Introduction
Following the identification of a gastrointestinal
submucosal tumor (SMT), periodic surveillance using endoscopy and
endoscopic ultrasonography (EUS) remains a major strategy, but the
use of this strategy is associated with multiple concerns,
including patient compliance and stress, cost-effectiveness, and
the risks associated with repeated endoscopic procedures and
delayed diagnosis of malignancy (1,2).
Furthermore, certain tumors exhibit malignant potential,
particularly those that originate from the muscularis propria (MP)
layer or are large in diameter (1).
Therefore, removing these SMTs is crucial. Current methods to
remove SMTs include surgery and endoscopic resection, compared with
the latter, surgical approaches are more invasive and associated
with increased costs and a longer hospital stay. Endoscopic
resection is a first-line treatment for SMTs ≤50 mm in diameter
(1,2).
Alternative methods include endoscopic submucosal dissection (ESD),
endoscopic submucosal excavation (ESE) and endoscopic
full-thickness resection, but these may be associated with
unsatisfactory outcomes due to incomplete resection and/or the risk
of perforation during the procedure (3–5).
Submucosal tunneling endoscopic resection (STER) has emerged as a
novel technique for treating upper gastrointestinal SMTs and has
yielded promising results (6–17). STER possesses multiple advantages over
other endoscopic methods, including the maintenance of mucosal
integrity, the facilitation of an increased rate of healing and a
decreased risk of pleural/abdominal infection. In addition, the
submucosal tunnel helps to maintain a clear visual field, which
facilitates an improved response to intraoperative bleeding. The
present study summarized the current status of STER, including its
applications, procedure, efficacy and complications.
Preoperative assessment
Prior to performing STER, the presence, originating
layer, size, and the presence or absence of malignancy-associated
risk features of the SMT should be confirmed. The SMT should also
be distinguished from extrinsic compression or hemangioma.
Esophagogastroduodenoscopy and colonoscopy may be used to locate
the lesion, and EUS and computerized tomography (CT) may be used to
determine the originating layer, size and risk features of the SMT
(1).
Applications of STER
STER for esophageal and cardia SMTs
≤35 mm
STER is a complicated procedure with a decreased
space for operation in the submucosal tunnel, and therefore was
initially performed for esophageal and cardia SMTs, with most
researchers recommending a maximum resectable lesion size of 35 mm
(6–10). With STER being increasingly applied
for patients with multiple types of SMT, STER has been modified
multiple times and its application has expanded further. The
patient selection diagram for candidates of STER at the Second
Xiangya Hospital of Central South University (Changsha, China) was
provided (Fig. 1).
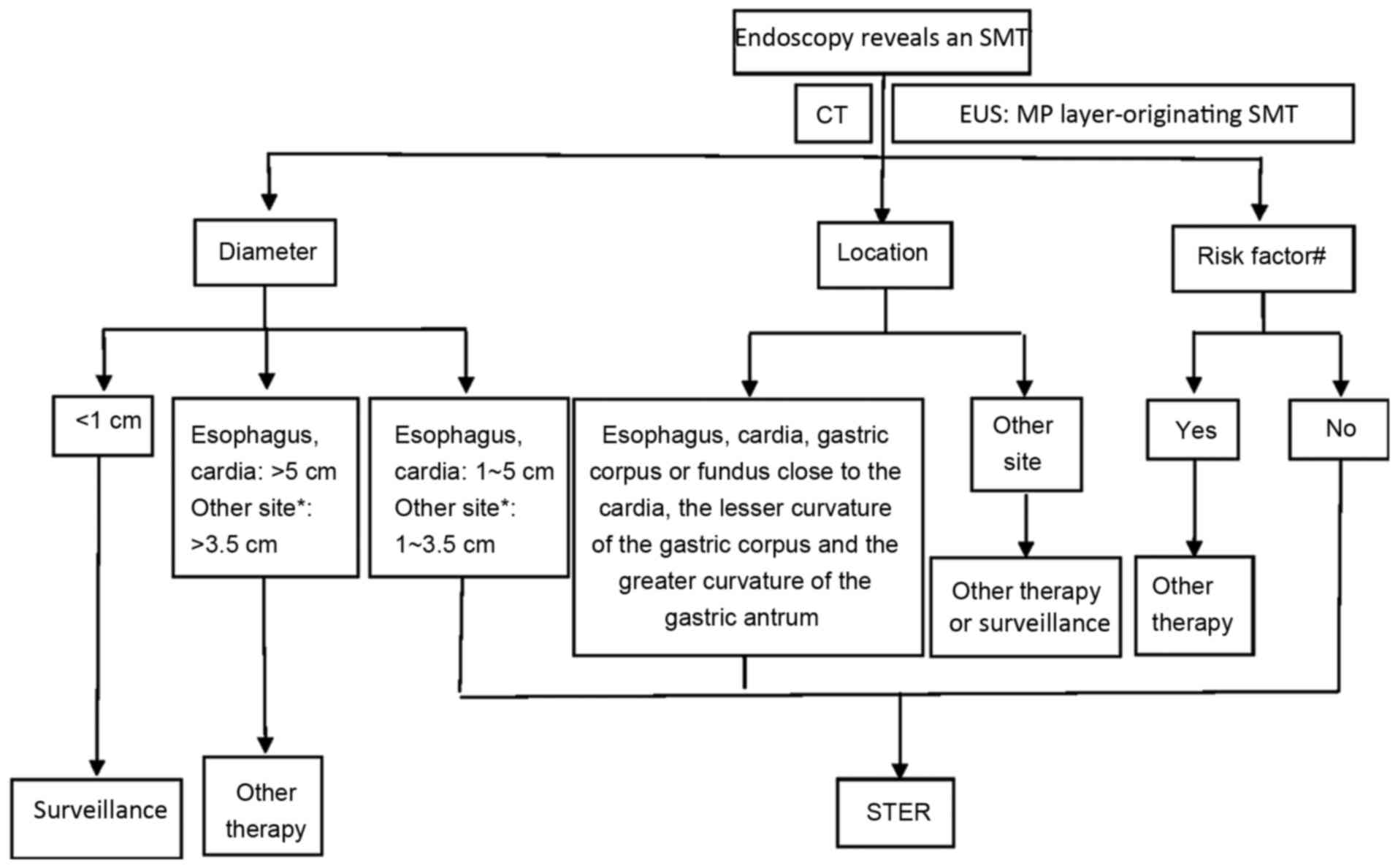 | Figure 1.Patient selection diagram for
candidates for STER at the Second Xiangya Hospital of Central South
University (Changsha, China). *Gastric corpus or fundus proximate
to the cardia, the lesser curvature of the gastric corpus and the
greater curvature of the gastric antrum. #Ulceration or
erosion at the tumor site; EUS reveals an irregular border, or
internal heterogeneity, including an anechoic area (i.e. necrosis),
echogenic loci (i.e. bleeding), heterogeneous enhancement or
regional lymph node swelling; CT reveals metastasis or invasion out
of the gastrointestinal tract; a Zubrod-Eastern Cooperative
Oncology Group Performance Status ≥2; patient exhibits severe
cardiopulmonary disease or blood coagulation disorders. SMT,
submucosal tumor; CT, computerized tomography; EUS, endoscopic
ultrasonography; MP, muscularis propria; STER, submucosal tunneling
endoscopic resection. |
STER for gastric SMTs
The stomach possesses specific anatomical and
physiological features, including a large lumen, increased
flexibility, an unfixed position and thick mucosa, that render
generating a submucosal tunnel more challenging compared with doing
so in the esophagus, and not all gastric SMTs are suitable for
STER. In addition to those in the cardia, STER may be used as a
treatment for SMTs located in the gastric corpus or fundus
proximate to the cardia, the lesser curvature of the gastric corpus
and the greater curvature of the gastric antrum. Lu et al
(18) treated 18 patients with
gastric fundus SMTs using STER; 19 tumors were removed, en bloc
resection was achieved for all the patients and the mean tumor size
was 21 mm (range, 8–50 mm). Lu et al (19) treated 45 patients with gastric SMTs
using STER, 43 cases were successfully treated and 47 tumors were
removed. The SMTs were all located in the cardia, the gastric
fundus proximate to the cardia or the gastric antrum. En bloc
resection was achieved for all the patients and the mean tumor size
was 14 mm (range, 5–50 mm). Li et al (20) reported on 32 patients with gastric
SMTs who were treated using STER without severe complications. Of
these SMTs, 12 were located in the gastric corpus proximate to the
cardia, 3 in the gastric fundus proximate to the cardia, 6 in the
lesser curvature of the gastric corpus and 11 in the greater
curvature of the gastric antrum. En bloc resection was achieved for
all the patients and the mean tumor size was 23 mm (range, 10–50
mm).
STER for multiple SMTs
Although the majority of the SMTs in the MP layer
are solitary, multiple studies have reported the presence of
multiple SMTs (11,13,18,19,21,22).
Chen et al (21) reported a
patient simultaneously exhibiting esophageal and cardia SMT, and
the two SMTs were successfully removed using STER with a single
tunnel. Zhang et al (22)
treated 23 patients with multiple SMTs in the upper
gastrointestinal tract using STER. A total of 49 SMTs were removed
and 3 of the patients exhibited three coexisting tumors.
STER for esophageal and cardia SMTs
>35 mm
Although the majority of researchers recommended a
maximum resectable lesion size of 35 mm during STER due to the
decreased space for operation in the submucosal tunnel, STER has
been applied multiple times for SMTs >35 mm, with the largest
SMT reported to undergo STER, to the best of our knowledge, being
70 mm (23–29). Wang et al (15) retrospectively analyzed the clinical
data of 80 patients with a total of 83 SMTs, 70 of which were ≤35
mm and 13 of which were >35 mm, and demonstrated that STER
resulted in a similar efficacy and rate of complications for SMTs
≤35 mm and those >35 mm, although an increased operative
duration was demonstrated for the latter compared with the
former.
STER for rectal SMTs
The rectum possesses a thin mucosa and a tortuous
lumen, thereby rendering the generation of a submucosal tunnel more
challenging compared with doing so in the esophagus. To the best of
our knowledge, only one center has reported the use of STER in
patients with rectal SMTs. Hu et al (30) treated 12 patients with rectal SMTs
using STER; en bloc resection was achieved for all the patients and
the median size of the resected tumors was 14 mm (range, 10–30 mm).
No severe complications were detected and no recurrence was
revealed during the 4–33 month follow up.
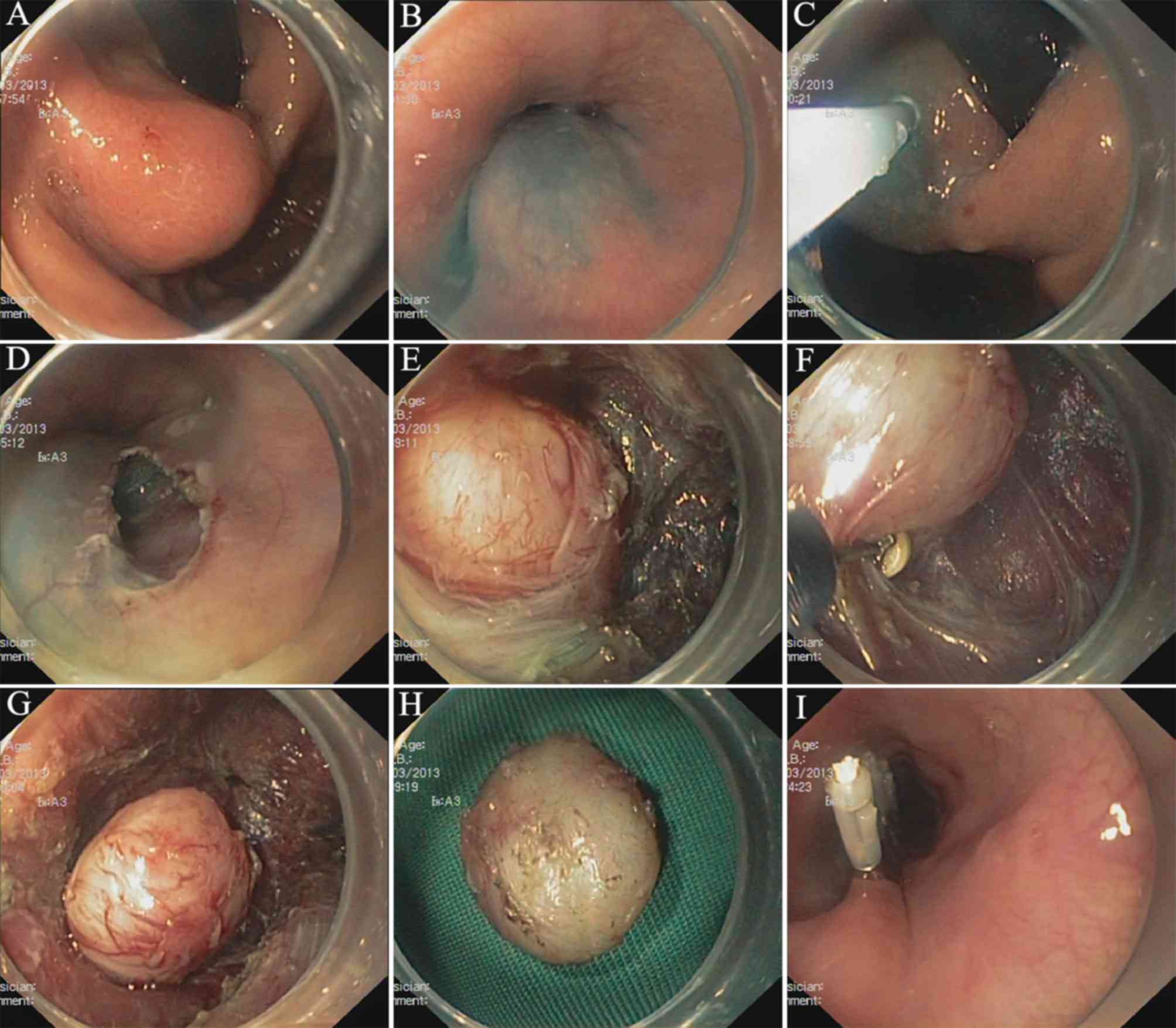
STER procedure
The STER procedure in The Second Xiangya Hospital of
Central South University (Fig. 2) is
typically performed with the patient in the supine or lateral
position under general anesthetic and with the airway intubated.
CO2 insufflation is recommended (29).
Identification of the tumor
The tumor is identified and accurately located. For
SMTs that are challenging to locate, including SMTs proximate to
the fundus of the stomach, the submucosal injection of indigo
carmine or methylene blue may be performed to help locate the tumor
and guide the direction of subsequent tunneling (20).
Submucosal injection
A fluid cushion is subsequently generated through a
submucosal injection consisting of saline solution with indigo
carmine 3–5 cm from the SMT. Typically, epinephrine is added to the
solution to decrease the risk of intraoperative bleeding. For
rectal or gastric SMTs, the submucosal injection is performed 2–3
cm from the SMT (19,20,30).
Generating tunnel entry
A 2 cm, longitudinal mucosa incision is used to
generate tunnel entry. A further submucosal dissection of ≥0.5 cm
along the sides of the longitudinal incision is made to facilitate
tumor extraction and gas diffusion (15,31). For
SMTs >35 mm, the mucosal incision may be increased to the size
of the short dimension of the tumor (32).
Generating the tunnel
A submucosal tunnel extending 2 cm from the tumor is
generated between the submucosal and MP layers using the ESD
method. The selection of ESD knives depends on surgical experience;
available knives include dual, hybrid, triangular-tip and hook
knives. The dissection plane should be maintained proximate to the
MP to decrease the risk of injury to the mucosal flap. The tunnel
should be sufficiently wide and its width should increase according
to the diameter of the SMT to ensure a satisfactory endoscopic view
of the SMT and sufficient space for resection and facilitate
submucosal dissection and gas diffusion (32).
Dissection of the tumor
The tumor is dissected at the MP layer. Complete
resection without damaging the tumor capsule is recommended. For
SMTs originating from the deep MP layer or exhibiting a tight
connection with the underlying MP or serosal layers, a
full-thickness resection, including the lesion, its underlying MP
and serosa is generally performed (10,15,32). For
patients with gastric SMTs undergoing full-thickness resection, the
SMT should be prevented from lodging in the abdominal cavity,
potentially by using laparoscopic assisted endoscopic surgery.
Removing the tumor
Although small SMTs may be easily removed from the
tunnel and extracted from the body, doing so for SMTs >35 mm in
the upper gastrointestinal tract may prove challenging. While
removing SMTs from the upper gastrointestinal tract, the tumor is
grasped such that its long dimensions are respectively parallel and
transverse to the long axis of the esophagus, and the tumor may be
easily extracted through the tunnel orifice and the upper
esophageal sphincter (32). If
preoperative imagery, endoscopy and clinical examination suggest a
benign tumor, a snare may be used following the completion of
resection to cut the tumor while still in the tunnel into ≥2 pieces
to facilitate its extraction from the tunnel (32). An alternative approach is to generate
a second ‘window’, either in the area of the tumor or through a
distal mucosal incision to facilitate en bloc extraction for large
leiomyomas (24,26).
Closing the tunnel entry
Following the removal of the SMT, the wound surface
should be repeatedly washed to decrease the risk of residual tumor
cells. Subsequently, several clips are applied to close the tunnel
entry.
Managing the resected tumor
The specimens are then fixed, embedded with
paraffin, and sectioned. Hematoxylin and eosin and
immunohistochemical staining are performed to detect cluster of
differentiation (CD) 34, CD117, S100 calcium binding proteins,
desmin, survival of motor neuron 1, marker of proliferation Ki-67
and gastrointestinal stromal tumor 1.
Postoperative management
The postoperative symptoms of patients are
monitored, including fever, chest or abdominal pain, dyspnea,
cyanosis, distention and peritonitis. Thoracoabdominal radiography,
second look endoscopy or CT is performed for selected patients with
postoperative symptoms 2 days following the operation. Generally,
patients are kept nil per os for 24 h, subsequently placed
on a liquid diet for multiple days to a week, and gradually
returned to a normal diet following this. Intravenous antibiotics
and potentially hemostatics are administered to patients for 3
days. For patients with upper gastrointestinal SMTs, intravenous
proton pump inhibitors are administered for 3–7 days and orally
administered for multiple weeks following this. For rectal SMTs, it
is necessary to ensure stools remain soft and defecation easy
(30).
Efficacy of STER
Currently, >20 studies have been published with
outcome data based on >700 patients (6–20,22,29,30,32–35).
In these studies, therapeutic success was recorded for >77% of
patients and en bloc resection was achieved in >85% of patients,
while irregularly shaped or larger tumors were risk factors in
piecemeal resection (33). Of the
SMTs reported in these studies, >95% were leimyomas or
gastrointestinal stromal tumors, while the other reported tumors
included lipomas, schwannomas, calcifying fibrous, glomus, granular
cell and nerve sheath tumors, proliferating collagen fibers,
degenerated nodes and aberrant pancreatic tissue. No recurrence was
detected for the patients of these studies. To the best of our
knowledge, there are no randomized, controlled trials comparing
STER with other treatments of SMTs, but three retrospective studies
have been published.
Comparing STER and ESD
Wang et al (34) retrospectively assessed the clinical
data of 39 patients with esophageal leiomyoma, 18 of which received
STER and 21 of which received ESD, and demonstrated that the
efficacy and complications of the two techniques were comparable,
though STER was associated with decreased operating time and
duration of hospital stay, and an increased rate of incision
healing compared with ESD.
Comparing STER and ESE
Lu et al (35)
retrospectively analyzed the clinical data of 77 patients with
upper gastrointestinal SMTs, 42 of which received STER and 35 of
which received ESE, and demonstrated that the efficacy and
complications of the two techniques were comparable, though STER
decreased air leakage for SMTs by >10 mm compared with ESE.
Comparing STER and video-assisted
thoracoscopic surgery (VATS)
Tan et al (32)
retrospectively evaluated the clinical data of 31 patients with
esophageal leiomyoma (diameter, 35–55 mm), 18 of which received
STER and 13 of which received VATS, and revealed that the efficacy
of the two techniques were comparable, though STER was associated
with decreased operation time, a reduced decrease in hemoglobin
level, and decreased cost and duration of hospital stay compared
with VATS.
Complications of STER
In the aforementioned >20 studies, STER has been
performed with a decreased rate of serious complications, and no
STER-associated mortality has been reported. Nonetheless, efforts
should be taken to decrease the risk of adverse events, recognize
them when they occur, and manage them appropriately following
identification. According to a previously published, large-scale
study consisting of 290 patients with SMTs who underwent STER, the
overall incidence of complications was 23.4% (68/290), and only
10.0% of procedures (29/290) required intervention for
complications (29). Furthermore, the
study demonstrated that irregular shape, the location of the tumor
in the deep MP layer, increased procedure time, and air
insufflation were risk factors for major STER-associated
complications.
Intraoperative complications
Aspiration
An important consideration in the use of STER is the
risk of aspiration during induction and intubation, and
communicating with the anesthesiologist is crucial to decrease
this. Standard airway protection methods should be used, including
a rapid induction sequence, to decrease the risk of aggressive
aspiration of mouth contents during intubation.
Bleeding
Bleeding may occur at any time during STER, but
usually results in <100 ml blood loss and may be immediately
controlled via coagulation with the tip of a knife. However, the
availability of electrosurgical hemostatic forceps for the
coagulation of larger vessels is essential. Chen et al
(29) reported a rate of 1.7% (5/290)
for major bleeding (>200 ml). All of those cases were managed
endoscopically and blood transfusion was not required.
Mucosal laceration
Mucosal lacerations may occasionally occur and the
majority are small (<1 cm) and may be closed using ≥1 clip. In
the aforementioned >20 studies, mucosal laceration occurred in a
total of 15 patients and in each case the laceration was closed
using clips without leakage (10,15,16,18,19,29).
Two case reports have reported on large esophageal mucosal
lacerations, which were managed using stent insertions (26,28).
Gas-associated complications
Gas-associated complications include subcutaneous
emphysema, pneumothorax, pneumoperitoneum, and mediastinal
emphysema. Gas-associated complications are the most common
complications of STER and may occur in ≤66.7% of patients
undergoing STER (10). Those patients
who undergo full-thickness resection exhibit an increased rate of
gas-associated complications, though the majority of these are
clinically insignificant and may resolve spontaneously (7,10,12,13,29).
Thoracic drainage is recommended for pneumothorax in patients with
lung collapse >30% and symptoms that include dyspnea, and lung
puncture is recommended for patients with pneumoperitoneum or
emphysema and more apparent symptoms (29). CO2 is recommended as the
insufflation gas instead of air. For CO2 insufflator
set-ups that allow adjustments to CO2 flow, the lower
flow setting should be set once the submucosal tunnel, and
particularly the MP, is breeched. Regardless of whether an
adjustable CO2 insufflator is used, the endoscopist
should use insufflation sparingly while in the submucosal tunnel.
In all the aforementioned studies, STER was not discontinued for
any patients exhibiting intraoperative complications.
Postoperative complications
Fistula
The most challenging potential complication of STER
is leakage from the associated fistula. In >700 patients
undergoing STER that have been described, leaks were uncommon, and
no leak-associated mortalities have been reported. Only one leak
(esophageal-pleural fistula; <0.2%) was reported and it was
managed using clips and thoracic drainage (29).
Infection
Infection includes mediastinitis, peritonitis,
subphrenic and intra-tunnel infections, and symptoms include
chest/abdominal pain and a fever >38°C. In all the
aforementioned >20 studies, significant infection was uncommon,
and mediastinitis, subphrenic and intra-tunnel infections have been
reported in 1, 1 and 2 patients, respectively (14,16,29). All
the reported cases of infection were controlled through
conservative management, and no infection-associated mortalities
have been reported.
Pleural or mediastinal effusion
The majority of effusions are reactive, but may be
considered a normal postoperative change and, as with peroral
endoscopic myotomy for treating patients with achalasia (36), clinically significant effusions are
uncommon. In the >700 patients undergoing STER that have been
described, 16 (2%) and 1 exhibited clinically significant pleural
and mediastinal effusion, respectively, and these cases of effusion
were treated using antibiotics and/or drainage (10,13,14,29).
Bleeding
Although postoperative bleeding is a potential
concern, no cases of postoperative bleeding have been reported in
the aforementioned >20 studies. Other, rare complications that
have been anecdotally presented and may be study-dependent and of
decreased general significance include wound pain and diverticulum
formation (15,16,29).
Conclusions
It is estimated that >1,000 STERs have been
performed globally over the last 5 years (37). The previous studies that reported the
outcomes of >700 STERs (mean follow up, 3.5–22.7 months),
demonstrating an en bloc resection rate of 85.7–100%, negligible
severe morbidity, and no mortality or recurrence. In addition, ≤10%
of the patients enrolled in these studies exhibited
intervention-requiring complications. These favorable outcomes
suggested that STER may represent a promising treatment for
patients with SMTs. However, STER remains a complicated endoscopic
surgery that requires a multidisciplinary team with expertise in
surgery and advanced endoscopy, and the patients for which STER is
performed should be selected carefully.
Glossary
Abbreviations
Abbreviations:
|
SMT
|
submucosal tumor
|
|
EUS
|
endoscopic ultrasonography
|
|
ESD
|
endoscopic submucosal dissection
|
|
ESE
|
endoscopic submucosal excavation
|
|
STER
|
submucosal tunneling endoscopic
resection
|
|
MP
|
muscularis propria
|
References
|
1
|
Nishida T, Kawai N, Yamaguchi S and
Nishida Y: Submucosal tumors: Comprehensive guide for the diagnosis
and therapy of gastrointestinal submucosal tumors. Dig Endosc.
25:479–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim GH: Endoscopic resection of
subepithelial tumors. Clin Endosc. 45:240–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD,
Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW and Liu JZ: Endoscopic
full-thickness resection without laparoscopic assistance for
gastric submucosal tumors originated from the muscularis propria.
Surg Endosc. 25:2926–2931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Q, Zhong YS, Yao LQ, Zhou PH, Xu MD
and Wang P: Endoscopic submucosal dissection for treatment of
esophageal submucosal tumors originating from the muscularis
propria layer. Gastrointest Endosc. 74:1194–1200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Ye LP, Zhu LH, Zhou XB, Mao XL
and Ding JX: Endoscopic muscularis excavation for subepithelial
tumors of the esophagogastric junction originating from the
muscularis propria layer. Dig Dis Sci. 58:1335–1340. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue H, Ikeda H, Hosoya T, Onimaru M,
Yoshida A, Eleftheriadis N, Maselli R and Kudo S: Submucosal
endoscopic tumor resection for subepithelial tumors in the
esophagus and cardia. Endoscopy. 44:225–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS,
Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ and Yao LQ: Submucosal
tunneling endoscopic resection: A new technique for treating upper
GI submucosal tumors originating from the muscularis propria layer
(with videos). Gastrointest Endosc. 75:195–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong W, Xiong Y, Zhi F, Liu S, Wang A and
Jiang B: Preliminary experience of endoscopic submucosal tunnel
dissection for upper gastrointestinal submucosal tumors. Endoscopy.
44:231–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou XB,
He SQ, Chen JY and Jin X: Submucosal tunnelling endoscopic
resection for the treatment of esophageal submucosal tumours
originating from the muscularis propria layer: An analysis of 15
cases. Dig Liver Dis. 45:119–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu BR, Song JT, Kong LJ, Pei FH, Wang XH
and Du YJ: Tunneling endoscopic muscularis dissection for
subepithelial tumors originating from the muscularis propria of the
esophagus and gastric cardia. Surg Endosc. 27:4354–4359. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiao CH, Yang SP, Li XL, Ding J, Xu YH,
Tao G, Chen L, Zhang DQ, He X, Chen WK and Shi RH: Preliminary
exploration on submucosal tunneling endoscopic resection for middle
and lower esophagus submucosal tumors. Zhonghua Yi Xue Za Zhi.
93:2388–2391. 2013.(In Chinese). PubMed/NCBI
|
|
12
|
Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X and
Chen JY: Submucosal tunneling endoscopic resection for small upper
gastrointestinal subepithelial tumors originating from the
muscularis propria layer. Surg Endosc. 28:524–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XY, Xu MD, Yao LQ, Zhou PH, Pleskow
D, Li QL, Zhang YQ, Chen WF and Zhong YS: Submucosal tunneling
endoscopic resection for submucosal tumors of the esophagogastric
junction originating from the muscularis propria layer: A
feasibility study (with videos). Surg Endosc. 28:1971–1977. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou DJ, Dai ZB, Wells MM, Yu DL, Zhang J
and Zhang L: Submucosal tunneling and endoscopic resection of
submucosal tumors at the esophagogastric junction. World J
Gastroenterol. 21:578–583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Tan Y, Zhou Y, Wang Y, Li C, Zhou
J, Zhang J and Liu D: Submucosal tunneling endoscopic resection for
upper gastrointestinal submucosal tumors originating from the
muscularis propria layer. Eur J Gastroenterol Hepatol. 27:776–780.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao H, Sheng H, Huang L, Jiang L, Xie Y
and Zhou P: Submucosal tunneling endoscopic resection in the
treatment of esophageal submucosal tumors originating from
muscularis propria layer. Zhonghua Wei Chang Wai Ke Za Zhi.
18:478–482. 2015.(In Chinese). PubMed/NCBI
|
|
17
|
Li B, Liu J, Lu Y, Hao J, Liu H, Jiang J,
Jiang Y, Qin C and Xu H: Submucosal tunneling endoscopic resection
for tumors of the esophagogastric junction. Minim Invasive Ther
Allied Technol. 25:141–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu J, Zheng M, Jiao T, Wang Y and Lu X:
Transcardiac tunneling technique for endoscopic submucosal
dissection of gastric fundus tumors arising from the muscularis
propria. Endoscopy. 46:888–892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu J, Jiao T, Li Y, Liu Y, Wang Y, Wang Y,
Zheng M and Lu X: Heading toward the right direction-solution
package for endoscopic submucosal tunneling resection in the
stomach. PLoS One. 10:e01198702015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li QL, Chen WF, Zhang C, Hu JW, Zhou PH,
Zhang YQ, Zhong YS, Yao LQ and Xu MD: Clinical impact of submucosal
tunneling endoscopic resection for the treatment of gastric
submucosal tumors originating from the muscularis propria layer
(with video). Surg Endosc. 29:3640–3646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Xu Z, Huo J and Liu D: Submucosal
tunneling endoscopic resection for simultaneous esophageal and
cardia submucosal tumors originating from the muscularis propria
layer (with video). Dig Endosc. 27:155–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang C, Hu JW, Chen T, Zhou PH, Zhong YS,
Zhang YQ, Chen WF, Li QL, Yao LQ and Xu MD: Submucosal tunneling
endoscopic resection for upper gastrointestinal multiple submucosal
tumors originating from the muscular propria layer: A feasibility
study. Indian J Cancer. 51 (Suppl 2):e52–e55. 2015.PubMed/NCBI
|
|
23
|
Tan Y and Liu D: En bloc submucosal
tunneling endoscopic resection for a giant esophageal leiomyoma.
Gastrointest Endosc. 82:3992015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ng JJ, Chiu PW, Shabbir A and So JB:
Removal of a large, 40-mm, submucosal leiomyoma using submucosal
tunneling endoscopic resection and extraction of specimen using a
distal mucosal incision. Endoscopy. 47 Suppl 1:E232–E233. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maydeo A, Sharma A, Bhandari S and Dhir V:
Submucosal tunneling and endoscopic resection of a large,
esophageal leiomyoma. Gastrointest Endosc. 82:9542015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan Y, Zhu H, Lv L and Liu D: Enlarging an
accidental mucosotomy to facilitate tumor extraction during
submucosal tunneling endoscopic resection for a giant esophageal
leiomyoma. Gastrointest Endosc. 83:248–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu H, Wei LL, Zhang YZ, Sha QM, Huang Y,
Qin CY and Xu HW: Submucosal tunnelling endoscopic resection (STER)
for the treatment of a case of huge esophageal tumor arising in the
muscularis propria: A case report and review of literature. Int J
Clin Exp Med. 8:15846–15851. 2015.PubMed/NCBI
|
|
28
|
Kumbhari V, Saxena P, Azola A, Messallam
AA, El Zein MH and Khashab MA: Submucosal tunneling endoscopic
resection of a giant esophageal leiomyoma. Gastrointest Endosc.
81:219–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen T, Zhang C, Yao LQ, Zhou PH, Zhong
YS, Zhang YQ, Chen WF, Li QL, Cai MY, Chu Y and Xu MD: Management
of the complications of submucosal tunneling endoscopic resection
for upper gastrointestinal submucosal tumors. Endoscopy.
48:149–155. 2016.PubMed/NCBI
|
|
30
|
Hu JW, Zhang C, Chen T, Zhou PH, Zhong YS,
Zhang YQ, Chen WF, Li QL, Yao LQ and Xu MD: Submucosal tunneling
endoscopic resection for the treatment of rectal submucosal tumors
originating from the muscular propria layer. J Cancer Res Ther. 10
Suppl:S281–S286. 2014. View Article : Google Scholar
|
|
31
|
Wang X, Tan Y, Zhang J and Liu D: Risk
factors for gas-related complications of peroral endoscopic myotomy
in achalasia. Neth J Med. 73:76–81. 2015.PubMed/NCBI
|
|
32
|
Tan Y, Lv L, Duan T, Zhou J, Peng D, Tang
Y and Liu D: Comparison between submucosal tunneling endoscopic
resection and video-assisted thoracoscopic surgery for large
esophageal leiomyoma originating from the muscularis propria layer.
Surg Endosc. 30:3121–3127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen T, Zhou PH, Chu Y, Zhang YQ, Chen WF,
Ji Y, Yao LQ and Xu MD: Long-term outcomes of submucosal tunneling
endoscopic resection for upper gastrointestinal submucosal tumors.
Ann Surg. 265:363–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Ren W, Zhang Z, Yu J, Li Y and
Song Y: Retrospective study of endoscopic submucosal tunnel
dissection (ESTD) for surgical resection of esophageal leiomyoma.
Surg Endosc. 27:4259–4266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu J, Jiao T, Zheng M and Lu X: Endoscopic
resection of submucosal tumors in muscularis propria: The choice
between direct excavation and tunneling resection. Surg Endosc.
28:3401–3407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang S, Zeng MS, Zhang ZY, Zhang HL, Liang
L and Zhang XW: Pneumomediastinum and pneumoperitoneum on computed
tomography after peroral endoscopic myotomy (POEM): Postoperative
changes or complications? Acta Radiol. 56:1216–1221. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lv X, Wang CH and Xie Y: Efficacy and
safety of submucosal tunneling endoscopic resection for upper
gastrointestinal submucosal tumors: A systematic review and
meta-analysis. Surg Endosc. 31:49–63. 2017. View Article : Google Scholar : PubMed/NCBI
|