Introduction
Cervical carcinoma is the second most prevalent
malignant tumor in females and has a high incidence rate in
developing countries (1,2). There is a continuous development process
from benign lesions to cervical intraepithelial neoplasia (CIN) and
finally carcinoma (3). In total ~30%
of CIN cases are resolved and only a small part of CIN cases
develop into carcinoma (4). Previous
studies have demonstrated that human papilloma virus (HPV)
infection and the inhibition of apoptosis were involved in the
occurrence and development of cervical cancer (5–9). CIN is a
group of precancerous lesions that are closely associated with
cervical carcinoma, including cervical dysplasia and primary
cervical carcinoma. However, the pathogenesis of CIN and carcinoma
remains to be elucidated. Ongoing research aims to elucidate the
mechanism underlying the development of cervical cancer and to
develop reliable biomarkers of cervical cancer for timely diagnosis
and treatment.
Apoptosis, a cellular program that serves an
important role in numerous pathological processes, including
tumorigenesis, involves the sequential activation of a family of
cysteine proteases known as caspases, whose proteolytic activity
promotes cell death (10). The
activity of these apoptotic proteins is downregulated by inhibitory
proteins, termed the inhibitors of apoptosis proteins (IAPs). IAPs
are highly conserved through evolution and have been reported to
bind caspases and prevent caspase activation to control the
induction of apoptosis (11). To
date, numerous IAPs have been identified, which include X-linked
inhibitor of apoptosis (XIAP), cellular IAP-1 (c-IAP1), cellular
IAP-2 (c-IAP2), testis specific IAP (Ts-IAP), survivin, livin and
BRUCE/Apollon. Among these, XIAP, as the most potent suppressor of
apoptosis, has been well characterized. Its baculoviral IAP repeat
(BIR) domains were reported to target and inhibit numerous caspases
(12). In addition, a previous study
demonstrated that the RING domain of XIAP has E3 ubiquitin ligase
activity, which destabilizes caspases following interaction with
the proteasome (13).
Second mitochondria-derived activator of caspase
(Smac), also termed as direct inhibitor of apoptosis-binding
protein with low PI (DIABLO), was identified from
mitochondria-released pro-apoptotic proteins (14). Smac is located in the intermembrane
space in the mitochondria and is released into the cytosol in the
presence of apoptotic stimuli. There, Smac interacts with IAPs and
induces the activation of caspases. Previous studies have revealed
that Smac interacts with mammalian IAPs, including XIAP, c-IAP1,
c-IAP2, melanoma-IAP and survivin, and disrupts the caspase
inhibition activity of IAPs (9,15–20). Furthermore, Smac promotes apoptosis by
binding to c-IAP1 and c-IAP2 via rapid degradation by
autoubiquitination (21). The
aforementioned findings indicate the significance of the balance
between IAPs and Smac.
Previous studies have identified an association
between the expression levels of XIAP and Smac in cervical
carcinoma suggesting there is a close association between XIAP and
Smac in the generation and development of tumors (22,23). The
increased expression level of XIAP was demonstrated to serve an
important role in the carcinogenesis and the development of
cervical carcinoma, which is associated with no or decreased Smac
protein expression levels (24,25).
However, the correlation analysis of these two protein factors in
cervical intraepithelial neoplasia and cervical carcinoma prognosis
remains to be elucidated.
The present study evaluated the expression levels of
XIAP and Smac in normal cervical epithelium, tissues of cervical
intraepithelial neoplasia and cervical carcinoma, and analyzed the
association between their expression levels and carcinogenesis,
development and prognosis of cervical carcinoma.
Materials and methods
Tumor samples
A total of 160 cervical tissue samples were obtained
from patients consecutively recruited at The First Affiliated
Hospital of Wenzhou Medical University (Wenzhou, China) between
January 2007 and March 2010. A total of 69 tissue samples were
associated with CIN (11 with CIN1, 25 with CIN2 and 33 with CIN3)
and 76 tissue samples were identified as cervical carcinoma (62 to
squamous cell and 14 to adenocarcinoma), A total of 15 cases of
normal cervical tissues were used as the control. The age of
patients ranged from 18–79 years, with an average of 40.05. None of
the patients received preoperative radiotherapy, chemotherapy or
other adjuvant therapy, and there were no significant baseline
differences between the 3 groups in age, body weight and the
existence of other internal diseases (Table I). The stages of cervical carcinoma
were categorized according to the International Gynecology and
Obstetrics Federation (FIGO) system (26) (http://www.figo.org/). The samples comprised 8 in
stage 1A, 19 in 1B, 29 in 2A, 12 in 2B and 8 in stage 3, including
34 cases of exogenic type, 13 endogenous types, 18 ulcerative type
and 11 cervical canal tissue samples. According to the
histopathological grade, the tumors included 13
well-differentiated, 26 middle-differentiated and 37
low-differentiated cases. The present study was approved by the
Ethics Committee of Wenzhou Hospital of Integrated Chinese and
Western Medicine (Wenzhou, China). Written informed consent was
obtained from all patients prior to enrollment in the present
study.
 | Table I.Study baseline data. |
Table I.
Study baseline data.
| Variable | Cervical
carcinoma | CIN | Normal cervical
tissue |
|---|
| No. patients
(n) | 76 | 69 | 15 |
| Average age ±
standard deviation (years) | 42.3±3.5 | 39.5±6.9 | 38.7±4.3 |
| No. patients with
hypertension | 15 | 18 | 3 |
| No. patients with
Diabetes Mellitus | 10 | 7 | 2 |
| No. patients that
smoke | 1 | 1 | 0 |
| No. patients that
drink alcohol | 4 | 6 | 2 |
| Average body weight
± standard deviation (kg) | 52.3±5.9 | 55.4±3.7 | 54±4.7 |
Histology
Histopathology was graded according to the World
Health Organization (27)
classification system. The tissue samples were diagnosed by two
senior pathologists from the First Affiliated Hospital of Wenzhou
Medical University who were blinded to the method at the time of
examination. If the two diagnoses did not match, then the two
additional senior pathological experts from the same hospital were
invited to discuss in order to make the final diagnosis.
Immunohistochemistry
Tissue sections <1 cm3 were obtained
from fresh cervical CIN and cervical cancer tissue samples, fixed
in 10% neutral formalin at room temperature for 24 h, then
dehydrated, embedded in paraffin, deparaffinized and rehydrated in
graded ethanol (100, 95, 85 and 75%). Antigen retrieval was
performed by heating the slides (10 min in a microwave oven, 122
mm) in citrate buffer at pH 6.0. Endogenous peroxidase activity was
blocked with 0.3% H2O2 for 10 min at room
temperature. Sections were incubated with primary antibodies at
1:200 dilutions (anti-XIAP; A-7: sc-55550) and anti-Smac (V-17:
sc-12683); Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C, washed with PBS and re-incubated with a secondary
antibody horseradish peroxidase (32230; dilution, 1:500; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 30 min at 37°C.
Diaminobenzidine staining was performed under close monitoring for
5 min at room temperature. Slides were finally counterstained with
hematoxylin at room temperature for 2 min and dehydrated in graded
ethanol (75, 85, 95 and 100%). Finally, the slides were imaged
using an AperioScanScope GL (Aperio Technologies, Vista, CA, USA)
at ×400 magnification.
Evaluation of XIAP/Smac expression
levels
XIAP/Smac immunoreactivity was evaluated by two
pathologists come from the First Affiliated Hospital of Wenzhou
Medical University blind to the procedure. To further validate the
staining of XIAP/Smac in tumor cells, the expression intensity was
graded according to the intensity of positive control and the
percentage of positive tumor cells. A total of 6 fields of view
were randomly selected and analyzed. The slides were first assessed
for expression intensity (0, negative; 1, less intense compared
with positive control; 2, equal intensity to control; 3, more
intense compared with control). Subsequently, the slides were
assessed for the rate of positive cells (0, <5%; 1, 5–25%; 2,
26–50%; 3, >50%; magnification, ×400) using an AperioScanScope
GL (Aperio Technologies). The multiplication product of two points
was used as the final assessment [0, negative (−); 1–4, weakly
positive (+); 5–8, moderate positive (++); 9–12, strong positive
(+++)].
Statistical analysis
In order to investigate the association between
clinical characteristics and XIAP/Smac-positive immunostaining, the
present study used one-way analysis of variance, χ2
tests and Spearman's ρ methods for the nonparametric bivariant
correlation analysis. The survival curve was drawn using the
Kaplan-Meier method, and the survival was analyzed by log-rank
test. The statistical package, SPSS version 17.0 (SPSS, Inc.,
Chicago, IL USA), was used for data analysis. P<0.05 was
considered to indicate a statistically significant difference, or
a=0.05 for bilateral analysis.
Results
XIAP expression level and prediction
of clinical outcome in cervical carcinoma
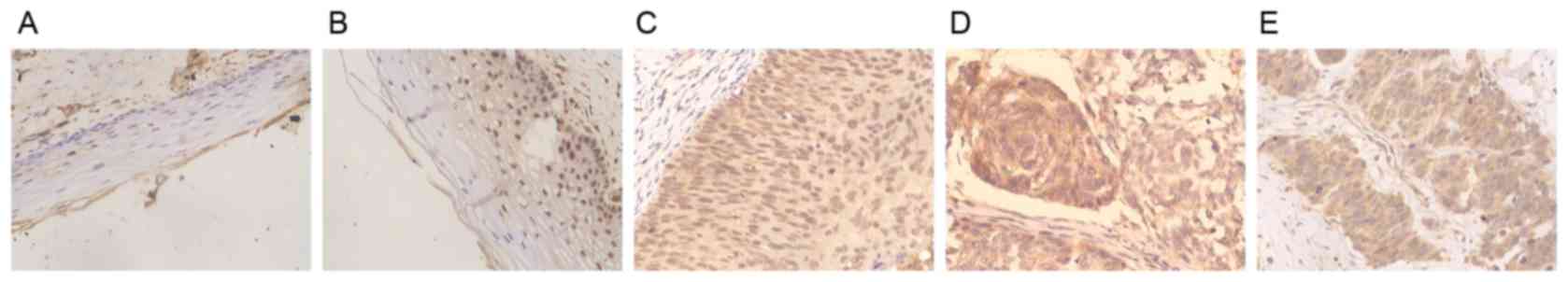
The present study demonstrated that XIAP was
typically localized in the cytoplasm, and its expression level
gradually increased in normal cervical tissue to CIN and then with
increasing cervical cancer stages, with the increasing development
of pathogenesis. Compared with negative or weak staining in normal
cervical tissue and CINI-II cervical carcinoma, the immunostaining
intensity of XIAP was moderate or strong in CINIII cervical
carcinoma, squamous carcinoma and adenocarcinoma (Fig. 1A-E). Cervical intraepithelial
neoplasia was also positively stained with XIAP (data not shown).
The expression level of XIAP exhibited an increase from normal
cervical squamous epithelium (20%, 3/15) to CIN stages (53.6%,
37/69) and again in cervical carcinoma (77.6%, 59/76; Table II). The expression level of XIAP
among the 3 groups was significantly different
(χ2=27.88; P<0.001), and significant differences were
identified between any two groups (P<0.05).
 | Table II.XIAP and Smac expression levels in
cervical cancer tissue samples. |
Table II.
XIAP and Smac expression levels in
cervical cancer tissue samples.
|
|
| No. XIAP-positive
samples | No. Smac-positive
samples |
|---|
|
|
|
|
|
|---|
|
Characteristics | No. patient samples
n | n (%) | P-value | n (%) | P-value |
|---|
| Normal cervical
tissue | 15 | 3 (20) |
<0.01a | 15 (100) |
<0.01a |
| FIGO stage | 69 | 37 (53.6) |
<0.05b | 39 (56.5) | <0.05b |
| CIN
I | 11 | 3 (27.3) |
| 8 (72.7) |
|
| CIN
II | 25 | 11 (44.0) |
| 16 (64.0) |
|
| CIN
III | 33 | 23 (69.7) |
| 15 (45.5) |
|
| Invasive cervical
carcinoma | 76 | 59 (77.6) |
| 27 (35.5) |
|
| Pathological
type |
|
| >0.05 |
| >0.05 |
|
Squamous carcinoma | 62 | 48 (77.4) |
| 21(33.9) |
|
|
Adenocarcinoma | 14 | 11 (78.6) |
| 6 (42.9) |
|
| Tumor grade |
|
|
<0.05b |
|
<0.05b |
| Well
differentiated | 13 | 6 (46.2) |
| 6 (46.2) |
|
|
Moderately differentiated | 26 | 20 (76.9) |
| 9 (34.6) |
|
| Poorly
differentiated | 37 | 33 (89.2) |
| 12 (32.4) |
|
| Clinical stage |
|
|
<0.05b |
|
<0.05b |
| Ia | 8 | 5 (62.5) |
| 4 (50.0) |
|
| Ib | 19 | 12 (63.2) |
| 10 (52.6) |
|
|
IIa | 29 | 23 (79.3) |
| 10 (34.5) |
|
|
IIb | 12 | 11 (91.7) |
| 2 (25.0) |
|
|
III | 8 | 8 (100.0) |
| 1 (12.5) |
|
| Tumor growth
type |
|
| >0.05 |
| >0.05 |
|
Exogenic type | 34 | 26 (76.5) |
| 13 (38.2) |
|
|
Endogenous type | 13 | 11 (84.6) |
| 4 (30.8) |
|
|
Ulcerative type | 18 | 14 (77.8) |
| 6 (33.3) |
|
|
Cervical canal | 11 | 8 (72.7) |
| 4 (36.4) |
|
| Lymphatic
metastasis |
|
|
<0.01c |
| >0.05 |
|
Negative | 29 | 11 (37.9) |
| 11 (37.9) |
|
|
Positive | 21 | 17 (81.0) |
| 9 (42.9) |
|
As XIAP demonstrated increasing levels of expression
in CINI-II and CINIII cervical carcinoma, the present study
suggested that XIAP expression level was associated with the FIGO
stage of the cancer. There were 36 CINI-II and 33 CINIII types in
the tissue samples, and 38.9% (14/36) of CINI-II tissue samples
were stained positively for XIAP, which was reduced compared with
the number of CINIII tissue samples positively for XIAP (69.7%,
23/33; Table I). This indicated that
the expression level of XIAP in CINIII was significantly increased
compared with that in CINI-II tissue samples (χ2=6.571;
P=0.016).
CIN was closely associated with precancerous
lesions, including cervical atypical hyperplasia, which is further
classified into poorly, moderately and well-differentiated grades
(28). In the present study, the
positive expression level of XIAP in well-differentiated cervical
atypical hyperplasia (66.7%, 26/39) was significantly different
compared with that in poorly differentiated hyperplasia (89.2%,
33/37; χ2=5.546; P=0.027; Table II).
The expression level of XIAP was associated with the
histological grade, the clinical stage and the presence of pelvic
lymph node metastasis of the cervical carcinoma. A total of 67.8%
(40/59) of cervical carcinoma with the clinical stage of I–IIa were
stained positively for XIAP, whereas 95.0% (19/20) in stage IIb-III
were XIAP-positive (Table II) and
this difference was statistically significant (χ2=4.715;
P=0.032). The positive expression level of XIAP in the lymph node
metastasis group was 81.0% (17/21), which was significantly higher
compared with tissue samples without lymph node metastasis in
cervical cancer groups (37.9%, 11/29; χ2=9.149; P=0.004;
Table II).
The present study further analyzed the association
between XIAP expression levels and the pathology or tumor type of
cervical carcinoma. The positive expression level of XIAP in
squamous carcinoma and adenocarcinoma was 77.4% (48/62) and 78.6%
(11/14), respectively (Table II;
χ2=0.009; P=1.000). The positive expression levels of
XIAP in tumor types of cervical carcinoma, including exogenic,
endogenous, ulcerative and cervical canal, were 76.5% (26/34),
84.6% (11/13), 77.8% (14/18) and 72.7% (8/11), respectively
(Table II); however, this difference
was not statistically significant (χ2=0.544;
P=0.909).
Smac expression levels and the
prediction of clinical outcome in cervical carcinoma
Smac is primarily localized in the cytoplasm
(14,29). The expression intensity of Smac was
strong in normal cervical tissue, whereas it decreased with an
increase in CIN stage and in cervical cancer, including in cervical
squamous carcinoma and adenocarcinoma tissue samples (Fig. 2A-E). The positive expression level of
Smac in normal cervical tissue samples, CIN stage tissue samples
and cervical cancer tissue samples was 100.00% (15/15), 56.5%
(39/69) and 35.5% (27/76), respectively (Table II). The expression level of Smac
among the three groups was significantly different
(χ2=22.521; P<0.001) and significant differences also
existed between any two groups (P<0.01).
The results of the present study demonstrated that
Smac had significantly increased expression in CINI-II tissues
(66.7%, 24/36) compared with in CINIII cervical carcinoma tissues
(45.5%, 15/33; χ2=5.115; P=0.030; Table II). Smac positive expression levels
in well-differentiated (48.7%, 19/39) and poorly differentiated
cervical atypical hyperplasia (21.6%, 8/37) were significantly
different (χ2=6.086; P=0.017; Table II). The clinical data revealed that
42.9% (24/56) of tissues from clinical stage I-IIa exhibited
positive staining of Smac and was significantly increased compared
with that of clinical stage IIb-III (15.0%, 3/20;
χ2=4.993; P=0.031).
Smac expression level was further analyzed in
squamous carcinoma (33.9%, 21/62) and adenocarcinoma (42.9%, 6/14)
but did not demonstrating a significant difference
(χ2=0.403; P=0.549; Table
II). The present study also revealed that the positive
expression level of Smac in the lymphatic metastasis group was
42.9% (9/21), similar to those without lymph node metastasis
(37.9%, 11/29; χ2=0.123; P=0.776; Table II). There was no significant
difference among the types of cervical carcinoma, which included
exogenic (38.2%, 13/34), endogenous (30.8%, 4/13), ulcerative
(33.3%, 6/18) and cervical canal types (36.4%, 4/11;
χ2=2.259; P=0.520; Table
II).
Associations between the expression
levels of XIAP and Smac and the prognosis of cervical cancer
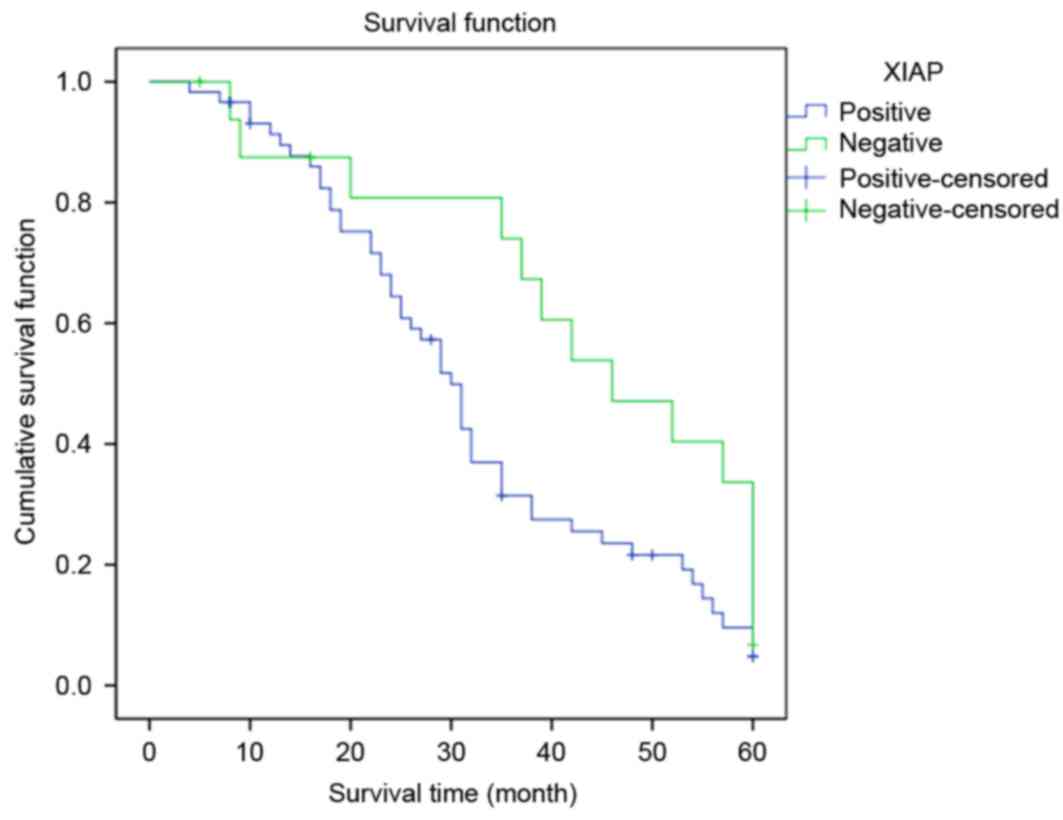
The survival time in the group with high XIAP
expression levels was significantly reduced compared with that of
the group with low XIAP expression levels (log rank=4.291; P=0.038;
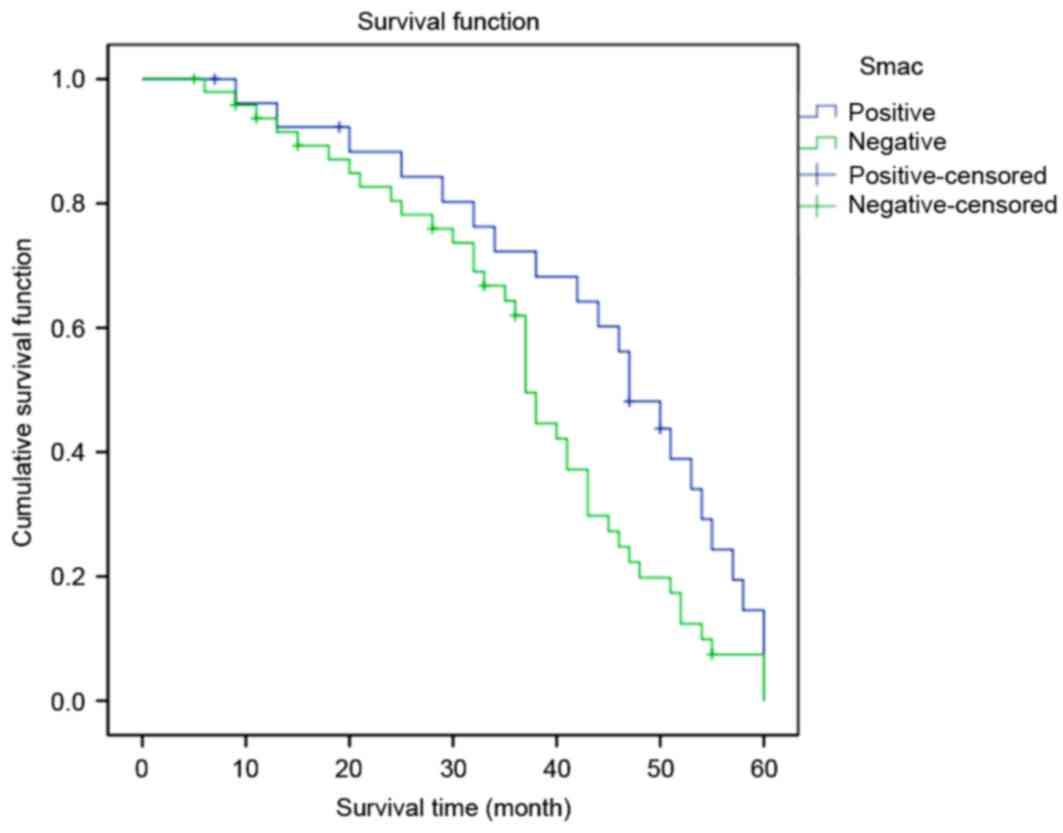
Fig. 3). Conversely, the survival
time in the group with high Smac expression levels was
significantly lower compared with that in the group with low Smac
expression levels (log rank=4.403; P=0.036; Fig. 4).
Associations between XIAP and Smac
expression levels
Among the 76 cervical carcinoma cases, 19 XIAP
positively-stained tumors were negative for Smac, whereas 5
XIAP-negative tumors were identified to be positively-stained for
Smac. The difference was statistically significant (Spearman
coefficient of correlation, r=−0.291; P=0.011), which indicated
that the expression levels of XIAP and Smac were negatively
associated with each other in cervical carcinoma.
Discussion
Proliferation enhancement and apoptosis inhibition
are the two primary mechanisms underlying tumorigenesis (30). Apoptosis is a cell death pathway that
cells are able activate. Compared with a cell proliferation
disorder, the inhibition of apoptosis serves an important role in
the occurrence, development and prognosis of tumors. The inhibition
of apoptosis and cell proliferation disrupts the balance between
cell growth and apoptosis to decrease the cell mortality rate. If
the physiological balance is not restored, it may induce an
increased number of cells with a growth advantage, which is an
important part of tumor formation (31,32).
Previous studies have demonstrated that the IAP
family has important roles in the gene regulation associated with
cell apoptosis (33,34). The IAP family is a class of endogenous
apoptosis inhibitory proteins, involved in tumor, neurodegenerative
and other diseases. XIAP is an effective inhibitor in the IAP
family. Its overexpression was established to be associated with
the occurrence and development of cervical cancer (35,36). The
present study demonstrated that XIAP was primarily localized in the
cytoplasm, and highly expressed in cervical intraepithelial
neoplasia and cervical cancer compared with that in the normal
cervical squamous epithelium. The present study identified that
XIAP expression levels were positively associated with the
malignancy of cervical cancer, indicating function for an increased
expression level of XIAP in cervical squamous tumorigenesis. The
present study also observed that XIAP expression level was closely
associated with lymphatic metastasis, indicating its expression
level was associated with the degree of malignancy and poor
prognosis. The results of the present study demonstrated that
CINIII stage may be pinnacle point in the malignant transformation
of intraepithelial neoplasia lesions. The present study
investigated the association between alterations of expression
levels of XIAP and the occurrence and development of tumors,
including lung cancer, gastrointestinal cancer and breast ductal
carcinoma. The results provided evidence supporting the potential
application of XIAP as a biomarker for the early diagnosis of
malignant tumors.
Smac was first reported as a pro-apoptotic protein
in July 2000 and is widely expressed in human normal tissues and
primarily located in the cell mitochondria (29). Smac has been observed to act
conversely to XIAP (37,38). Previous studies investigating Smac in
numerous tumor tissues suggested that the low expression of Smac
may inhibit the apoptosis of tumor cells (39–42). The
present study demonstrated that Smac is expressed differently among
tissues samples of normal epithelium, CIN and cervical squamous
cell carcinoma. Its expression levels were negatively associated
with the malignancy of cervical cancer, suggesting that the low
expression levels of Smac may be associated with the occurrence of
cervical squamous cell carcinoma, and the sustained low expression
levels may contribute to malignant tumor development. However, the
results of the present study also revealed that Smac may not serve
significant role in the lymphatic metastasis and invasion of
cervical squamous cell carcinoma.
Once cells were exposed to anticancer drugs,
ultraviolet irradiation and another apoptosis signal stimulation,
the active Smac/DIABLO protein is released from mitochondria into
the cytoplasm and promotes apoptosis via its interaction with IAPs.
The N-terminus of Smac is able to identify and interact with the
BIR domain of IAPs. By binding to IAPs, Smac reduces the inhibitive
activity of IAPs on caspase-9, caspase3 and caspase7, and thereby
activates caspases and promotes apoptosis. However, certain studies
revealed there may be other mechanisms underlying the apoptotic
activity of Smac (43–45).
The survival curves in the present study suggested
that a reduced expression level of XIAP or an increased expression
level of Smac provides a significant survival advantage. Increased
expression levels of XIAP or reduced expression levels of Smac were
observed in patients with cervical cancer at advanced stages or
patients with low differentiation or pelvic lymph node metastasis.
Therefore, it was suggested that a high expression level of XIAP
and a simultaneous low expression level of Smac in cervical cancer
may be associated with the progression and prognosis of the
disease.
The present study revealed negative associations
between the expression levels of XIAP and Smac in cervical cancer,
suggesting a potential interaction between these proteins. The
negative correlation between XIAP and Smac expression levels and
the association with their increased or decreased expression level
with cervical cancer, suggested the ratio of XIAP/Smac may be a
potential prognostic indicator for cervical cancer. Further studies
are required to verify this and investigate the mechanisms
underlying the regulation and functions of XIAP/Smac in cervical
cancer.
In conclusion, the present study demonstrated that
XIAP expression levels were positively associated with the
malignancy of cervical cancer, whereas Smac expression levels
revealed a converse association. In addition, the expression levels
of XIAP were negatively associated with that of Smac in cervical
cancer. Further studies focusing on elucidating the interaction
between XIAP and Smac in cervical cancer are required.
Acknowledgements
The authors would like to thank Professor Jing Jin
from Wenzhou Medical University (Wenzhou, China) for her support.
The present study was supported by the Municipal Science and
Technology Foundation of Wenzhou City, Wenzhou, China (grant no.
Y20140110) and the Science Technology Department of Zhejiang
Province, China (grant no. 2013C33098).
References
|
1
|
Husain RS and Ramakrishnan V: Global
variation of human papillomavirus genotypes and selected genes
involved in cervical malignancies. Ann Glob Health. 81:675–683.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmad F and Stewart DE: Predictors of
clinical breast examination among South Asian immigrant women. J
Immigr Health. 6:119–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gadducci A, Guerrieri ME and Greco C:
Tissue biomarkers as prognostic variables of cervical cancer. Crit
Rev Oncol Hematol. 86:104–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kyrgiou M, Mitra A, Arbyn M, Stasinou SM,
Martin-Hirsch P, Bennett P and Paraskevaidis E: Fertility and early
pregnancy outcomes after treatment for cervical intraepithelial
neoplasia: Systematic review and meta-analysis. BMJ. 349:g61922014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shoji Y, Saegusa M, Takano Y, Ohbu M and
Okayasu I: Correlation of apoptosis with tumour cell
differentiation, progression, and HPV infection incervical
carcinoma. J Clin Pathol. 49:134–138. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saslow D, Solomon D, Lawson HW, Killackey
M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur
DC, et al: American cancer society, American society for colposcopy
and cervical pathology, and American society for clinical pathology
screening guidelines for the prevention and early detection of
cervical cancer. Am J Clin Pathol. 137:516–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manzo-Merino J, Contreras-Paredes A,
Vázquez-Ulloa E, Rocha-Zavaleta L, Fuentes-Gonzalez AM and Lizano
M: The role of signaling pathways in cervical cancer and molecular
therapeutic targets. Arch Med Res. 45:525–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Massad LS, Einstein MH, Huh WK, Katki HA,
Kinney WK, Schiffman M, Solomon D, Wentzensen N and Lawson HW: 2012
ASCCP Consensus Guidelines Conference: 2012 updated consensus
guidelines for the management of abnormal cervical cancer screening
tests and cancer precursors. J Low Genit Tract Dis. 17 5 Suppl
1:S1–S27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang P and Yue Y: Human papillomavirus
oncoproteins and apoptosis (Review). Exp Ther Med. 7:3–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu SS, Tsang BK, Cheung AN, Xue WC, Cheng
DK, Ng TY, Wong LC and Ngan HY: Anti-apoptotic proteins, apoptotic
and proliferative parameters and their prognostic significance in
cervical carcinoma. Eur J Cancer. 37:1104–1110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang YL and Li XM: The IAP family:
Endogenous caspase inhibitors with multiple biological activities.
Cell Res. 10:169–177. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Silke J and Meier P: Inhibitor of
apoptosis (IAP) proteins-modulators of cell death and inflammation.
Cold Spring Harb Perspect Biol. 5:a0087302013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lukacs C, Belunis C, Crowther R, Danho W,
Gao L, Goggin B, Janson CA, Li S, Remiszewski S, Schutt A, et al:
The structure of XIAP BIR2: Understanding the selectivity of the
BIR domains. Acta Crystallogr D Biol Crystallogr. 69:1717–1725.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krepler C, Chunduru SK, Halloran MB, He X,
Xiao M, Vultur A, Villanueva J, Mitsuuchi Y, Neiman EM, Benetatos
C, et al: The novel SMAC mimetic birinapant exhibits potent
activity against human melanoma cells. Clin Cancer Res.
19:1784–1794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lecis D, Drago C, Manzoni L, Seneci P,
Scolastico C, Mastrangelo E, Bolognesi M, Anichini A, Kashkar H,
Walczak H and Delia D: Novel SMAC-mimetics synergistically
stimulate melanoma cell death in combination with TRAIL and
Bortezomib. Br J Cancer. 102:1707–1716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Finlay D, Vamos M, González-López M,
Ardecky RJ, Ganji SR, Yuan H, Su Y, Cooley TR, Hauser CT, Welsh K,
et al: Small-molecule IAP antagonists sensitize cancer cells to
TRAIL-induced apoptosis: Roles of XIAP and cIAPs. Mol Cancer Ther.
13:5–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shintani M, Sangawa A, Yamao N and
Kamoshida S: Smac/DIABLO expression in human gastrointestinal
carcinoma: Association with clinicopathological parameters and
survivin expression. Oncol Lett. 8:2581–2586. 2014.PubMed/NCBI
|
|
19
|
Fulda S: Smac mimetics as IAP antagonists.
Semin Cell Dev Bio. 39:132–138. 2015. View Article : Google Scholar
|
|
20
|
Saelens X, Festjens N, Walle L Vande, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang QH and Du C: Smac/DIABLO selectively
reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and
livin in HeLa cells. J Biol Chem. 279:16963–16970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki Y, Nakabayashi Y and Takahashi R:
Ubiquitin-protein ligase activity of X-linked inhibitor of
apoptosis protein promotes proteasomal degradation of caspase-3 and
enhances its anti-apoptotic effect in Fas-induced cell death. Proc
Natl Acad Sci USA. 98:8662–8667. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
MacFarlane M, Merrison W, Bratton SB and
Cohen GM: Proteasome-mediated degradation of Smac during apoptosis:
XIAP promotes Smac ubiquitination in vitro. J Biol Chem.
277:36611–36616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baldus SE, Schwarz E, Lohrey C, Zapatka M,
Landsberg S, Hahn SA, Schmidt D, Dienes HP, Schmiegel WH and
Schwarte-Waldhoff I: Smad4 deficiency in cervical carcinoma cells.
Oncogene. 24:810–819. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Phillipps HR and Hurst PR: XIAP: A
potential determinant of ovarian follicular fate. Reproduction.
144:165–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pecorelli S, Zigliani L and Odicino F:
Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol
Obstet. 105:107–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Böcker W: WHO classification of breast
tumors and tumors of the female genital organs: Pathology
andgenetics. Verh Dtsch Ges Pathol. 86:116–119. 2002.(In German).
PubMed/NCBI
|
|
28
|
Wright TC Jr, Cox JT, Massad LS, Carlson
J, Twiggs LB and Wilkinson EJ: 2001 ASCCP-sponsored Consensus
Workshop: 2001 Consensus guidelines forthe management of women with
cervical intraepithelial neoplasia. J Low Genit Tract Dis.
7:154–167. 2003.PubMed/NCBI
|
|
29
|
Verhagen AM, Ekert PG, Pakusch M, Silke J,
Connolly LM, Reid GE, Moritz RL, Simpson RJ and Vaux DL:
Identification of DIA-BLO, a mammalian protein that promotes
apoptosis by binding toand antagonizing IAP proteins. Cell.
102:43–55. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malchenko S, Galat V, Seftor EA, Vanin EF,
Costa FF, Seftor RE, Soares MB and Hendrix MJ: Cancer hallmarks in
induced pluripotent cells: New insights. J Cell Physiol.
225:390–393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang X, Hao Y, Ferenczy A, Tang SC and
Pater A: Overexpression of anti-apoptotic gene BAG-1 in human
cervical cancer. Exp Cell Res. 247:200–207. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Espinosa M, Cantú D, Herrera N, Lopez CM,
De la Garza JG, Maldonado V and Melendez-Zajgla J: Inhibitors of
apoptosis proteins in human cervical cancer. BMC Cancer. 6:452006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Silke J and Vucic D: IAP family of cell
death and signaling regulators. Methods Enzymol. 545:35–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei Y, Fan T and Yu M: Inhibitor of
apoptosis proteins and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 40:278–288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Estornes Y and Bertrand MJ: IAPs,
regulators of innate immunity and inflammation. Semin Cell Dev
Biol. 39:106–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cavin LG, Wang F, Factor VM, Kaur S,
Venkatraman M, Thorgeirsson SS and Arsura M: Transforming growth
factor-alpha inhibits the intrinsic pathway of c-Myc-induced
apoptosis through activation of nuclear factor-kappaB in murine
hepatocellular carcinomas. Mol Cancer Res. 3:403–412. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Elsawy MA, Tikhonova IG, Martin L and
Walker B: Smac-derived Aza-peptide as an aminopeptidase-resistant
XIAP BIR3 antagonist. Protein Pept Lett. 22:836–843. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hamacher-Brady A, Choe SC, Krijnse-Locker
J and Brady NR: Intramitochondrial recruitment of endolysosomes
mediates Smac degradation and constitutes a novel intrinsic
apoptosis antagonizing function of XIAP E3 ligase. Cell Death
Differ. 21:1862–1876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fulda S, Wick W, Weller M and Debatin KM:
Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced
apoptosis and induce regression of malignant glioma in vivo. Nat
Med. 8:808–815. 2002.PubMed/NCBI
|
|
40
|
Schliep S, Decker T, Schneller F, Wagner H
and Häcker G: Functional evaluation of the role of inhibitor of
apoptosis proteins in chronic lymphocytic leukemia. Exp Hematol.
32:556–562. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kempkensteffen C, Hinz S, Christoph F,
Krause H, Magheli A, Schrader M, Schostak M, Miller K and Weikert
S: Expression levels of the mitochondrial IAP
antagonistsSmac/DIABLO and Omi/HtrA2 in clear-cell renal cell
carcinomas and their prognostic value. J Cancer Res Clin Oncol.
134:543–550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bao ST, Gui SQ and Lin MS: Relationship
between expression of Smac and Survivin and apoptosis of primary
hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
5:580–583. 2006.PubMed/NCBI
|
|
43
|
Vucic D, Stennicke HR, Pisabarro MT,
Salvesen GS and Dixit VM: ML-IAP, a novel inhibitor of apoptosis
that is preferentially expressed in human melanomas. Cur Biol.
10:1359–1366. 2000. View Article : Google Scholar
|
|
44
|
LaCasse EC, Mahoney DJ, Cheung HH,
Plenchette S, Baird S and Korneluk RG: IAP-targeted thera-pies for
cancer. Oncogene. 27:6252–6275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vince JE, Wong WW, Khan N, Feltham R, Chau
D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, et
al: IAP antagonists target cIAP1 to induce TNFalpha-dependent
apoptosis. Cell. 13:682–693. 2007. View Article : Google Scholar
|