Introduction
Bladder cancer ranks 13th in the causes for
cancer-associated mortality worldwide and is the most common type
of urological cancer in China (1).
Muscle-invasive bladder cancer constitutes ~30% of newly diagnosed
cases of bladder cancer (2).
Approximately 10% of non-invasive bladder cancer cases eventually
progress to invasive cancer following the transurethral resection
of the bladder tumor (3). Compared
with non-invasive bladder cancer, patients with invasive disease
have a poor prognosis, with a 5-year survival rate of 50% (4). Systemic chemotherapy remains the major
therapeutic option for muscle-invasive bladder cancer in
neoadjuvant and adjuvant settings, as well as for metastatic
disease. Although it often leads to an initial therapeutic success
in patients with metastatic bladder cancer, 60–70% of responding
patients relapse within the first year of treatment, with a median
survival time of 12–14 months (5).
This limited efficacy appears to be largely associated with drug
resistance of the tumor during treatment. Therefore, there is an
urgent requirement to develop chemosensitization strategies.
Mitomycin-C (MMC) is widely used as a
chemotherapeutic drug in the treatment of bladder cancer. However,
only a limited number of patients were microscopically free of
tumor cells following MMC treatment. The development of resistance
to MCC is a major concern in bladder cancer therapy, and the
mechanism remains largely unclear. Several methods have been
demonstrated to increase the anticancer efficacy of MMC therapy in
bladder cancer (6,7). In our previous study, it was revealed
that as a DNA methyltransferase inhibitor, 5-Aza-2′-deoxycitidine
(5-Aza-CdR) could inhibit the proliferation, migration and invasion
of the T24 bladder cancer cell line (8). However, whether 5-Aza-CdR could affect
the chemosensitivity of T24 cells was not studied. Therefore, the
present study aimed to investigate the effects of 5-Aza-CdR on the
MMC chemosensitivity of bladder cancer T24 cells. The underlying
mechanisms were also investigated.
Materials and methods
Cell culture and chemicals
MMC was purchased from the Zhejiang Haizheng Group
Co., Ltd. (Taizhou, China). It was dissolved in physiological
saline to a concentration of 0.01 mg/ml, and then stored until use
at −4°C. 5-Aza-CdR was purchased from Sigma-Aldrich (Merck KGaA;
Darmstadt, Germany). It was dissolved in physiological saline to a
concentration of 0.25, 1 and 10 µmol/l, and then stored until use
at −20°C, protected from light.
T24 human bladder cancer cells were obtained from
Beijing Dingguo Changsheng Biotechnology Co., Ltd. (Beijing,
China). The cells were cultured in Dulbecco's modified Eagle's
medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) with high
glucose (4,500 mg/l), supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cell
culture was conducted with 5% CO2 at 37°C.
T24 cells were harvested in the exponential phase.
Cells were seeded in 96-well plates at a density of
2×104 cells per well. Following incubation overnight in
the previously specified conditions, the cells were treated with
0.01 mg/ml MMC and 5-Aza-CdR at different concentrations (including
0, 0.25, 1 and 10 µmol/l). T24 cells without MMC and 5-Aza-CdR
served as a control. At 48 h, the cells were obtained for
subsequent experiments.
MTT assay
Cells were treated with 20 µl MTT dye
(Sigma-Aldrich; Merck KGaA) and incubated at 37°C for 4 h. The
supernatant was then removed, and 100 µl of dimethylsulfoxide was
added to every well to dissolve the formazan product. The
absorbance was recorded at a wavelength of 570 nm using a
microplate reader (Bio-Rad Laboratories, Inc.). The following
calculation was used: Cellular inhibition rate, % = (1-treated
group absorbance/control group absorbance) ×100.
Flow cytometric analysis
The cells were washed twice with PBS, harvested with
pancreatin and centrifugated (164.3 × g, 5 min) at room
temperature, and resuspended. The samples were incubated with 5 µl
Annexin V-fluorescein isothiocyanate and 5 µl propidium iodide for
exactly 5 min at room temperature in the dark and then measured on
a FACS Vantage Flow Cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Western blotting
The cells were lysed with lysis buffer (ProMab
Biotechnologies, Inc., Richmond, CA, USA). The total protein
concentration was quantified by a bicinchoninic acid protein assay.
Appropriate amounts of protein (20–40 µg per well) were separated
with 10% SDS-PAGE. The proteins were then transferred to a
polyvinylidene difluoride membrane. The membranes were blocked at
37°C for 3 h in a blocking solution consisting of 5% nonfat milk
and TBS with 0.1% Tween-20. The primary antibodies, including mouse
anti-P-gp (cat. no. ab80594; dilution, 1:1,000), mouse anti-MRP1
(cat. no. ab24102; dilution, 1:1,000), rabbit anti-beclin 1 (cat.
no. ab62557; dilution, 1:1,000), mouse anti-p62 (cat. no. ab56416;
dilution, 1:1,000), rabbit anti-ATG5 (cat. no. ab108327; dilution,
1:1,000) and mouse anti-GAPDH (cat. no. ab8245; dilution, 1:1,000)
(all from Abcam, Cambridge, UK) were incubated overnight with the
membrane at 4°C, followed by corresponding horseradish
peroxidase-conjugated secondary antibodies goat anti-rabbit
immunoglobulin (Ig)G/horse radish peroxidase (HRP) (cat. no.
ab6721; dilution, 1:2,000) and goat anti-mouse IgG/HRP (cat. no.
ab6789; dilution, 1:2,000) (all from Abcam) at room temperature for
1 h. Immunoreactive bands were detected using an Enhanced
Chemiluminescence-plus kit (GE Healthcare, Chicago, IL, USA)
according to the manufacturer's protocol. The chemiluminescence was
analyzed using ChemiDoc XRS system with Image Lab Software version
6.0 (Bio-Rad Laboratories, Inc., Hercules, CA). The levels of the
target protein were presented as the relative density vs.
GAPDH.
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. Data are presented as the mean ± standard
deviation. The significance of the data was determined by Student's
t-test and one-way analysis of variance. The post hoc test was
performed using Student-Newman-Keuls method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of 5-Aza-CdR on MMC-induced
proliferation inhibition in T24 cells
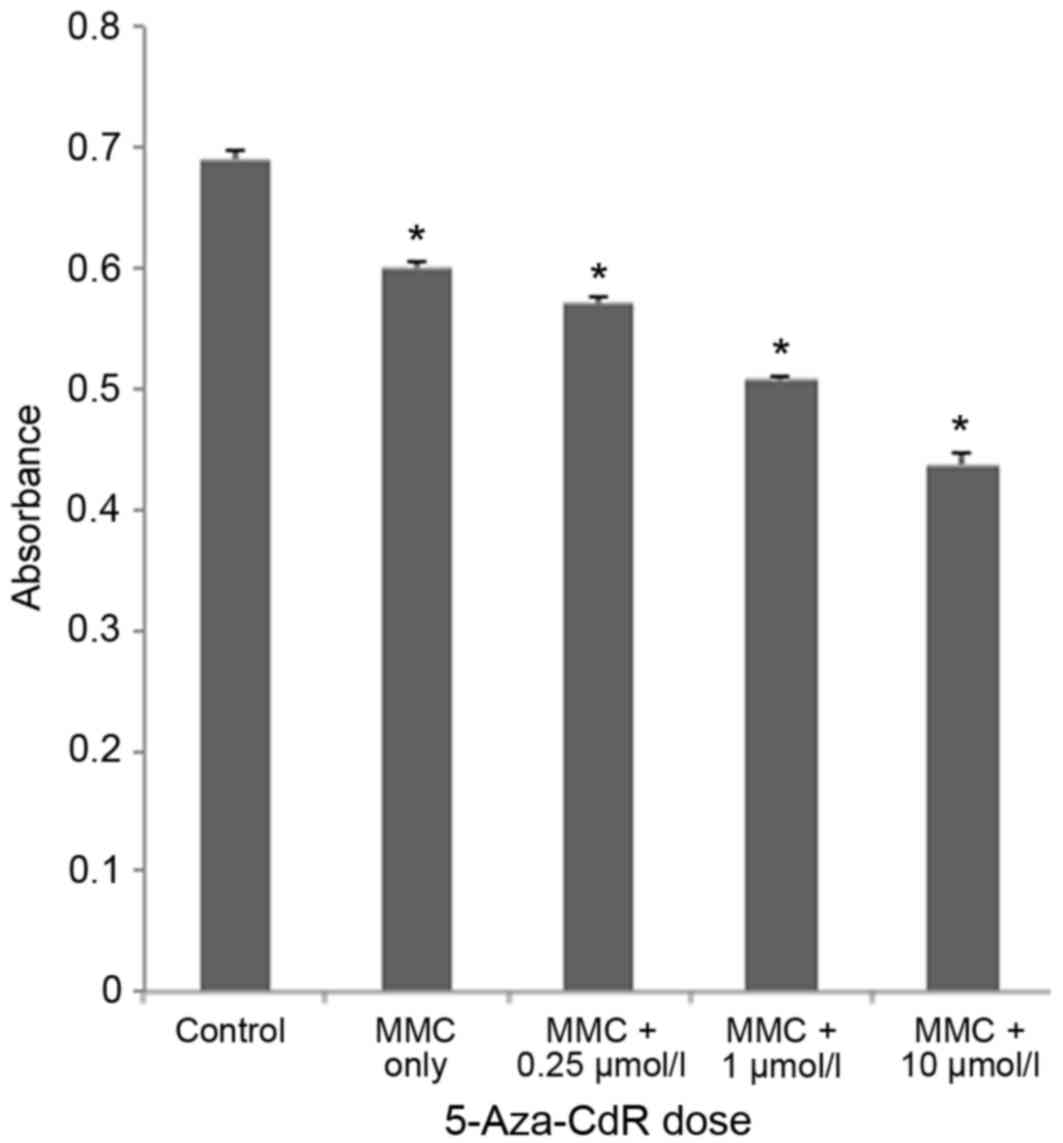
To investigate the effects of 5-Aza-CdR treatment,
an MTT assay was performed subsequent to treating T24 cells with
different concentrations of 5-Aza-CdR (0, 0.25, 1 and 10 µmol/l)
and MMC (0.01 mg/ml). 5-Aza-CdR enhanced MMC-induced proliferation
inhibition in T24 cells (Fig. 1). The
rate of T24 cell growth inhibition was 1.63±0.01 with MMC only, and
5.0±0.04, 15.50±0.06 and 27.16±0.11%, with 0.025, 1 or 10 µmol/l
5-Aza-CdR, respectively; growth inhibition was thus induced in a
dose-dependent manner by 5-Aza-CdR (P<0.05).
Effects of 5-Aza-CdR on MMC-induced
apoptosis in T24 cells
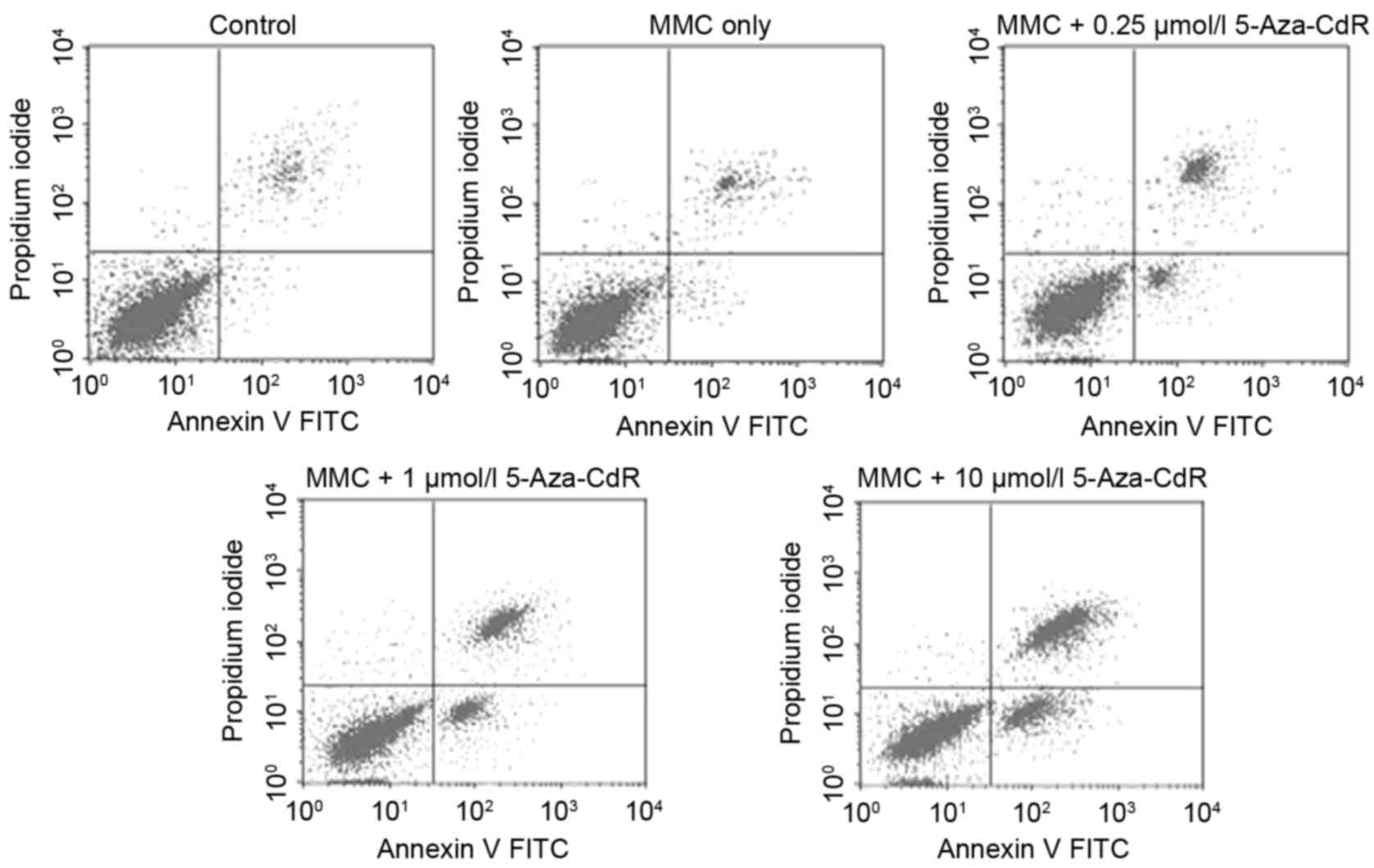
To determine whether 5-Aza-CdR could enhance
MMC-induced apoptosis in T24 cells, flow cytometry was performed to
identify the apoptotic rate of cells. The apoptotic rate increased
in a 5-Aza-CdR-dose-dependent manner, including early and late
apoptotic cell death (P<0.05; Fig.
2). In the control group, 3.23±0.08% of cells underwent
apoptosis, whereas 4.85±0.04, 8.81±0.06, 21.14±0.05 and 38.17±0.07%
of cells underwent apoptosis in the 0, 0.25, 1 and 10 µmol/l
5-Aza-CdR groups, respectively.
Effects of 5-Aza-CdR on the expression
of chemoresistance-associated proteins in T24 cells
To elucidate the potential mechanism for
5-Aza-CdR-mediated chemosensitivity alterations, western blot
analysis was performed to examine the effects on P-gp and MRP1
expression. The results demonstrated that with increasing
5-Aza-CdR, the expression levels of P-gp and MRP1 were
significantly decreased (P<0.05; Fig.
3).
Effects of 5-Aza-CdR on the expression
of autophagy-associated proteins in T24 cells
The expression level of the autophagy-associated
proteins beclin 1, p62 and ATG5 was determined with western blot
analysis. Data revealed that with increasing 5-Aza-CdR
concentration, the expression levels of beclin 1, p62 and ATG5 were
all significantly decreased (P<0.05; Fig. 4).
 | Figure 4.Western blotting analysis of the
expression of beclin 1, p62 and ATG5 protein in T24 cells. (A)
Beclin 1, p62 and ATG5 expression levels in T24 cells were measured
by western blotting. 1 is the control, 2 MMC only, 3 MMC + 0.25
µmol/l, 4 MMC + 1 µmol/l and 5 MMC + 10 µmol/l 5-Aza-2′
deoxycitidine. GAPDH was used as an internal control. (B) The
relative protein levels, normalized to GAPDH. Beclin 1, p62 and
ATG5 expression levels were decreased with increasing 5-Aza-CdR
treatment. The data are presented as the mean ± standard deviation
(n=3). *P<0.05 vs. control. p62, nucleoporin 62; ATG5, autophagy
5; MMC, mitomycin-C; 5-Aza-CdR, 5-Aza-2′-deoxycitidine. |
Discussion
Resistance to chemotherapeutic drugs is an important
reason for clinical chemotherapy failure in bladder cancer. In the
present study, it was identified that 5-Aza-CdR may enhance the MMC
sensitivity of T24 bladder cancer cells. The apoptosis of T24 cells
was significantly promoted by combined treatment with MMC and
5-Aza-CdR, compared with those treated by MMC alone. Furthermore,
with increasing 5-Aza-CdR concentrations, the cellular inhibition
rates increased in a dose-dependent manner. This data indicated
that 5-Aza-CdR serves a role in the enhancement of MMC
chemosensitivity of bladder cancer T24 cells.
To investigate the mechanism of 5-Aza-CdR in the
chemosensitivity of bladder cancer T24 cells, western blotting was
used to detect the expression of P-gp and MRP1 protein, which are
associated with chemotherapeutic resistance. The data revealed that
the expression levels of P-gp and MRP1 protein in T24 cells
following treatment with MMC were significantly decreased in a
dose-dependent manner with increasing 5-Aza-CdR concentration. P-gp
and MRP1 protein belong to a family of ATP-dependent efflux
transporters termed the ATP-binding cassette (ABC) family of
membrane transport proteins (9).
Members of the ABC transporter family have the capacity to efflux
small molecules, causing drug accumulation in the cell (including
the accumulation of anticancer drugs) to decrease, and thus
contribute to drug resistance (10).
The clinical significance of P-gp and MRP1 in drug resistance is
supported by evidence that their expression indicates an adverse
prognosis in patients with a range of types of cancer (11,12). Yang
et al (13) demonstrated that
Nsc23925 could prevent the development of paclitaxel resistance by
inhibiting the expression of P-gp and enhancing apoptosis.
Therefore, it was concluded from the data of the present study that
5-Aza-CdR enhances MMC chemosensitivity of T24 cells by suppressing
P-gp and MRP1 expression.
Based on this result, studying the mechanism for the
effect of 5-Aza-CdR on the chemosensitivity of T24 bladder cancer
cells may yield clinical value. The expression levels of beclin 1,
p62 and ATG5 protein were then detected, which were associated with
autophagy. It was demonstrated that the expression levels of beclin
1, p62 and ATG5 protein in T24 cells were decreased in a
dose-dependent manner following treatment with MMC and increasing
5-Aza-CdR concentrations. Beclin 1, p62 and ATG5 are considered as
the key regulators of autophagic cell death (14–16).
Autophagy is a lysosome-dependent self-digesting system primarily
responsible for the removal and recycling of long-lived proteins,
and damaged or obsolete intracellular organelles, in order to
maintain cell homeostasis (17). The
exact role of autophagy in cancer remains controversial. A number
of studies provide evidence that autophagy suppresses tumorigenesis
(18,19), whereas other studies propose that
autophagy is associated with tumor development and protects tumor
cells from apoptosis (20,21). In addition, a role for autophagy in
the chemosensitivity of cancer cells has been identified; Wu et
al (22) reported that autophagy
may facilitate the resistance of lung adenocarcinoma cells to
cisplatin treatment by the activation of the AMP-activated protein
kinase/mechanistic target of rapamycin signaling pathway. Yang
et al (23) demonstrated that
the inhibition of autophagy could reduce pancreatic cancer stem
cell activity and potentiate the tumoricidal effect of gemcitabine.
In the present study, the expression of beclin 1, p62 and ATG5 in
T24 cells was decreased in a dose-dependent manner following
treatment with MMC and increasing 5-Aza-CdR treatment, indicating
the reduced autophagy activity. Based on the regulatory role of
autophagy in chemosensitivity, it was speculated that 5-Aza-CdR
enhanced MMC chemosensitivity of T24 cells partially by suppression
of autophagy. Future studies involving autophagy and
chemosensitivity are warranted to confirm the conclusions of the
present study.
In our previous study, 5-Aza-CdR was revealed to
exhibit an inhibitory effect on the proliferation, migration and
invasion of T24 bladder cancer cells (8). In the present study, it was demonstrated
that 5-Aza-CdR could enhance the cytotoxicity of MMC in T24 cells.
This effect may be partially mediated by the suppression of drug
resistance- and autophagy-associated proteins. Although the
mechanism remains to be clarified, the conclusions of the present
study may provide a new therapeutic option to overcome
chemoresistance in bladder cancer.
Acknowledgements
The present study was supported by the Science
Project of Hengyang City (grant no. 2016KJ34) and the National
Natural Science Foundation of China (grant no. 81602241).
References
|
1
|
Chen Y, Yang Y, Liu L, Wang S, Song H and
Liu X: Tumor suppressor in lung cancer-1 is a prognostic predictor
for the recurrence and progression of non-muscle-invasive bladder
cancer. Urol Int. 96:142–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jayaratna IS, Navai N and Dinney CP: Risk
based neoadjuvant chemotherapy in muscle invasive bladder cancer.
Transl Androl Urol. 4:273–282. 2015.PubMed/NCBI
|
|
3
|
Izumi K, Ito Y, Miyamoto H, Miyoshi Y, Ota
J, Moriyama M, Murai T, Hayashi H, Inayama Y, Ohashi K, et al:
Expression of androgen receptor in non-muscle-invasive bladder
cancer predicts the preventive effect of androgen deprivation
therapy on tumor recurrence. Oncotarget. 7:14153–14160. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Byun SJ, Kim JH, Oh YK and Kim BH:
Concurrent chemoradiotherapy improves survival outcome in
muscle-invasive bladder cancer. Radiat Oncol J. 33:294–300. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun L, Lu J, Niu Z, Ding K, Bi D, Liu S,
Li J, Wu F, Zhang H, Zhao Z and Ding S: A Potent chemotherapeutic
strategy with Eg5 inhibitor against gemcitabine resistant bladder
cancer. PLoS One. 10:e01444842015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vasquez JL, Gehl J and Hermann GG:
Electroporation enhances mitomycin C cytotoxicity on T24 bladder
cancer cell line: A potential improvement of intravesical
chemotherapy in bladder cancer. Bioelectrochemistry. 88:127–133.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gederaas OA, Søgaard CD, Viset T, Bachke
S, Bruheim P, Arum CJ and Otterlei M: Increased anticancer efficacy
of intravesical mitomycin c therapy when combined with a PCNA
targeting peptide. Transl Oncol. 7:812–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Qi F, Cao Y, Zu X, Chen M, Li Z
and Qi L: 5-Aza-2′-deoxycytidine enhances maspin expression and
inhibits proliferation, migration, and invasion of the bladder
cancer T24 cell line. Cancer Biother Radiopharm. 28:343–350. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Higgins CF: ABC transporters: From
microorganisms to man. Annu Rev Cell Biol. 8:67–113. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sedláková I, Laco J, Caltová K, Červinka
M, Tošner J, Řezáč A and Špaček J: Clinical significance of the
resistance proteins LRP, Pgp, MRP1, MRP3 and MRP5 in epithelial
ovarian cancer. Int J Gynecol Cancer. 25:236–243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kovalev AA, Tsvetaeva DA and Grudinskaja
TV: Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the
development of primary and acquired multiple drug resistance in
patients with early and metastatic breast cancer. Exp Oncol.
35:287–290. 2013.PubMed/NCBI
|
|
12
|
Roundhill E and Burchill S: Membrane
expression of MRP-1, but not MRP-1 splicing or Pgp expression,
predicts survival in patients with ESFT. Br J Cancer. 109:195–206.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang X, Shen J, Gao Y, Feng Y, Guan Y,
Zhang Z, Mankin H, Hornicek FJ and Duan Z: Nsc23925 prevents the
development of paclitaxel resistance by inhibiting the introduction
of P-glycoprotein and enhancing apoptosis. Int J Cancer.
137:2029–2039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katagiri H, Nakayama K, Razia S, Nakamura
K, Sato E, Ishibashi T, Ishikawa M, Iida K, Ishikawa N, Otsuki Y,
et al: Loss of autophagy-related protein Beclin 1 may define poor
prognosis in ovarian clear cell carcinomas. Int J Oncol.
47:2037–2044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartsch G, Jennewein L, Harter PN,
Antonietti P, Blaheta RA, Kvasnicka HM, Kögel D, Haferkamp A,
Mittelbronn M and Mani J: Autophagy-associated proteins BAG3 and
p62 in testicular cancer. Oncol Rep. 35:1629–1635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ge J and Chen Z, Huang J, Chen J, Yuan W,
Deng Z and Chen Z: Upregulation of autophagy-related gene-5 (ATG-5)
is associated with chemoresistance in human gastric cancer. PLoS
One. 9:e1102932014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levine B: Cell biology: Autophagy and
cancer. Nature. 446:745–747. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Z, Zhao T, Liu H and Zhang L:
Ginsenoside Rh2 inhibits hepatocellular carcinoma through β-catenin
and autophagy. Sci Rep. 6:193832016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang SJ, Ou-Yang F, Tu HP, Lin CH, Huang
SH, Kostoro J, Hou MF, Chai CY and Kwan AL: Decreased expression of
autophagy protein LC3 and stemness
(CD44+/CD24−/low) indicate poor prognosis in
triple-negative breast cancer. Hum Pathol. 48:48–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masuda GO, Yashiro M, Kitayama K, Miki Y,
Kasashima H, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T,
Sakurai K, et al: Clinicopathological correlations of
autophagy-related proteins LC3, Beclin 1 and p62 in gastric cancer.
Anticancer Res. 36:129–136. 2016.PubMed/NCBI
|
|
21
|
Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y
and Fan Y: Upregulated SMYD3 promotes bladder cancer progression by
targeting BCLAF1 and activating autophagy. Tumour Biol.
37:7371–7381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu T, Wang MC, Jing L, Liu ZY, Guo H, Liu
Y, Bai YY, Cheng YZ, Nan KJ and Liang X: Autophagy facilitates lung
adenocarcinoma resistance to cisplatin treatment by activation of
AMPK/mTOR signaling pathway. Drug Des Devel Ther. 9:6421–6431.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang MC, Wang HC, Hou YC, Tung HL, Chiu TJ
and Shan YS: Blockade of autophagy reduces pancreatic cancer stem
cell activity and potentiates the tumoricidal effect of
gemcitabine. Mol Cancer. 14:1792015. View Article : Google Scholar : PubMed/NCBI
|