10) and acts as a carcinogen if injected
into mice younger than two weeks (when hepatocytes are
proliferating) (8). When administered
later, tumor-promoting agents may be required (11). The age, sex and genetics of the mice
serve roles in the early stages of DEN-induced HCC (12).
Although DEN is the chemical most widely used to
induce liver cancer in mice, the commonly used method for using DEN
to establish the liver cancer model has limitations: DEN is
typically injected into postnatal rats and mice <2 weeks old in
order to induce HCC (8), whereas
human HCC is typically diagnosed in adults (3). Therefore, although this DEN model is
used and considered one of the best chemical models to induce
hepatocarcinoma in laboratory rodents, it is not ideal for studying
human HCC (13,14). Secondly, when DEN is administered
later, tumor-promoting agents are required, for instance:
CCl4, pentobarbital, partial hepatectomy or high fat
diet feeding (11,15). Thirdly, these conventional methods
require a long period (5–10 months) to induce HCC (13).
Previous research suggests that excessive alcohol
use over a prolonged period of time typically results in alcoholic
liver disease (ALD), which includes steatosis, steatohepatitis,
acute alcoholic steatohepatitis, alcoholic fibrosis and cirrhosis
(Laennec's cirrhosis) (16). Multiple
pathogenic factors are involved in the development of ALD; alcohol
and its metabolites induce reactive oxygen species production and
hepatocyte injury through mitochondrial damage and endoplasmic
reticulum stress (16). In addition,
there is early activation of chemokines, particularly interleukin
(IL)8, which contributes to recruitment of neutrophil leukocytes,
and monocyte chemoattractant protein-1, which recruits macrophages
in the liver (17,18). Activation of Kupffer cells (KCs) has
been identified as a central element in the pathogenesis of ALD
(19,20). Previous studies have indicated that
bacterial endotoxin-lipopolysaccharide (LPS), through Toll-like
receptor 4 (TLR4), activate KCs and recruit macrophages in the
liver, and that the level of LPS is increased in the portal and the
systemic circulation following excessive alcohol intake (21,22). These
observations indicate that LPS derived from the gut is a central
mediator of inflammation in alcoholic steatohepatitis. Advanced ALD
predisposes to hepatocellular cancer; LPS-TLR4 interactions and
stem cell Nanog expression serve mechanistic roles in animal models
(23,24).
In the present study, an HCC model of adult male
BALB/c mouse was induced using the combination of alcohol with a
conventional chemical-induced mouse liver cancer model. Induced
lesions were subsequently analyzed using histology. The present
study aimed to evaluate macroscopic, microscopic and
ultrastructural hepatic changes in a BALB/c mouse strain induced by
alcohol/DEN/CCl4, and to report the histological
features of pre-neoplastic and neoplastic lesions.
Materials and methods
Animals and experimental
conditions
Previous studies have demonstrated that men exhibit
higher rates of HCC compared with women, with male:female ratios
ranging from 2:1 to 4:1 (14).
Therefore, the present study used 80 male mice as subjects.
Six-week-old specific pathogen-free BALB/c male mice (20–25 g) were
provided by the Animal Center of the Fourth Military Medical
University (Xi'an, China) (25).
Animals were housed in a specific pathogen-free facility,
maintained at a temperature of 23±2°C, 50±10% humidity, and given
free access to water and a regular chow diet, with 14 h light/10 h
dark and hardwood bedding. Mice were handled in accordance with
institutional guidelines. All animal experiments were approved by
the Institutional Animal Care and Use Committee at Fourth Military
Medical University (Xi'an, China) and were in accordance with the
Declaration of the National Institutes of Health Guide for Care and
Use of Laboratory Animals (Publication No. 85–23, revised
1985).
Experimental mouse model
procedures
Previous to the present procedures, mice did not
receive any treatment. The subsequent quarantine period lasted for
one week. At seven weeks of age, 80 mice were identified with ear
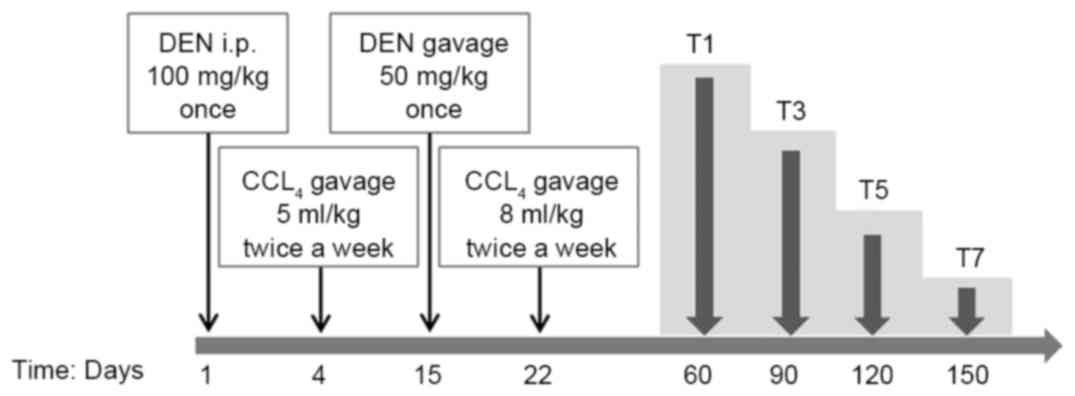
cuts and randomly divided into 8 groups, as depicted in Fig. 1. On day 1, groups T1, 3, 5 and 7
(experiment groups) were intraperitoneally (i.p.) injected with 100
mg/kg bodyweight of DEN (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). From day 4, CCl4 (5 ml/kg, dissolved in olive
oil) was administered via gavage twice a week. On day 15, 50 mg/kg
bodyweight of DEN was administered via gavage once and 9% alcohol
was administered instead of drinking water. From day 22, the dose
of CCl4 was administered up to 8 ml/kg via gavage twice
a week. The reason why two different routes were used to
administrate DEN is as follows: For higher incidence of HCC, more
DEN should be administrated; due to toxicity of DEN and tolerance
to DEN/CCl4 of mice (8).
Saline solution was used as a substitute for DEN, CCl4
and 9% alcohol for groups T2, 4, 6 and 8 (control groups) compared
with experiment groups T1, 3, 5 and 7. Mouse weights were noted
weekly.
Sample collection and histological
processing
On day 60, the first groups (T1 and 2) were
sacrificed by means of a lethal i.p. dose of sodium pentobarbital.
The remaining groups were sacrificed, correspondingly, at the
following days: 90 (T3 and 4), 120 (T5 and 6), 150 (T7 and 8) days
following the first DEN injection by the same method mentioned
above.
All animals were submitted to necropsies and all
macroscopic lesions were recorded. The liver, lungs, spleen,
stomach, intestine, pancreas and kidneys were collected and fixed
in 10% neutral buffered formalin for 48 h and then samples were
routinely processed and paraffin-embedded at room temperature.
Relative organ weights were estimated as the ratio of the organ
weight to total mouse bodyweight according to Da Costa et al
(15). Macroscopically visible
hepatic nodules were counted and measured using a caliper to
determine their largest diameters.
On day 150, macroscopically visible hepatic nodules
of T7 and the livers tissues of T8 were diced into 1 mm3
sections, excised and prefixed in 2.5% glutaraldehyde for 3 h at
room temperature. Subsequently, post-fixation was performed in cold
1% aqueous osmium tetroxide for 1 h at 4°C. Following rinsing with
phosphate buffer, tissue samples were dehydrated in graded ethanol
(50, 70, 90 and 100%; 5 min for each) and embedded in Epon 812 for
12 h at room temperature. After sectioning into 50 nm sections,
grids were hand stained with 2% uranyl acetate in 50% methanol for
10 min and 1% lead citrate for 7 min at room temperature.
Histological evaluation
Representative histological sections (4 µm-thick)
were obtained and stained with hematoxylin and eosin for
examination under light microscopy by two different researchers in
a blinded fashion and results were compared. Steps of staining with
hematoxylin and eosin were as follows and at room temperature:
Dewaxing (xylene: I, II, III; 5 min for each), hydrating (ethanol:
100, 95, 90, 80 and 0%; 5 min for each), hematoxylin staining (15
sec), rinsing with water, adding 1% hydrochloric acid alcohol (3–5
sec), eosin staining (2 min), rinsing with water, dehydrating in
graded ethanol (70, 90, 95 and 100%; 5 min for each),
deparaffinized in xylene (I, II, III; 5 min for each), mounting and
coverslipping (neutral balsam).
Images of ultrastructural hepatic tissue samples (T7
and T8) were captured using a JEOL JEM-2000EX transmission electron
microscope (JEOL USA, Inc., Peabody, MA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
The samples of all experiment groups and T8 (150
days, control group) mice were used to perform RT-PCR analysis.
Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc. Waltham, MA, USA), according to the
manufacturer's protocol. RNA (1 µg) was reverse transcribed into
cDNA according to the instructions of the
SuperScriptTMIII Reverse Transcriptase kit (Invitrogen;
Thermo Fisher Scientific, Inc.). The PCR primers used were as
follows: For mAFP, forward 5′-CTGGCGATGGGTGTTTAG-3′ and reverse
5′-TGGTTGTTGCCTGGAGGT-3′; for β-actin, forward
5′-AGTGTGACGTTGACATCCGTA-3′ and reverse
5′-CCAGAGCAGTAATCTCCTTCT-3′. The PCR reaction was conducted at 94°C
for 5 min, followed by 40 cycles at 94°C for 30 sec, at 56°C for 45
sec and at 72°C for 2 min. PCR was performed using a Bio-Rad
iCycler IQ™ 5 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
Takara Ex Taq® (Takara Bio, Inc., Otsu, Japan),
according to the manufacturer's instructions. β-actin was used as
endogenous control in this study. PCR products were separated on a
1% agarose gel, and visualized and photographed under ultraviolet
light. ImageJ software (1.49n) was used for quantification
(National Institutes of Health, Bethesda, MD, USA).
Measurement of blood glucose
level
On day 150, the blood glucose levels of group 7 and
8 were measured prior to sacrifice. Blood was collected with a
minimum volume (1 µl) from the tail-vein. The glucose level was
measured using the Accu-Chek Performa blood glucose monitoring
system (glucometer; Roche Diagnostics GmbH, Mannheim, Germany).
Statistical analysis
Data was analyzed using a two-tailed paired t-test
using GraphPad Prism software, version 5.01 (GraphPad Software,
Inc., La Jolla, CA, USA). The results are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Alcohol/DEN/CCl4 treatment caused a
significant loss of body weight and a significant increase in the
liver/body weight ratios of mice
During the experimental protocol, the mortality rate
was 7.5% (3/40 mice, one mouse following gavage administration of
CCl4 in T1, and two mice following gavage administration
of DEN in T3 and T7) and occurred exclusively in the
alcohol/DEN/CCl4-treated groups. A post-mortem
evaluation of animals that succumbed during the experiment was
conducted. An anatomic observation of mice that succumbed
prematurely during the experiment was made, and the liver, lungs,
spleen, stomach, intestine, pancreas and kidneys were collected and
observed. Hemorrhage and edema of the intestine and hepatomegaly
were observed. Due to male competitive behavior, despite the
environmental enrichment, some sporadic injuries associated with
the establishment of hierarchy and territory defense were noted,
resulting in focal loss of hair (barbering behavior). The liver,
lungs, spleen, stomach, intestine, pancreas and kidneys were
collected and were carefully examined. The livers and spleens
exhibited abnormal changes in weight. Therefore only the changes of
liver and spleen are exhibited, and the data of other organs isn't
included in the results.
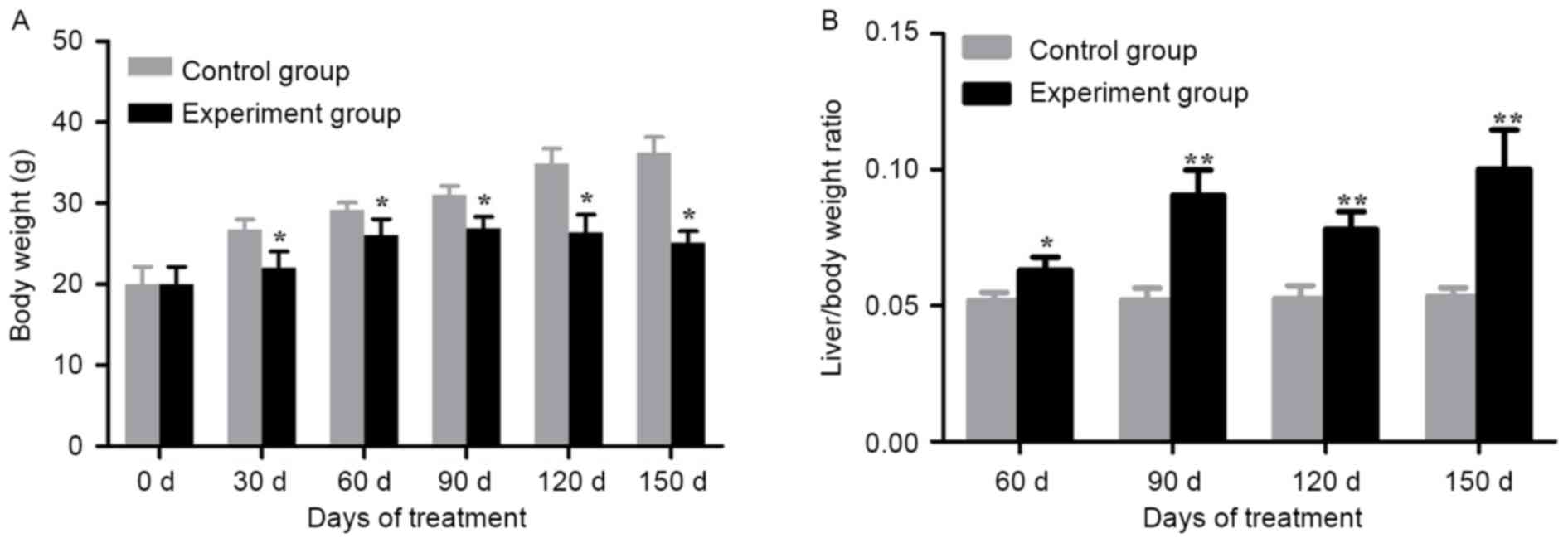
Alcohol/DEN/CCl4-treated groups
demonstrated a significant loss in body weight compared with the
control groups on day 30, 60, 90, 120 and 150 (P<0.05; Fig. 2A). In addition, the
alcohol/DEN/CCl4-treated mice exhibited a significant
increase in liver/body weight ratios compared with the control
groups (P<0.05 for day 60, P<0.01 for day 90, 120 and 150;
Fig. 2B).
Macroscopic and microscopic effects of
alcohol/DEN/CCl4 on mouse livers
Exposure to alcohol/DEN/CCl4 resulted in
a sequence of lesions that evolved over time, from chronic toxic
lesions observed from T1 (60 days) onwards, fibrosis observed from
T3 (90 days), cirrhosis observed from T5 (120 days), to the
occurrence of HCC observed from T7 (150 days) (data not shown).
Control mice (T2, 4, 6, 8) did not exhibit any significant lesions.
T1 (experiment group, 60 days) exhibited hepatitis (90%, one
succumbed following gavage administration of CCl4), T3
(experiment group, 90 days) exhibited liver fibrosis (90%, one
succumbed following gavage administration of DEN), T5 (experiment
group, 120 days) exhibited liver cirrhosis (100%), T7 (experiment
group, 150 days) exhibited high or middle differentiation group of
HCC (90%, one succumbed following gavage administration of DEN),
which means that the malignant transformation rate of the surviving
mice in T7 (experimental group, 150 days) was 100% and the liver
lesions were classified as the high or middle differentiation group
of HCC according to the International Classification of Rodent
Tumors and the update on precursors and early lesion on HCC
(15) under light microscopy by two
pathologists (Department of Pathology, Xijing Hospital, Fourth
Military Medical University) in a blinded fashion.
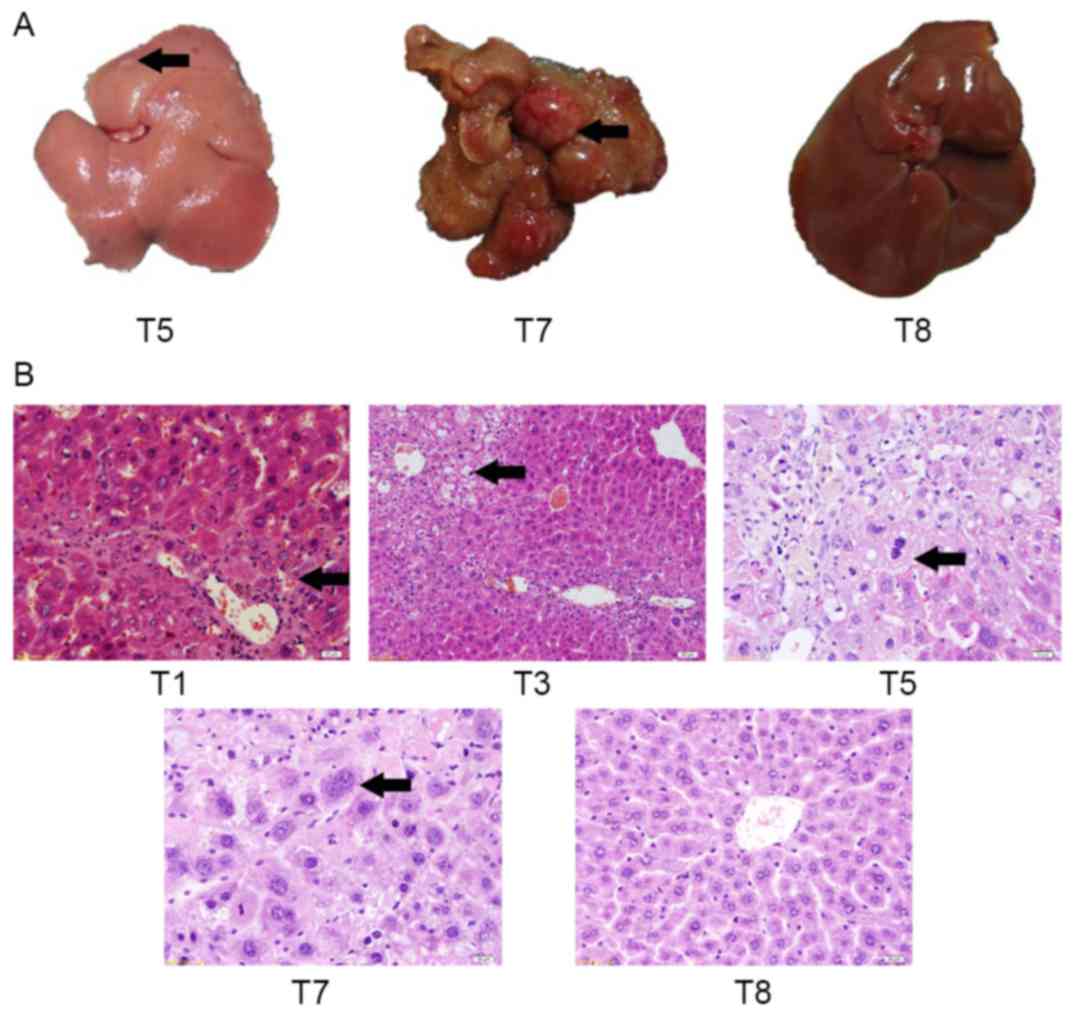
To assess gross changes in liver morphology,
macroscopic lesions in liver tissue were identified in T5 and T7
groups, and in the T7 control group (Fig.
3A). At day 150, control mice (T8) did not exhibit any evident
hepatic lesions. At day 120, the liver from an
alcohol/DEN/CCl4-treated mouse (T5) exhibited an
irregular hepatic surface (several small nodules and granular
appearance). At day 150, the liver from an
alcohol/DEN/CCl4-treated mouse (T7) exhibited multifocal
lesions and solitary nodules with a maximum diameter of 10 mm.
Histological hepatic changes of mice in each
experiment group were the same (Fig.
3B). Spotty and focal necrosis was identified in liver tissue,
and inflammatory cells infiltrated in portal duct areas among mice
of the T1 group, which suggested that hepatitis occurred.
Lymphocyte and monocyte are a major type of infiltrate in the
present study.
Compared with the T1 group, the necrotic area of
hepatic lobule, fat droplets of hepatic cells and inflammatory cell
infiltration were increased in the T2 group. The dysplasia of
connective tissue was observed, but the structure of liver lobule
remained normal, which suggested hepatitis increased with
appearance of liver fibrosis.
Lobules of liver in the mice of T5 group exhibited a
disordered arrangement of hepatocytes and a pile of deposition of
fibrous tissue, which suggested the presence of liver cirrhosis.
Flipid droplets, hydropic degeneration, necrosis and regeneration
of hepatocytes were found. Certain regenerated hepatocytes
exhibited a larger volume and binucleate eggs. It is suggested that
liver lesions became liver cirrhosis in the present study.
Histologically, the liver lesions of T7 group were
classified as the high or middle differentiation group of HCC.
These lesions arose within diffuse dysplastic areas, and exhibited
invasive growth and a multifocal distribution. The liver lesions
were composed of moderately to highly pleomorphic cells disposed in
solid nests, trabeculae or multifocal pseudo-acinar structures,
supported by a loose fibrovascular stroma. The present study
observes that malignant cells were characterized by a large
nucleus, an irregular size and shape (pleomorphism), and an
irregular border. In addition, malignant cells exhibited a small
cytoplasm, frequently with vacuoles and consequently exhibit an
increased nuclear-cytoplasmic (N/C) ratio. Some tumor giant cells
and irregular mitosis were observed.
Changes in ultrastructure and mAFP in
alcohol/DEN/CCl4-treated and control mice livers
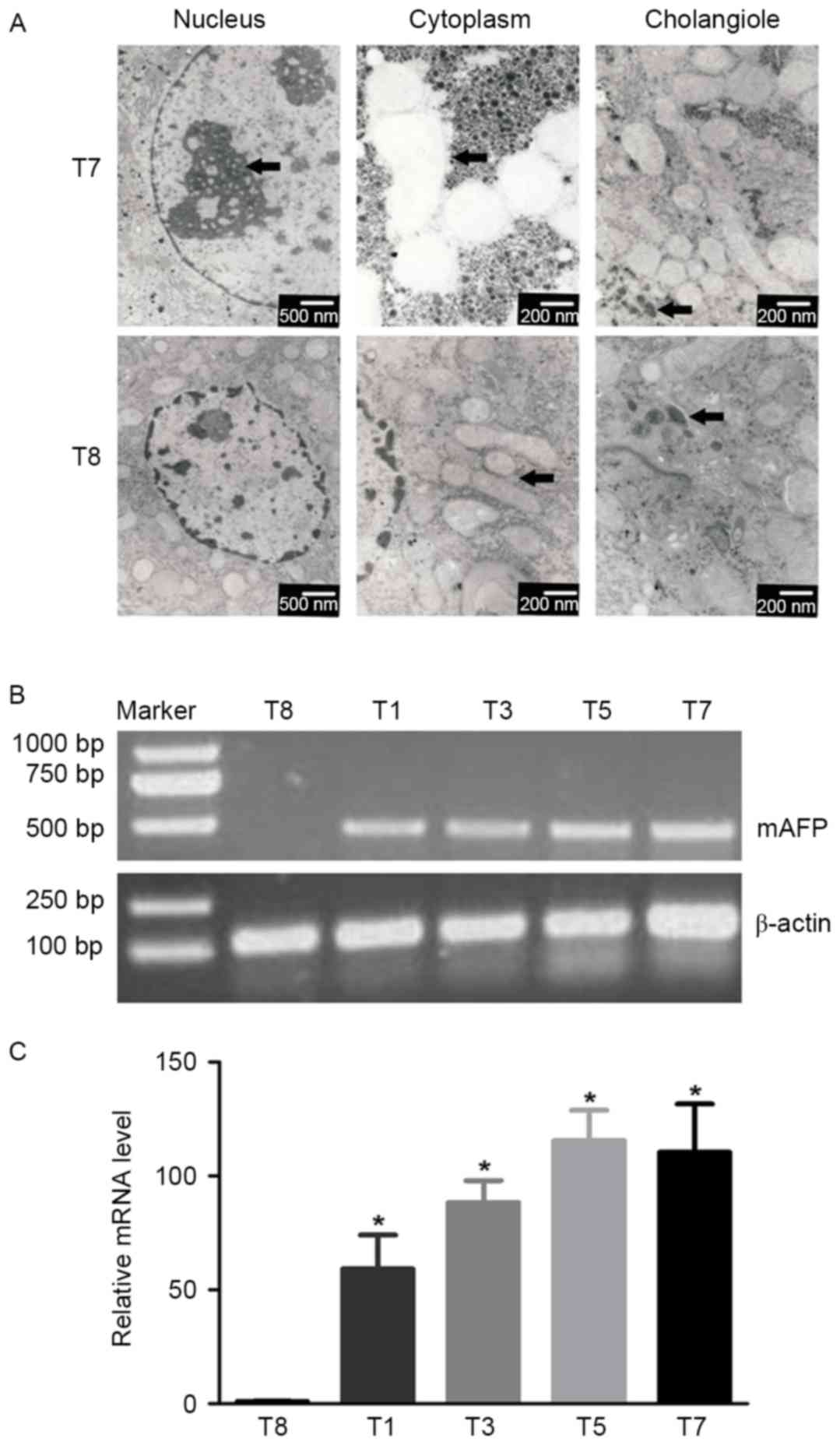
Electron microscopy revealed specific
ultrastructural changes in hepatic cells under experimental
conditions. Examples of a normal hepatic cell from group T8 and a
malignant hepatic cell of group T7, and their organelles are
illustrated in Fig. 4A. The
hepatocytes of group T8 were polygonal, with oval-shaped nuclei in
the center accompanied by more euchromatin and less
heterochromatin. The cytoplasm was crowded with mitochondria, rough
and smooth endoplasmic reticulum, golgi apparatus, ribosomes and
glycogen particles. The lumen of the cholangioles were filled with
numerous microvilli of hepatocytes.
Malignant cells of group T7 were characterized by a
large nucleus and a small cytoplasmic amount resulting in an
increased N/C ratio; the mitochondrial structure was loose and
vacuolated, and certain mitochondrial cristae were broken or
absent; the cholangioles exhibited poor development, which resulted
in cholestasis and less microvilli (arrowhead).
The expression of mAFP in liver tissues of
alcohol/DEN/CCl4-treated and control mice was examined.
Liver inflammation, fibrosis, cirrhosis and hepatocellular
carcinoma can cause AFP elevation compared with normal liver
(26). The expression of AFP mRNA
detected by RT-PCR was used to test whether AFP was expressed
during the whole experimental process following treatment with
alcohol/DEN/CCl4. The mAFP expression in the liver
tissues of treated mice was significantly increased compared with
that in control mice, as determined by RT-PCR (P<0.05; Fig. 4B).
Changes in other internal organs in
alcohol/DEN/CCl4-treated and control mice
The lungs, spleen, stomach, intestine, pancreas and
kidneys were collected and were carefully examined. Only the spleen
and pancreas exhibited abnormal changes in histology or weight. The
pancreas of control mice at T8 exhibited normal architecture and
cells; however, the pancreas of DEN-treated mouse at T3 (90 days),
T5 (120 days), and T7 (150 days) exhibited sporadic hydropic
degeneration (arrowhead), mild hydropic degeneration (larger size
but lower degree, arrowhead) or moderate hydropic degeneration
(arrowhead), respectively (Fig.
5A).
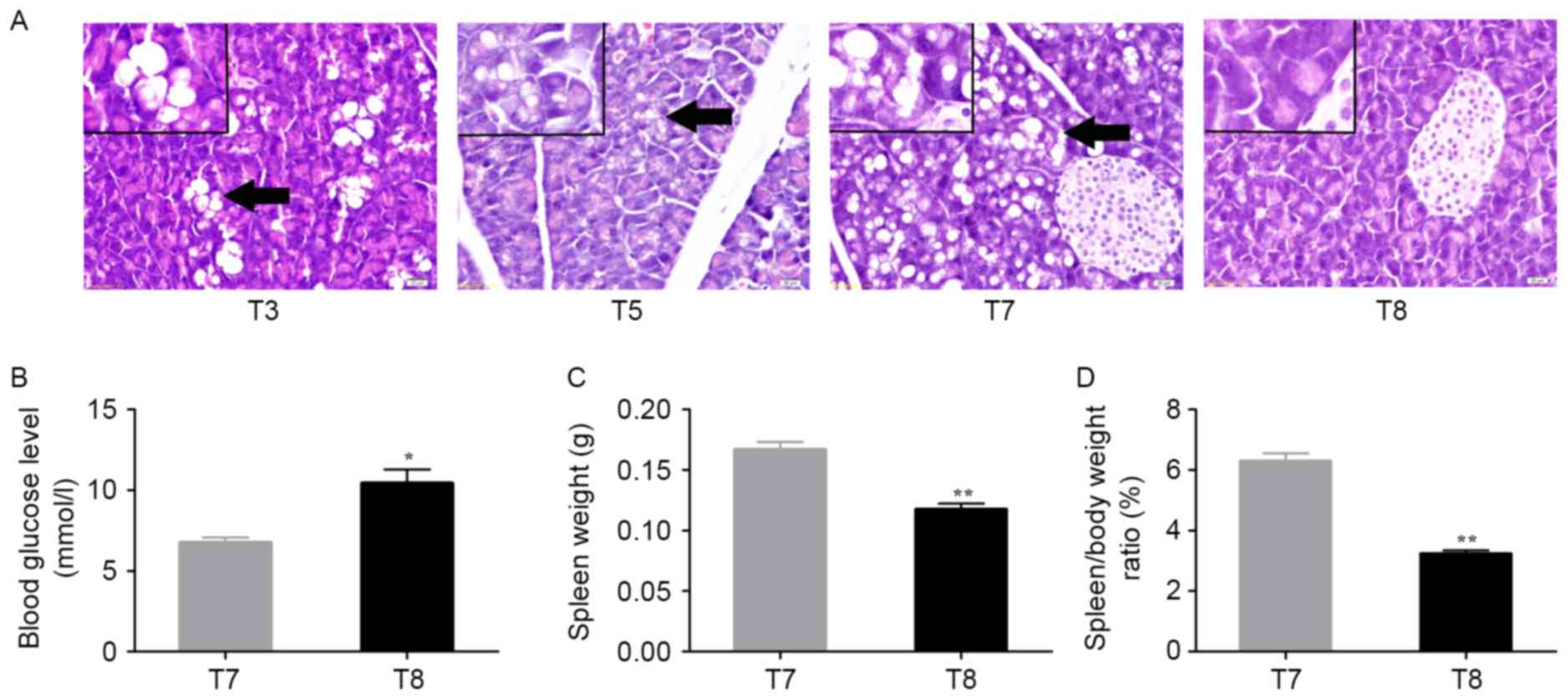 | Figure 5.Changes in pancreas and spleen in
alcohol/DEN/CCl4-treated and control mice. (A)
Histological pancreatic changes in
alcohol/DEN/CCl4-treated and control mice. The pancreas
of control mice at T8 exhibited normal architecture and cells. The
pancreas of DEN-treated mouse at T3 (90 days), T5 (120 days), and
T7 (150 days) exhibited a sporadic hydropic degeneration
(arrowhead), mild hydropic degeneration (bigger size but lower
degree, arrowhead), or moderate hydropic degeneration (arrowhead)
respectively. Hematoxylin and eosin staining. Magnification, ×400.
Scale bar=20 µm. (B) Analysis of blood glucose levels between
treated and control mice at 150d (*P<0.05, a two-tailed paired
t-test). (C) Analysis of spleen weight between treated and control
mice at day 150 (**P<0.01, a two-tailed paired t-test). (D)
Analysis of spleen/body weight ratio (%) between treated and
control mice at 150 days (**P<0.01, a two-tailed paired t-test).
Time in days (post-treatment): T3: 90 days; T5: 120 days; T7/8: 150
days. DEN, diethylnitrosamine. |
On day 150, the alcohol/DEN/CCl4-treated
mice of T7 exhibited a decreased blood glucose level (P<0.05;
Fig. 5B), but a significant increase
in spleen weight (P<0.01; Fig. 5C)
and spleen/body weight ratios (P<0.01; Fig. 5D) compared with control mice of T8 at
150 days.
Discussion
The present study aimed to evaluate the macroscopic,
microscopic and ultrastructural hepatic changes induced by
alcohol/DEN/CCl4 in adult male BALB/c mice, which is a
novel method of inducing liver cancer in a mouse model.
The genotoxic drug DEN is the most widely used
chemical to induce liver cancer in mice (8). In the model of the present study, HCC
development is similar to that in patients and typically follows a
slow multistep sequence, in which cycles of necrosis and
regeneration promote neoplastic transformation (8,11,15). The progression from early dysplastic
lesions to fully malignant tumors is associated with an increased
occurrence of genomic alterations (10,27,28). The
similarities and differences between the model of the present study
and the conventional one (DEN + phenoparpital) are that DEN and
phenoparpital were typically used in postnatal rats and mice
younger than 2 weeks for inducing HCC, whereas
DEN/CCl4/alcohol in the present study were used in adult
mice older than 7 weeks. DEN and phenoparpital takes 6–9 months to
induce HCC, whereas DEN/CCl4/alcohol in the present
study took 5 months to induce HCC (29).
CCl4 is a tumor-promoting agent that is
typically associated with classic experimental models for
steatohepatitis and liver fibrosis (8). If used for the HCC model,
CCl4 may be used for >12 months and is typically
combined with DEN or genetic models (8). The mechanism involves the generation of
free radicals during CCl4 metabolism by cellular
cytochrome P450 in the liver, including trichloromethyl and
oxygen-centered lipid radicals, which result in lipid peroxidation,
DNA modification, mitochondrial damage and even cell death
(30).
Alcohol is associated with HCC via the development
of cirrhosis; however, the published evidence does not support a
role for alcohol as a direct carcinogen for HCC (31). The mechanism of alcohol-induced
hepatotoxicity includes interactions between the direct toxic
effects and alcohol metabolites on various cell types in the liver,
induction of reactive oxygen species, upregulation of the
inflammatory cascade, in addition to other cell-specific effects in
the liver (32).
DEN is a chemical carcinogenic agent, and
CCl4 and alcohol are tumor-promoting agents (8). By using chemical carcinogenic agents and
tumor-promoting agents together, the present study developed an
AFP-secreting HCC model in the BALB/c mouse.
The findings of the present study are similar to
those of Da Costa et al (15),
who observed that DEN-induced hepatic lesions in mice, from initial
lesions to malignant neoplasms. However, the present study
presented some differences from their studies. At day 150,
following the first DEN injection (T7, 150 days following first DEN
exposure), an AFP-secreting HCC model developed faster compared
with just using DEN, by using adult mice not postnatal mice. The
malignant transformation rate of the survival mice in experimental
groups was 100%, and the liver lesions were classified as the high
or middle differentiation group of HCC. These lesions arose within
diffuse dysplastic areas, and exhibited invasive growth and a
multifocal distribution. The ultrastructural changes of the induced
liver neoplasms model were similar to those of human liver cancer
(8). Fatty degeneration was observed
in the pancreas, and the blood glucose levels were reduced compared
with the control, which may be similar to paraneoplastic syndrome
(PNS) in humans (31). In addition to
liver dysfunction, the histological changes in the pancreas may
account for why the blood glucose levels were reduced. The treated
mice demonstrated a significant increase in spleen weight and
spleen/body weight ratios compared with control mice at day 150,
which may be similar to spleen changes in humans when HCC occurs
(31).
The present study had certain limitations. The
sample size was relatively small. The changes of liver lesion's
ultrastructure, blood glucose level and spleen weight were observed
only at day 150. The level of insulin was not detected. Although
there is previously published research concerning the DEN-only
group (15), the DEN-only group was
not selected as a control for comparison. In future, studies
including all the relevant controls for models of HCC induction are
required in order to confirm these preliminary observations. The
reasons for the ultrastructure and measurement of blood glucose
level only being performed at 150 days following treatment is that
the lobules of liver in the mice of T5 group (120 days) were
observed to exhibit a disordered arrangement of hepatocytes and a
pile of deposition of fibrous tissue, which suggested liver
cirrhosis. Therefore, changes in ultrastructure in the T7 groups
(150 days) were detected to confirm the presence of HCC. The
present study aimed to detect changes in pancreatic structure;
however, the pancreases of DEN-treated mice at T3 (90 days) and T5
(120 days) exhibited sporadic hydropic degeneration or mild
hydropic degeneration, respectively. It would have been appropriate
to investigate whether changes in structure could cause changes in
blood glucose level; however, the whole blood of mice had not been
preserved. In future studies, the results of this preliminary study
would be further confirmed with complete haematological studies,
analyzing the levels of liver biomarkers such as alanine
transaminase, bilirubin, albumin and alkaline phosphatase, and
improved pathohistological methods and quantification of AFP at the
mRNA (RT-quantitative PCR) and protein (ELISA,
immunohistochemistry) level.
In conclusion, the present study used chemical
carcinogenic agents and tumor-promoting agents together to
successfully develop an AFP-secreting HCC model in adult male
BALB/c mouse for the first time, to the best of our knowledge. This
method used less time for inducement and the malignant
transformation rate of the surviving mice in the experimental
groups was 100%. The disease process and ultrastructural changes
met the criterion of the human liver cancer process (2). In addition, the changes of blood glucose
levels were similar to PNS in humans and the increase in spleen
weight was similar to spleen changes during human HCC (31). Therefore, this model may be an ideal
experimental animal model for studying the occurrence and
development of liver cancer, and may be a novel animal model for
studying PNS in primary hepatic carcinoma.
Acknowledgements
The present study was supported by the National
National Science Foundation of China (grant nos. 31300830 and
81371615).
References
|
1
|
Nordenstedt H, White DL and El-Serag HB:
The changing pattern of epidemiology in hepatocellular carcinoma.
Dig Liver Dis. 42 Suppl 3:S206–S214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dhanasekaran R, Limaye A and Cabrera R:
Hepatocellular carcinoma: Current trends in worldwide epidemiology,
risk factors, diagnosis, and therapeutics. Hepat Med. 4:19–37.
2012.PubMed/NCBI
|
|
3
|
Kassebaum NJ, Bertozzi-Villa A, Coggeshall
MS, Shackelford KA, Steiner C, Heuton KR, Gonzalez-Medina D, Barber
R, Huynh C, Dicker D, et al: Global, regional, and national levels
and causes of maternal mortality during 1990–2013: A systematic
analysis for the Global Burden of Disease Study 2013. Lancet.
384:980–1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sherman M: The resurrection of
alphafetoprotein. J hepatol. 52:939–940. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertino G, Neri S, Bruno CM, Ardiri AM,
Calvagno GS, Malaguarnera M, Toro A, Malaguarnera M, Clementi S,
Bertino N and Di Carlo I: Diagnostic and prognostic value of
alpha-fetoprotein, des-γ-carboxy prothrombin and squamous cell
carcinoma antigen immunoglobulin M complexes in hepatocellular
carcinoma. Minerva Med. 102:363–371. 2011.PubMed/NCBI
|
|
8
|
Bakiri L and Wagner EF: Mouse models for
liver cancer. Mol Oncol. 7:206–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rajewsky MF, Dauber W and Frankenberg H:
Liver carcinogenesis by diethylnitrosamine in the rat. Science.
152:83–85. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi Y, Chen X, Chan CY, Li D, Yuan C, Yu F,
Lin MC, Yew DT, Kung HF and Lai L: Two-dimensional differential gel
electrophoresis/analysis of diethylnitrosamine induced rat
hepatocellular carcinoma. Int J Cancer. 122:2682–2688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park EJ, Lee JH, Yu GY, He G, Ali SR,
Holzer RG, Osterreicher CH, Takahashi H and Karin M: Dietary and
genetic obesity promote liver inflammation and tumorigenesis by
enhancing IL-6 and TNF expression. Cell. 140:197–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diwan BA, Rice JM, Ohshima M and Ward JM:
Interstrain differences in susceptibility to liver carcinogenesis
initiated by N-nitrosodiethylamine and its promotion by
phenobarbital in C57BL/6NCr, C3H/HeNCrMTV- and DBA/2NCr mice.
Carcinogenesis. 7:215–220. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pok S, Wen V, Shackel N, Alsop A, Pyakurel
P, Fahrer A, Farrell GC and Teoh NC: Cyclin E facilitates
dysplastic hepatocytes to bypass G1/S checkpoint in
hepatocarcinogenesis. J Gastroenterol Hepatol. 28:1545–1554. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong VW and Janssen HL: Can we use HCC
risk scores to individualize surveillance in chronic hepatitis B
infection? J Hepatol. 63:722–732. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Da Costa RM, Paula-Santos N, Rocha AF,
Colaco A, Lopes C and Oliveira PA: The N-nitrosodiethylamine mouse
model: Sketching a timeline of evolution of chemically-induced
hepatic lesions. Anticancer Res. 34:7029–7037. 2014.PubMed/NCBI
|
|
16
|
Szabo G: Gut-liver axis in alcoholic liver
disease. Gastroenterology. 148:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mandrekar P, Ambade A, Lim A, Szabo G and
Catalano D: An essential role for monocyte chemoattractant
protein-1 in alcoholic liver injury: Regulation of proinflammatory
cytokines and hepatic steatosis in mice. Hepatology. 54:2185–2197.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szabo G, Petrasek J and Bala S: Innate
immunity and alcoholic liver disease. Dig Dis. 30 Suppl 1:S55–S60.
2012. View Article : Google Scholar
|
|
19
|
Wheeler MD, Kono H, Yin M, Nakagami M,
Uesugi T, Arteel GE, Gäbele E, Rusyn I, Yamashina S, Froh M, et al:
The role of Kupffer cell oxidant production in early
ethanol-induced liver disease. Free Radic Biol Med. 31:1544–1549.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Enomoto N, Ikejima K, Bradford BU, Rivera
CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H, et
al: Role of Kupffer cells and gut-derived endotoxins in alcoholic
liver injury. J Gastroenterol Hepatol. 15 Suppl:D20–D25. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uesugi T, Froh M, Arteel GE, Bradford BU
and Thurman RG: Toll-like receptor 4 is involved in the mechanism
of early alcohol-induced liver injury in mice. Hepatology.
34:101–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petrasek J, Mandrekar P and Szabo G:
Toll-like receptors in the pathogenesis of alcoholic liver disease.
Gastroenterol Res Pract. 2010:pii:7103812010. View Article : Google Scholar
|
|
23
|
Dapito DH, Mencin A, Gwak GY, Pradere JP,
Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A,
Bataller R, et al: Promotion of hepatocellular carcinoma by the
intestinal microbiota and TLR4. Cancer Cell. 21:504–516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Machida K, Tsukamoto H, Mkrtchyan H, Duan
L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH, et
al: Toll-like receptor 4 mediates synergism between alcohol and HCV
in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl
Acad Sci USA. 106:1548–1553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kapanadze T, Gamrekelashvili J, Ma C, Chan
C, Zhao F, Hewitt S, Zender L, Kapoor V, Felsher DW, Manns MP, et
al: Regulation of accumulation and function of myeloid derived
suppressor cells in different murine models of hepatocellular
carcinoma. J Hepatol. 59:1007–1013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Serag HB, Kanwal F, Davila JA, Kramer J
and Richardson P: A new laboratory-based algorithm to predict
development of hepatocellular carcinoma in patients with hepatitis
C and cirrhosis. Gastroenterology. 146:1249–1255.e1. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teufel A, Maass T, Strand S, Kanzler S,
Galante T, Becker K, Strand D, Biesterfeld S, Westphal H and Galle
PR: Liver-specific Ldb1 deletion results in enhanced liver cancer
development. J Hepatol. 53:1078–1084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai Z, Lou Q, Wang F, Li E, Sun J, Fang H,
Xi J and Ju L: N-acetylcysteine protects against liver injure
induced by carbon tetrachloride via activation of the Nrf2/HO-1
pathway. Int J Clin Exp Pathol. 8:8655–8662. 2015.PubMed/NCBI
|
|
31
|
Saran U, Humar B, Kolly P and Dufour JF:
Hepatocellular Carcinoma and Lifestyles. J Hepatol. 64:203–214.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Neuman MG, Maor Y, Nanau RM, Melzer E,
Mell H, Opris M, Cohen L and Malnick S: Alcoholic liver disease:
Role of cytokines. Biomolecules. 5:2023–2034. 2015. View Article : Google Scholar : PubMed/NCBI
|